Unraveling Calcium Absorption and Distribution in Peach and Nectarine during Fruit Development through 44Ca Isotope Labeling
Abstract
:1. Introduction
2. Results
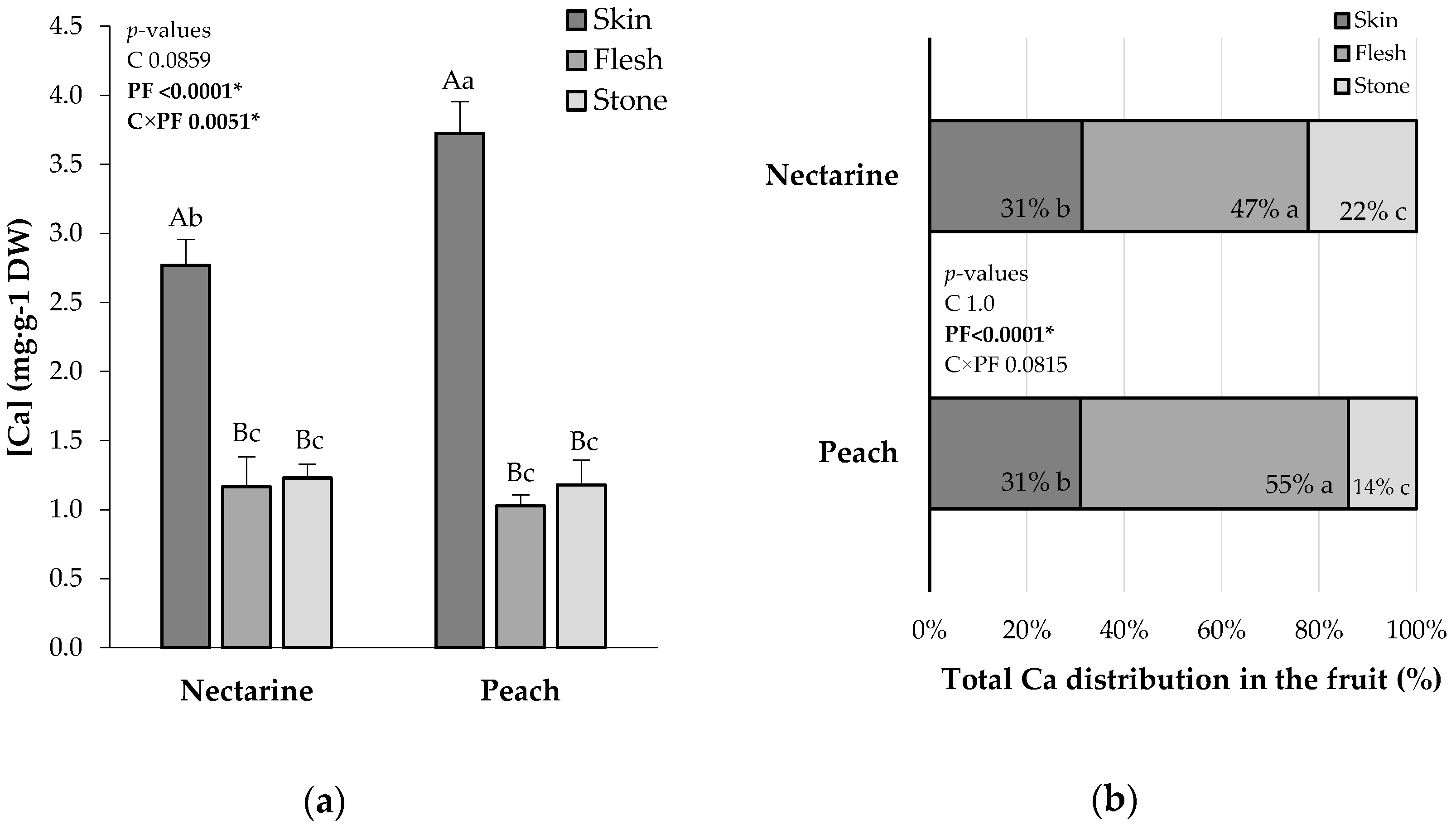
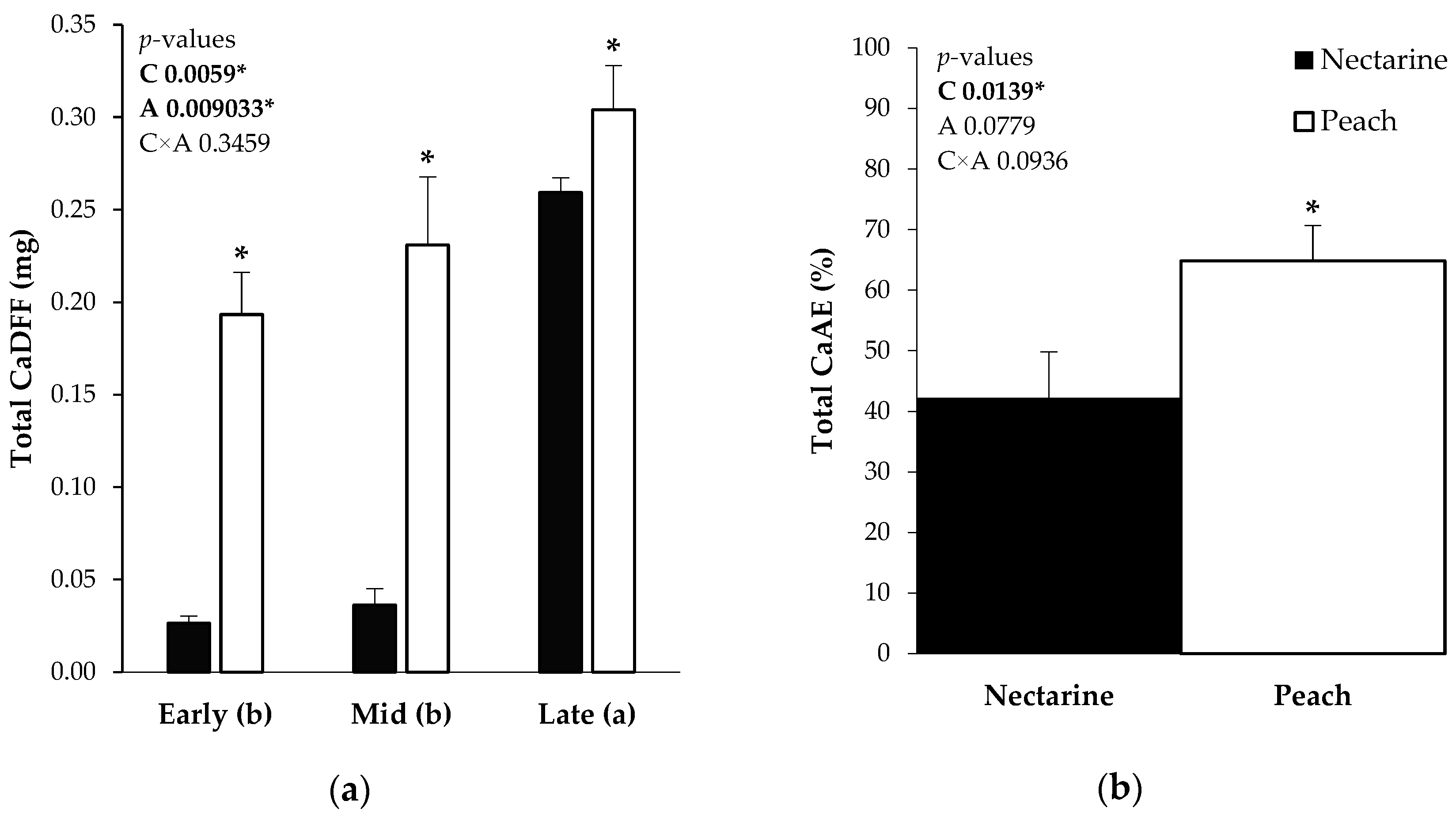
2.1. Total Ca and Ca Derived from the Foliar Fertilizer
2.2. Ca Absorption Efficiency and Ca Fruit Distribution from 44CaCl2 Application
3. Discussion
4. Materials and Methods
4.1. Experimental Conditions and Plant Material
4.2. 44CaCl2 Preparation and Applications
4.3. Collection and Processing of Samples
4.4. Ca and 44Ca-Labeled Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reig, G.; Iglesias, I.; Gatius, F.; Alegre, S. Antioxidant capacity, quality, and anthocyanin and nutrient contents of several peach cultivars [Prunus persica (L.) Batsch] grown in Spain. J. Agric. Food Chem. 2013, 61, 6344–6357. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Minas, I.; Cirilli, M.; Torres, R.; Bassi, D.; Costa, G. Peach for the future: A specialty crop revisited. Sci. Hortic. 2022, 305, 111390. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Martínez-Romero, D.; Castillo, S.; Guillen, F.; Valero, D. Effect of preharvest sprays containing calcium, magnesium, and titanium on the quality of peaches and nectarines at harvest and during postharvest storage. J. Sci. Food Agric. 2004, 84, 1270–1276. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Mignani, I.; Diamantidis, G.; Tzavella-Klonari, K. The effect of preharvest calcium sprays on quality attributes, physicochemical aspects of cell wall components and susceptibility to brown rot of peach fruits (Prunus persica L. cv. Andross). Sci. Hortic. 2005, 107, 43–50. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Wang, R.; Zeng, Y.; Kang, L.; Chen, Z. Nano-calcium alleviates the cracking of nectarine fruit and improves fruit quality. Plant Physiol. Biochem. 2023, 196, 370–380. [Google Scholar] [CrossRef]
- Fernández, V.; Díaz, A.; Blanco, Á.; Val, J. Surface application of calcium-containing gels to improve quality of late maturing peach cultivars. J. Sci. Food Agric. 2009, 89, 2323–2330. [Google Scholar] [CrossRef]
- Val, J.; Fernández, V. In-season calcium-spray formulations improve calcium balance and fruit quality traits of peach. J. Plant Nutr. Soil Sci. 2011, 174, 465–472. [Google Scholar] [CrossRef]
- Peris, M.; Alegre, S. Factors affecting corky spot on nectarine fruits in the Ebro valley in Spain. In VII International Peach Symposium; International Society for Horticultural Science: Lleida, Spain, 2009; Volume 962, pp. 557–562. [Google Scholar]
- Elmer, P.A.G.; Spiers, T.M.; Wood, P.N. Effects of pre-harvest foliar calcium sprays on fruit calcium levels and brown rot of peaches. Crop Prot. 2007, 26, 11–18. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Xiloyannis, C. Fruit calcium accumulation coupled and uncoupled from its transpiration in kiwifruit. J. Plant Physiol. 2015, 181, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, T.; Voulgarakis, A.; Karaiskos, D.; Chatzistathis, T.; Manthos, I.; Dichala, O.; Mpountla, A. Foliar calcium fertilizers impact on several fruit quality characteristics and leaf and fruit nutritional status of the ‘Hayward’kiwifruit cultivar. Agronomy 2021, 11, 235. [Google Scholar] [CrossRef]
- Casero, T.; Torres, E.; Alegre, S.; Recasens, I. Macronutrient accumulation dynamics in apple fruits. J. Plant Nutr. 2017, 40, 2468–2476. [Google Scholar] [CrossRef]
- Dražeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Causes and effects of changes in xylem functionality in apple fruit. Ann. Bot. 2004, 93, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Miqueloto, A.; do Amarante, C.V.T.; Steffens, C.A.; dos Santos, A.; Mitcham, E. Relationship between xylem functionality, calcium content and the incidence of bitter pit in apple fruit. Sci. Hortic. 2014, 165, 319–323. [Google Scholar] [CrossRef]
- Ali, I.; Abbasi, N.A.; Hafiz, I. Application of calcium chloride at different phenological stages alleviates chilling injury and delays climacteric ripening in peach fruit during low-temperature storage. Int. J. Fruit Sci. 2021, 21, 1040–1058. [Google Scholar] [CrossRef]
- Torres, E.; Recasens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; ur Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Kalcsits, L.; Van Der Heijden, G.; Reid, M.; Mullin, K. Calcium absorption during fruit development in ‘Honeycrisp’apple measured using 44Ca as a stable isotope tracer. HortScience 2017, 52, 1804–1809. [Google Scholar] [CrossRef]
- Matteo, M.; Zoffoli, J.P.; Van der Heijden, G.; Ayala, M. Calcium absorption by fruit and leaves of sweet cherry trees (Prunus avium L.) by isotope labeling. Sci. Hortic. 2024, 329, 113026. [Google Scholar] [CrossRef]
- Bonomelli, C.; Fernández, V.; Capurro, F.; Palma, C.; Videla, X.; Rojas-Silva, X.; Mártiz, J. Absorption and distribution of calcium (45Ca) applied to the surface of orange (Citrus sinensis) fruits at different developmental stages. Agronomy 2022, 12, 150. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Significance of fruit transpiration on calcium nutrition in developing apricot fruit. J. Plant Nutr. Soil Sci. 2010, 173, 618–622. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 182808. [Google Scholar] [CrossRef]
- Song, W.; Yi, J.; Kurniadinata, O.F.; Wang, H.; Huang, X. Linking fruit Ca uptake capacity to fruit growth and pedicel anatomy, a cross-species study. Front. Plant Sci. 2018, 9, 361537. [Google Scholar] [CrossRef] [PubMed]
- Morandi, B.; Rieger, M.; Grappadelli, L.C. Vascular flows and transpiration affect peach (Prunus persica Batsch.) fruit daily growth. J. Exp. Bot. 2007, 58, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Gibert, C.; Génard, M.; Vercambre, G.; Lescourret, F. Quantification and modelling of the stomatal, cuticular and crack components of peach fruit surface conductance. Funct. Plant Biol. 2010, 37, 264–274. [Google Scholar] [CrossRef]
- Fogle, H.W.; Faust, M. Fruit Growth and Cracking in Nectarines1. J. Am. Soc. Hortic. Sci. 1976, 101, 434–438. [Google Scholar] [CrossRef]
- Fernández, V.; Pimentel, C.; Bahamonde, H.A. Salt hydration and drop drying of two model calcium salts: Implications for foliar nutrient absorption and deposition. J. Plant Nutr. Soil Sci. 2020, 183, 592–601. [Google Scholar] [CrossRef]
- Santos, E.; Montanha, G.S.; Agostinho, L.F.; Polezi, S.; Marques, J.P.R.; de Carvalho, H.W.P. Foliar Calcium Absorption by Tomato Plants: Comparing the Effects of Calcium Sources and Adjuvant Usage. Plants 2023, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Vicente, A.R.; Echeverría, G.; Crisosto, C.H. Postharvest supply-chain management protocols and handling of physiological disorders. In Peach; GB:CABI: Wallingford, UK, 2023; pp. 206–225. [Google Scholar]
- Fernández, V.; Khayet, M.; Montero-Prado, P.; Heredia-Guerrero, J.A.; Liakopoulos, G.; Karabourniotis, G.; Heredia, A. New insights into the properties of pubescent surfaces: Peach fruit as a model. Plant Physiol. 2011, 156, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.T.; Schnabel, G. Infrequent occurrence of peach skin streaking and the role of rainwater attributes on symptom development. Plant Dis. 2019, 103, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, G.; Legout, A.; Midwood, A.J.; Craig, C.A.; Pollier, B.; Ranger, J.; Dambrine, E. Mg and Ca root uptake and vertical transfer in soils assessed by an in situ ecosystem-scale multi-isotopic (26 Mg & 44 Ca) tracing experiment in a beech stand (Breuil-Chenue, France). Plant Soil 2013, 369, 33–45. [Google Scholar]
- Van Der Heijden, G.; Legout, A.; Pollier, B.; Ranger, J.; Dambrine, E. The dynamics of calcium and magnesium inputs by throughfall in a forest ecosystem on base poor soil are very slow and conservative: Evidence from an isotopic tracing experiment (26 Mg and 44 Ca). Biogeochemistry 2014, 118, 413–442. [Google Scholar] [CrossRef]
- Carrasco-Cuello, F.; Jené, L.; Dolcet-Sanjuan, R.; Quiñones, A.; Rufat, J.; Torres, E. Differential response to calcium-labelled (44Ca) uptake and allocation in two peach rootstocks in relation to transpiration under in vitro conditions. Sci. Hortic. 2024, 326, 112718. [Google Scholar] [CrossRef]
- Morales, J.; Martínez-Alcántara, B.; Bermejo, A.; Millos, J.; Legaz, F.; Quiñones, A. Effect of Calcium Fertilization on Calcium Uptake and Its Partitioning in Citrus Trees. Agronomy 2023, 13, 2971. [Google Scholar] [CrossRef]

| Cultivar | Harvest Date | Calcium Application | Application Date | Phenological Stage (BBCH) | Fruit Diameter (mm) | |
|---|---|---|---|---|---|---|
 | Nectarine ‘Esmeralda’ | 09/05/2023 172 DAFB | Early | 06/19/2023 94 DAFB | 73 | 40 |
| Mid | 07/19/2023 124 DAFB | 75 | 48 | |||
| Late | 08/30/2023 166 DAFB | 78 | 61 | |||
 | Peach ‘Tardibelle’ | 09/13/2023 175 DAFB | Early | 06/19/2023 88 DAFB | 73 | 42 |
| Mid | 07/19/2023 119 DAFB | 75 | 48 | |||
| Late | 08/30/2023 161 DAFB | 78 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Cuello, F.; Van der Heijden, G.; Rufat, J.; Torres, E. Unraveling Calcium Absorption and Distribution in Peach and Nectarine during Fruit Development through 44Ca Isotope Labeling. Plants 2024, 13, 2287. https://doi.org/10.3390/plants13162287
Carrasco-Cuello F, Van der Heijden G, Rufat J, Torres E. Unraveling Calcium Absorption and Distribution in Peach and Nectarine during Fruit Development through 44Ca Isotope Labeling. Plants. 2024; 13(16):2287. https://doi.org/10.3390/plants13162287
Chicago/Turabian StyleCarrasco-Cuello, Francisca, Gregory Van der Heijden, Josep Rufat, and Estanis Torres. 2024. "Unraveling Calcium Absorption and Distribution in Peach and Nectarine during Fruit Development through 44Ca Isotope Labeling" Plants 13, no. 16: 2287. https://doi.org/10.3390/plants13162287





