Olive Leaf Mottling Virus: A New Member of the Genus Olivavirus
Abstract
1. Introduction
2. Results
2.1. Virus-like Symptoms in Olive Trees
2.2. Identification of a New Virus by HTS Analysis of Symptomatic Olive Plants
2.3. Genome Organization of OLMV
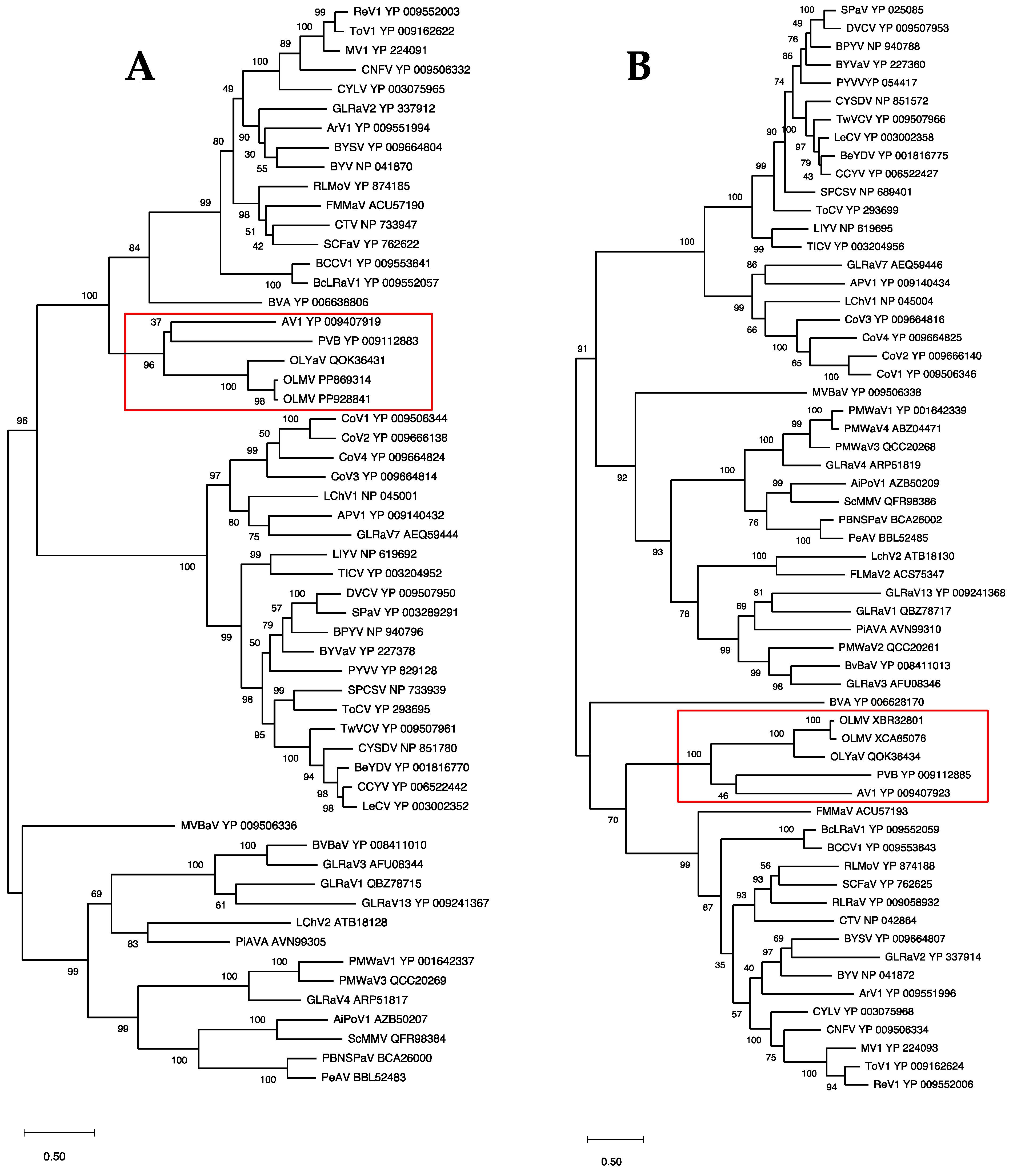
2.4. OLMV Is a New Member of the Genus Olivavirus
2.5. RT-PCR Detection of OLMV in Olive Samples
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plan Testing for Fungal Presence
4.3. RNA Purification and Preparation of HTS Libraries
4.4. Analysis of HTS Datasets and Genome Organization of Olive Leaf Mottling Virus
4.5. Analysis of Encoded Proteins and Phylogenetic Analysis of Olive Leaf Mottling Virus
4.6. Detection of OLMV and OLYaV by RT-PCR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/faostat (accessed on 25 July 2024).
- Faggioli, F.; Barba, M. An elongated virus isolated from olive (Olea europaea L.). Acta Hortic. 1995, 386, 593–599. [Google Scholar] [CrossRef]
- Savino, V.; Sabanadzovic, S.; Scarito, G.; Laviola, C.; Martelli, G.P. Two olive yellows of possible viral origin in Sicily. Inf. Fitopatol. 1996, 46, 55–59. [Google Scholar]
- Martelli, G.P. Infectious diseases and certification of olive: An overview. EPPO Bull. 1999, 29, 127–133. [Google Scholar] [CrossRef]
- Ruiz-García, A.B.; Candresse, T.; Canales, C.; Morán, F.; Machado de Oliveira, C.; Bertolini, E.; Olmos, A. Molecular characterization of the complete coding sequence of olive leaf yellowing-associated virus. Plants 2020, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.P. Infectious diseases of olive. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Transworld Research Network: Kerala, India, 2011; pp. 71–88. [Google Scholar]
- Chiumenti, M.; Greco, C.; De Stradis, A.; Loconsole, G.; Cavalieri, V.; Altamura, G.; Zicca, S.; Saldarelli, P.; Saponari, M. Olea europaea geminivirus: A novel bipartite geminivirid infecting olive trees. Viruses 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Xylogianni, E.; Margaria, P.; Knierim, D.; Sareli, K.; Winter, S.; Chatzivassiliou, E.K. Virus surveys in olive orchards in Greece identify olive virus T, a novel member of the genus Tepovirus. Pathog. 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Marte, M.; Gadani, E.; Savino, V.; Ruigini, E. Strawberry latent ringspot virus associated with a new disease of olive in Central Italy. Plant Dis. 1986, 70, 171–172. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2008, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.W.; Peremyslov, V.V.; Mushegian, A.R.; Dawson, W.O.; Dolja, V.V. Functional specialization and evolution of leader proteinases in the family Closteroviridae. J. Virol. 2001, 75, 12153–12160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Dolja, V.V.; Kreuze, J.F.; Valkonen, J.P. Comparative and functional genomics of closteroviruses. Virus Res. 2006, 117, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, A.; Suzaki, K. An assemblage of divergent variants of a novel putative closterovirus from American persimmon. Virus Genes 2015, 51, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Blouin, A.G.; Biccheri, R.; Khalifa, M.E.; Pearson, M.N.; Poggi Pollini, C.; Hamiaux, C.; Cohen, D.; Ratti, C. Characterization of actinidia virus 1, a new member of the family Closteroviridae encoding a thaumatin-like protein. Arch. Virol. 2018, 163, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability, molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, A.B.; Canales, C.; Morán, F.; Ruiz-Torres, M.; Herrera-Mármol, M.; Olmos, A. Characterization of Spanish olive virome by high throughput sequencing opens new insights and uncertainties. Viruses 2021, 13, 2233. [Google Scholar] [CrossRef] [PubMed]



| Virus | Isolate | Origin | ORF1a | RdRp (ORF1b) | Thaumatin-like Protein (ORF2) | HSP70h (ORF4) | HSP90h (ORF5) | CP (ORF6) |
|---|---|---|---|---|---|---|---|---|
| OLYaV | CS1 (MT809205) | Brazil | 94.58% | 98.61% | 89.47% | 88.79% | 87.02% | 97.86% |
| OL2 (OK569886) | Greece | 96.26% | 98.21% | 94.21% | 97.79% | 96.69% | 97.44% | |
| IVIA158 (PP855214) | Spain | 95.79% | 98.20% | 93.68% | 96.94% | 95.93% | 99.57% | |
| IVIA160 (PP855215) | Spain | 87.80% | 96.61% | 87.89% | 89.64% | 87.02% | 97.44% | |
| J168.2 (PP855216) | Spain | 95.93% | 98.61% | 94.74% | 97.28% | 95.93% | 98.29% | |
| J167.44 (PP855724) | Spain | 95.68% | 97.41% | 93.68% | 96.94% | 96.71% | 96.58% | |
| J168.4 (PP855725) | Spain | 95.71% | 97.22% | 94.21% | 97.11% | 97.09% | 97.01% | |
| OLMV | OLMV158 (PP869314) | Spain | 44.96% | 71.99% | 58.97% | 61.97% | 55.43% | 60.34% |
| OLMV167.95 (PP928841) | Spain | 45.73% | 72.20% | 59.49% | 61.46% | 56.40% | 60.76% |
| nt Position 1 | V1 2/Nº Isolates 3 | V2 4/Nº Isolates | aa Modification 5 |
|---|---|---|---|
| 31 | G/16 | A/4 | G → S |
| 44 | T/12 | A/4 | F → Y |
| 92 | G/19 | C/1 | S → T |
| 173 | C/19 | T/1 | S → F |
| 337 | A/19 | G/1 | I → V |
| 338 | T/17 | C/3 | I → T |
| 400 | A/18 | T/2 | M → L |
| 402 | G/19 | A/1 | M → I |
| 412 | A/19 | T/1 | T → S |
| 478 | T/19 | C/1 | C → R |
| 496 | T/19 | G/1 | S → A |
| 638 | G/15 | A/5 | R → K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-García, A.B.; Candresse, T.; Malagón, J.; Ruiz-Torres, M.; Paz, S.; Pérez-Sierra, A.; Olmos, A. Olive Leaf Mottling Virus: A New Member of the Genus Olivavirus. Plants 2024, 13, 2290. https://doi.org/10.3390/plants13162290
Ruiz-García AB, Candresse T, Malagón J, Ruiz-Torres M, Paz S, Pérez-Sierra A, Olmos A. Olive Leaf Mottling Virus: A New Member of the Genus Olivavirus. Plants. 2024; 13(16):2290. https://doi.org/10.3390/plants13162290
Chicago/Turabian StyleRuiz-García, Ana Belén, Thierry Candresse, José Malagón, Manuel Ruiz-Torres, Sergio Paz, Ana Pérez-Sierra, and Antonio Olmos. 2024. "Olive Leaf Mottling Virus: A New Member of the Genus Olivavirus" Plants 13, no. 16: 2290. https://doi.org/10.3390/plants13162290
APA StyleRuiz-García, A. B., Candresse, T., Malagón, J., Ruiz-Torres, M., Paz, S., Pérez-Sierra, A., & Olmos, A. (2024). Olive Leaf Mottling Virus: A New Member of the Genus Olivavirus. Plants, 13(16), 2290. https://doi.org/10.3390/plants13162290






