Physiological Responses of Crotalaria spp. to the Presence of High Aluminum Availability in the Soil
Abstract
:1. Introduction
2. Material and Methods
2.1. Installation of the Experiment and Soil Preparation
- NC = required amount of limestone per hectare;
- T = cation exchange capacity of the soil at pH 7.00;
- V2 = base saturation percentage (%), required for the crop in question (V2 = 60%);
- V1 = current base saturation percentage (%) of the soil.
2.2. Plant Material and Seed Treatment
2.3. Experimental Design
2.4. Gas Exchange
2.5. Collection and Biometric Evaluations
2.6. Pigments
2.7. Extraction of Soluble Compounds and Quantification
2.7.1. Ureides: Allantoin and Allantoic Acid
2.7.2. Proteins
2.7.3. Amino Acids
2.8. Aluminum Analysis in Plant Biomass
2.9. Aluminum Accumulation in Dry Biomass, Transfer Factor, and Tolerance Index
2.10. Statistical Analysis
3. Results
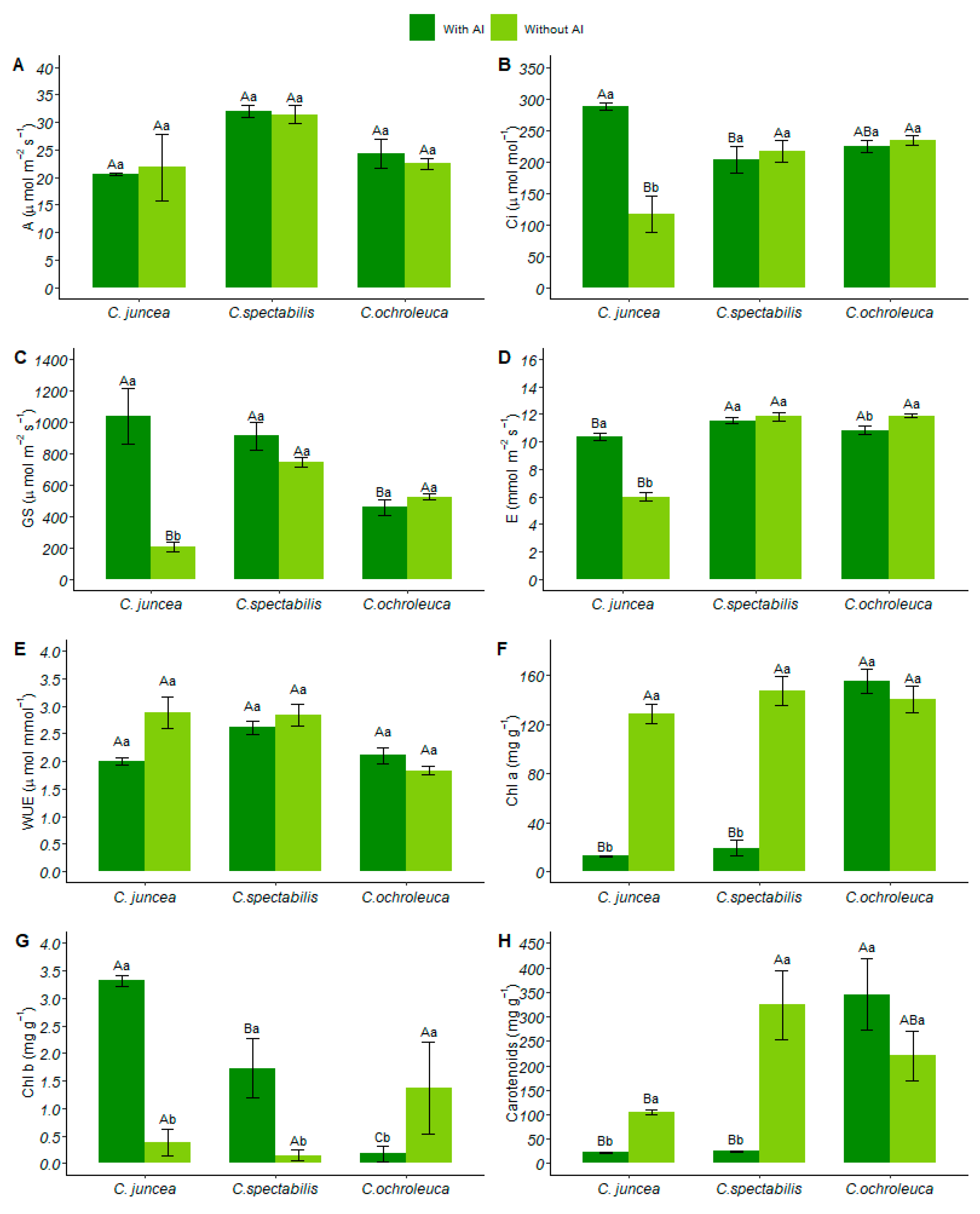
3.1. Gas Exchange
3.2. Pigments
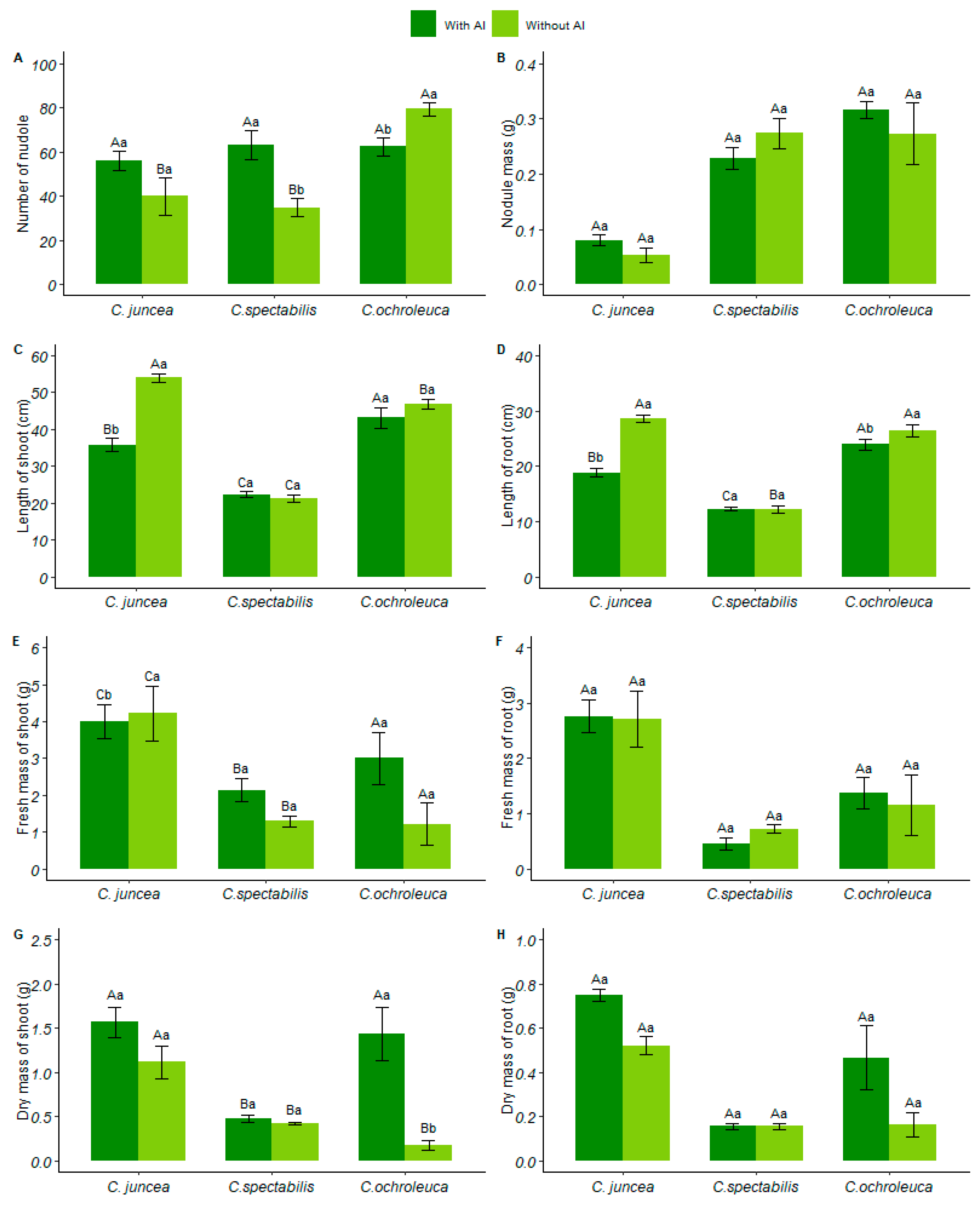
3.3. Biometrics
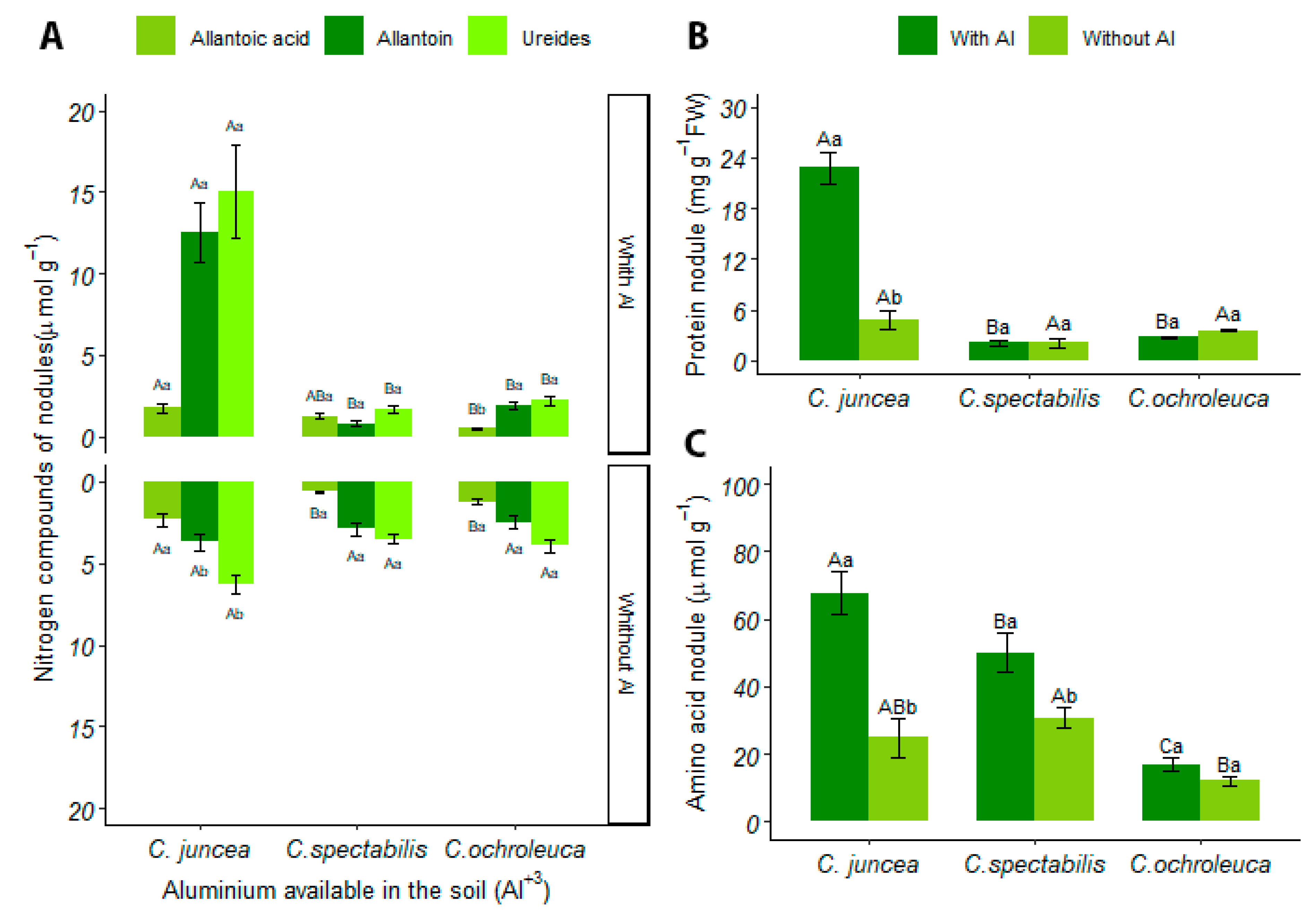
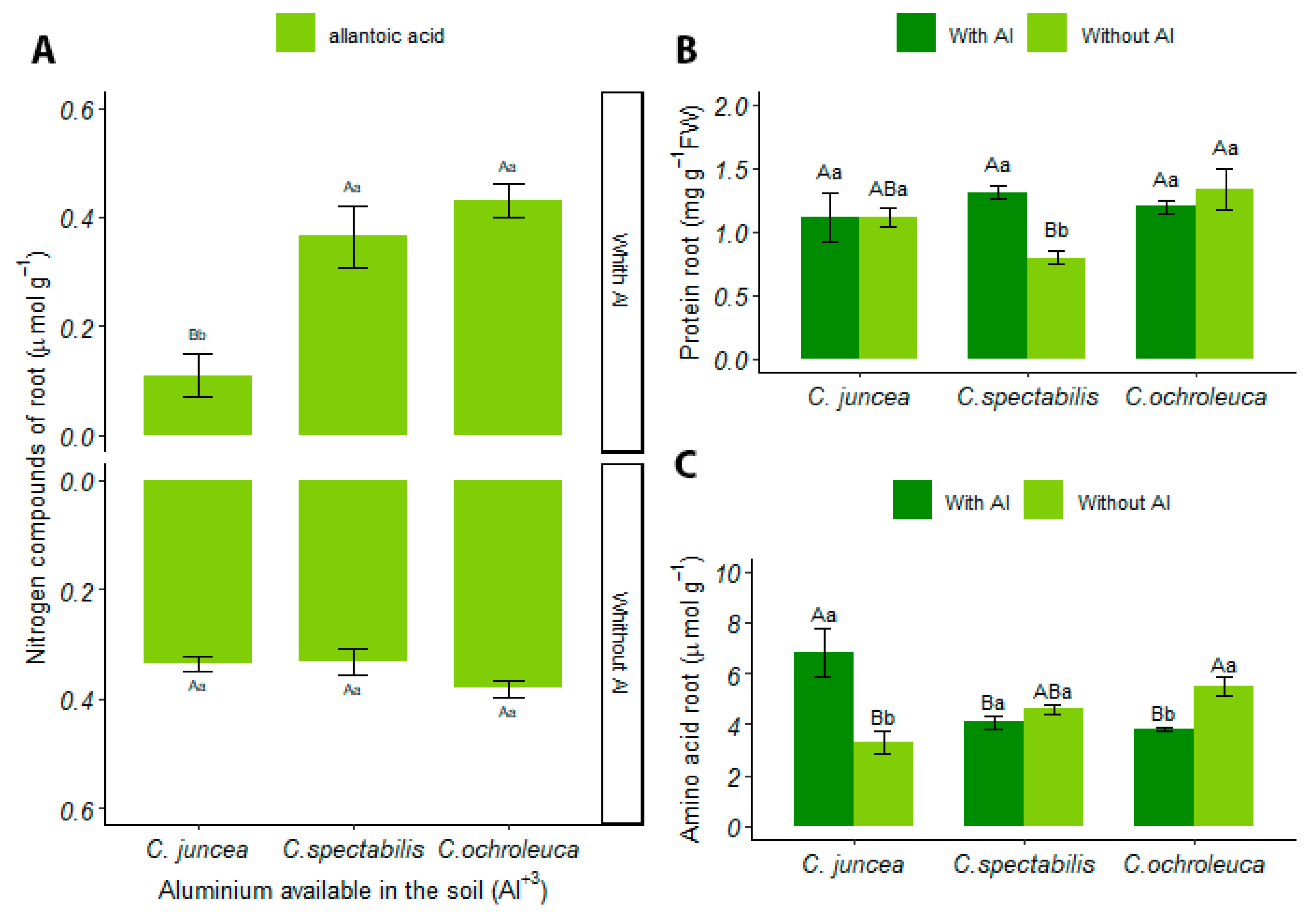
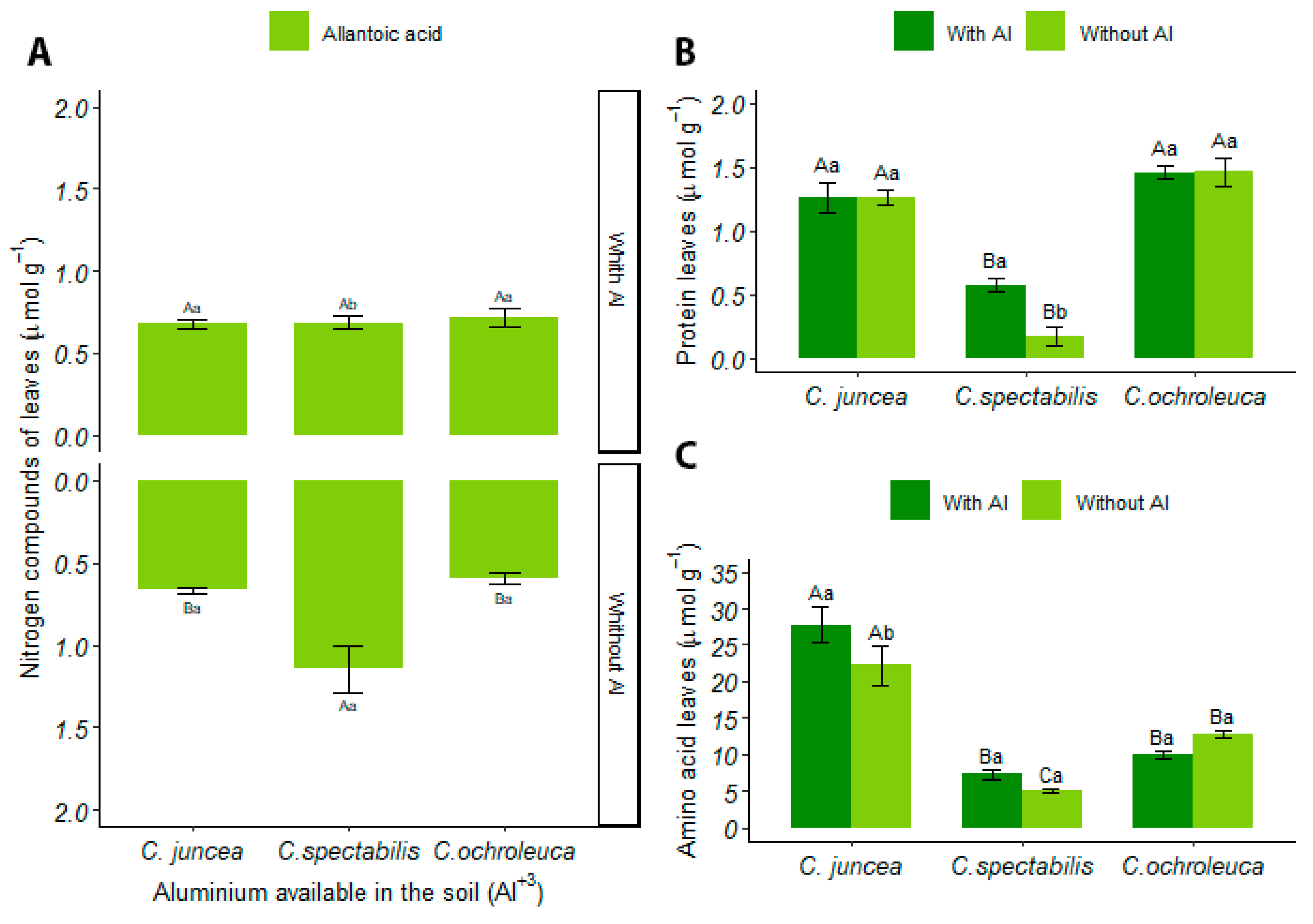
3.4. Quantification of Compounds
3.4.1. Nodules
3.4.2. Root
3.4.3. Leaves
3.5. Aluminum Accumulation in Biomass and Tolerance Index
4. Discussion
4.1. Aluminum Influence on the Photosynthetic Process and Plant Growth
4.2. Nodulation and Translocation of Nitrogenous Compounds in Plants
4.3. Aluminum Tolerance and Accumulation in Plant Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, Y. Aluminum Toxicity in Plant Cells: Mechanisms of Cell Death and Inhibition of Cell Elongation. Soil Sci. Plant Nutr. 2019, 65, 41–55. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum Toxicity in Plants and Its Possible Mitigation in Acid Soils by Biochar: A Review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef]
- Yang, J.; Fan, W.; Zheng, S. Mechanisms and Regulation of Aluminum-Induced Secretion of Organic Acid Anions from Plant Roots. J. Zhejiang Univ.-Sci. B 2019, 20, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Baluška, F.; Matsumoto, H. Aluminum Stress Signaling in Plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J. The Aluminum Tolerance and Detoxification Mechanisms in Plants; Recent Advances and Prospects. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhang, J.H.; Sun, L.M.; Zhu, L.F.; Abliz, B.; Hu, W.J.; Zhong, C.; Bai, Z.G.; Sajid, H.; Cao, X.C.; et al. Hydrogen Sulfide Alleviates Aluminum Toxicity via Decreasing Apoplast and Symplast Al Contents in Rice. Front. Plant Sci. 2018, 9, 294. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Wander, A.E.; da Cunha, C.A. Locais de concentração de atividades agropecuárias na região centro-oeste. Rev. Tecnol. E Soc. 2016, 12, 129–144. [Google Scholar] [CrossRef]
- Martirosyan, G.S.; Sarikyan, K.M.; Adjemyan, G.J.; Hakobyan, A.H.; Pahlevanyan, A.M. The Influence of Green Manure Crops on the Growth, Development, Yield and Fruits Quality of Eggplant. IOP Conf. Ser. Earth Environ. Sci. 2023, 1229, 012006. [Google Scholar] [CrossRef]
- Gut, G.A.P.; Neto, J.V.E.; Santos, R.D.S.; Melo, R.F.D.; Nogueira, D.M.; Difante, G.D.S.; Gurgel, A.L.C.; Santana, Í.L.O. Intercrops of Grass with Legumes as Green Manure for Agroecological Systems. Aust. J. Crop Sci. 2022, 16, 922–927. [Google Scholar] [CrossRef]
- Le Roux, M.; Wyk, B.-E. A Taxonomic Revision of Amphitrichae, a New Section of Crotalaria (Fabaceae). Syst. Bot. 2013, 38, 638–652. [Google Scholar] [CrossRef]
- Yaradua, S.; Alzahrani, D.; Bello, A. Phylogenetic Position of West African Species of the Genus Crotalaria L. (Crotalarieae, Fabaceae) Based on the Current Infrageneric Classification. Pak. J. Bot. 1453, 51, 1453–1458. [Google Scholar] [CrossRef]
- Silva, T.; Macêdo, G.; Soares, N.; Fonseca, M.; Lacerda, G.; Veloso, M.D.D.; Reis, A.; Pimenta, M.; Arrudas, S. Phytoremediation Potential of Crotalaria Juncea Plants in Lead-Contaminated Soils. J. Agric. Sci. 2021, 13, 27. [Google Scholar] [CrossRef]
- Cardoso, P.F.; Gratão, P.L.; Gomes-Junior, R.A.; Medici, L.O.; Azevedo, R.A. Response of Crotalaria Juncea to Nickel Exposure. Braz. J. Plant Physiol. 2005, 17, 267–272. [Google Scholar] [CrossRef]
- Uraguchi, S.; Watanabe, I.; Yoshitomi, A.; Kiyono, M.; Kuno, K. Characteristics of Cadmium Accumulation and Tolerance in Novel Cd-Accumulating Crops, Avena Strigosa and Crotalaria Juncea. J. Exp. Bot. 2006, 57, 2955–2965. [Google Scholar] [CrossRef]
- Franke, I.L.; de Souza Marinho, J.T. Adubação verde com Crotalária juncea no cultivo do milho e pastagem em sistema de integração lavoura pecuária na agricultura familiar no Acre. Cad. Agroecol. 2020, 15, 1–6. [Google Scholar]
- Lindino, C.A.; Tomczak, A.P.; Gonçalves Junior, A.C. Soil phytoremediation using Crotalaria spectabilis for removal of cadmium and lead. Sci. Agrar. Parana. 2012, 11, 25–32. [Google Scholar]
- Meda, A.R.; Furlani, P.R. Tolerance to Aluminum Toxicity by Tropical Leguminous Plants Used as Cover Crops. Braz. Arch. Biol. Technol. 2005, 48, 309–317. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasilia, Brazil, 2018; ISBN 978-85-7035-817-2. [Google Scholar]
- van RAIJ, B.; de ANDRADE, J.C.; Cantarella, H.; Quaggio, J.A. ANÁLISE QUÍMICA PARA AVALIAÇÃO DA FERTILIDADE DE SOLOS TROPICAIS. 300; SCIRP: Wuhan, China, 2001. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A. Manual de métodos de análise de solo.; Embrapa: Brasilia, Brazil, 2017; ISBN 978-85-7035-771-7. [Google Scholar]
- de Sousa, D.M.G.; Lobato, E. Cerrado: Correção do solo e adubação; Brasília, DF: Embrapa Informação Tecnológica; Embrapa: Brasilia, Brazil, 2004; ISBN 978-85-7383-230-3. [Google Scholar]
- Prado, C.H.B.A.; Casali, C. Fisiologia Vegetal: Práticas Em Relações Hídricas, Fotossíntese e Nutrição Mineral; Universidades en Brasil: São Paulo, Brazil, 2006; ISBN 978-85-204-1553-5. [Google Scholar]
- Bomfim, N.C.P.; Aguilar, J.V.; Ferreira, T.C.; dos Santos, B.S.; de Paiva, W.d.S.; de Souza, L.A.; Camargos, L.S. Root Development in Leucaena Leucocephala (Lam.) de Wit Enhances Copper Accumulation. Environ. Sci. Pollut. Res. 2023, 30, 80245–80260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.-X.; Wang, J.; Qi, C.; Wang, X.; Wang, G.; Li, M.; Li, X.; Guo, Y.-D. BoALMT1, an Al-Induced Malate Transporter in Cabbage, Enhances Aluminum Tolerance in Arabidopsis Thaliana. Front. Plant Sci. 2018, 8, 8,435–444. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, K.J.; DAY, W.; Leach, J.E. A Portable System for Measuring the Photosynthesis and Transpiration of Graminaceous Leaves. J. Exp. Bot. 1980, 31, 1441–1453. [Google Scholar] [CrossRef]
- Marshall, B.; Biscoe, P.V. A Model for C3 Leaves Describing the Dependence of Net Photosynthesis on Irradiance. J. Exp. Bot. 1980, 31, 29–39. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and Estimation of Amino Acids in Crude Plant Extracts by Thin-Layer Electrophoresis and Chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van Der Drift, C. Differential Analyses of Glyoxylate Derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. de Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações.; POTAFOS: Atwater, CA, USA, 1997. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 August 2024).
- Bressan, A.C.G.; de Oliveira Carvalho Bittencourt, B.M.; Silva, G.S.; Habermann, G. Could the Absence of Aluminum (Al) Impair the Development of an Al-Accumulating Woody Species from Brazilian Savanna? Theor. Exp. Plant Physiol. 2021, 33, 281–292. [Google Scholar] [CrossRef]
- Banhos, O.F.A.A.; de O. Carvalho, B.M.; da Veiga, E.B.; Bressan, A.C.G.; Tanaka, F.A.O.; Habermann, G. A Diminuição Da Assimilação de CO2 Induzida Pelo Alumínio No Limoeiro “Cravo” Está Associada à Baixa Condutância Estomática e Não Ao Baixo Desempenho Fotoquímico. Sci. Hortic. 2016, 205, 133–140. [Google Scholar] [CrossRef]
- Silva, C.M.S.; Zhang, C.; Habermann, G.; Delhaize, E.; Ryan, P.R. Does the Major Aluminium-Resistance Gene in Wheat, TaALMT1, Also Confer Tolerance to Alkaline Soils? Plant Soil 2018, 424, 451–462. [Google Scholar] [CrossRef]
- Dong, Z.; Shi, L.; Wang, Y.; Chen, L.; Cai, Z.; Wang, Y.; Jin, J.; Li, X. Identification and Dynamic Regulation of microRNAs Involved in Salt Stress Responses in Functional Soybean Nodules by High-Throughput Sequencing. Int. J. Mol. Sci. 2013, 14, 2717–2738. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Zheng, Z.; Dong, J.; Song, L.; Sui, L.; Nussaume, L.; Desnos, T.; Liu, D. Genetic Dissection of Fe-Dependent Signaling in Root Developmental Responses to Phosphate Deficiency. Plant Physiol. 2019, 179, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Pinto, G.; Dias, M.C.; Correia, C.M.; Moutinho-Pereira, J.; Pinto-Carnide, O.; Santos, C. Aluminium Long-Term Stress Differently Affects Photosynthesis in Rye Genotypes. Plant Physiol. Biochem. 2012, 54, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.A.; Ainsworth, E.A.; Amtmann, A.; Cavanagh, A.P.; Driever, S.M.; Ferguson, J.N.; Kromdijk, J.; Lawson, T.; Leakey, A.D.B.; Matthews, J.S.A.; et al. A Guide to Photosynthetic Gas Exchange Measurements: Fundamental Principles, Best Practice and Potential Pitfalls. Plant Cell Environ. 2024, 9, 3344–3364. [Google Scholar] [CrossRef]
- Tominaga, J.; Shimada, H.; Kawamitsu, Y. Direct Measurement of Intercellular CO2 Concentration in a Gas-Exchange System Resolves Overestimation Using the Standard Method. J. Exp. Bot. 2018, 69, 1981–1991. [Google Scholar] [CrossRef]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of Leaf Anatomy in Determining Mesophyll Diffusion Conductance to CO2 across Species: Quantitative Limitations and Scaling up by Models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Flexas, J.; Galmés, J.; Niinemets, U.; Sancho-Knapik, D.; Barredo, G.; Villarroya, D.; Gil-Pelegrín, E. Leaf Anatomical Properties in Relation to Differences in Mesophyll Conductance to CO2 and Photosynthesis in Two Related Mediterranean Abies Species. Plant Cell Environ. 2012, 35, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 Diffusion Pathway inside Leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Hanba, Y.; Miyazawa, S.-I.; Kogami, H.; Terashima, I. Effects of Leaf Age on Internal CO2 Transfer Conductance and Photosynthesis in Tree Species Having Different Types of Shoot Phenology. Funct. Plant Biol. 2001, 28, 1075–1084. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf Functional Anatomy in Relation to Photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.L.; Bravo, L.A.; Cavieres, L.A.; Vallejos, V.; Sanhueza, C.; Font-Carrascosa, M.; Gil-Pelegrín, E.; Javier Peguero-Pina, J.; Galmés, J. Photosynthetic Limitations in Two Antarctic Vascular Plants: Importance of Leaf Anatomical Traits and Rubisco Kinetic Parameters. J. Exp. Bot. 2017, 68, 2871–2883. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Baroli, I.; Price, G.D.; Badger, M.R.; von Caemmerer, S. The Contribution of Photosynthesis to the Red Light Response of Stomatal Conductance. Plant Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef]
- Lawson, T.; von Caemmerer, S.; Baroli, I. Photosynthesis and Stomatal Behaviour. In Progress in Botany 72; Lüttge, U.E., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 265–304. ISBN 978-3-642-13145-5. [Google Scholar]
- Overdieck, D. Water Use Efficiency and Stomatal Conductance. In CO2, Temperature, and Trees: Experimental Approaches; Overdieck, D., Ed.; Springer: Singapore, 2016; pp. 57–64. ISBN 978-981-10-1860-2. [Google Scholar]
- Tuzet, A.; Perrier, A.; Leuning, R. A Coupled Model of Stomatal Conductance, Photosynthesis and Transpiration. Plant Cell Environ. 2003, 26, 1097–1116. [Google Scholar] [CrossRef]
- Ridolfi, M.; Garrec, J.-P. Consequences of an Excess Al and a Deficiency in Ca and Mg for Stomatal Functioning and Net Carbon Assimilation of Beech Leaves. Ann. For. Sci. 2000, 57, 209–218. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Liu, P.; Yang, Y.S.; Xu, G.-D. Effect of Al in Soil on Photosynthesis and Related Morphological and Physiological Characteristics of Two Soybean Genotypes. Bot. Stud. 2007, 48, 2156. [Google Scholar]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic Acid Metabolism in Plants: From Adaptive Physiology to Transgenic Varieties for Cultivation in Extreme Soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of Proline Function in Higher Plants under Extreme Temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fundamentos de Fisiologia Vegetal, 6th ed.; Artmed Editora: Brasilia, Brazil, 2021; ISBN 9786581335113. [Google Scholar]
- Sharma, V.; Yadav, M.; Kumari, N. Aluminium Fluoride Induced Changes in Chlorophyll a Fluorescence, Antioxidants and Psb A Gene Expression of Brassica Juncea Cultivars. J. Plant Interact. 2018, 13, 472–482. [Google Scholar] [CrossRef]
- Ali, B.; Hasan, S.A.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. Um Papel Dos Brassinosteróides Na Melhoria Do Estresse Do Alumínio Por Meio Do Sistema Antioxidante No Feijão Mungo ( Vigna Radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159. [Google Scholar] [CrossRef]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-Induced Changes in Chlorophyll Fluorescence, Photosynthetic Pigments, and Thylakoid Membrane Proteins of Vigna Radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef]
- Ram, A.; Verma, P.; Gadi, B. Effect of Fluoride and Salicylic Acid on Seedling Growth and Biochemical Parameters of Watermelon (Citrullus Lanatus). Fluoride 2014, 47, 49–55. [Google Scholar]
- Lu, M.-Z.; Carter, A.M.; Tegeder, M. Altering Ureide Transport in Nodulated Soybean Results in Whole-Plant Adjustments of Metabolism, Assimilate Partitioning, and Sink Strength. J. Plant Physiol. 2022, 269, 153613. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Atkins, C.A.; Smith, P.M.C. Translocation in Legumes: Assimilates, Nutrients, and Signaling Molecules. Plant Physiol. 2007, 144, 550–561. [Google Scholar] [CrossRef]
- Ferreira, T.C.; Aguilar, J.V.; Souza, L.A.; Justino, G.C.; Aguiar, L.F.; Camargos, L.S. pH Effects on Nodulation and Biological Nitrogen Fixation in Calopogonium Mucunoides. Braz. J. Bot. 2016, 39, 1015–1020. [Google Scholar] [CrossRef]
- Brychkova, G.; Alikulov, Z.; Fluhr, R.; Sagi, M. A Critical Role for Ureides in Dark and Senescence-Induced Purine Remobilization Is Unmasked in the Atxdh1 Arabidopsis Mutant. Plant J. 2008, 54, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Ishiga, Y.; Watanabe, S.; Konishi, T.; Egusa, M.; Akiyoshi, N.; Matsuura, T.; Mori, I.C.; Hirayama, T.; Kaminaka, H.; et al. Allantoin, a Stress-Related Purine Metabolite, Can Activate Jasmonate Signaling in a MYC2-Regulated and Abscisic Acid-Dependent Manner. J. Exp. Bot. 2016, 67, 2519–2532. [Google Scholar] [CrossRef]
- Alamillo, J.M.; Díaz-Leal, J.L.; Sánchez-Moran, M.V.; Pineda, M. Molecular Analysis of Ureide Accumulation under Drought Stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Matsumoto, M.; Hakomori, Y.; Takagi, H.; Shimada, H.; Sakamoto, A. The Purine Metabolite Allantoin Enhances Abiotic Stress Tolerance through Synergistic Activation of Abscisic Acid Metabolism. Plant Cell Environ. 2014, 37, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, L.M.; Prévost, D. Nodulation and Nitrogen Fixation in Extreme Environments. Plant Soil 1994, 161, 115–125. [Google Scholar] [CrossRef]
- Lin, M.-H.; Gresshoff, P.M.; Ferguson, B.J. Systemic Regulation of Soybean Nodulation by Acidic Growth Conditions. Plant Physiol. 2012, 160, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.J.; Lea, P.J.; Rosenberg, C.; Trinchant, J.-C. Nodule Formation and Function. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 101–146. ISBN 978-3-662-04064-5. [Google Scholar]
- Plaxton, W.C.; Podestá, F.E. The Functional Organization and Control of Plant Respiration. Crit. Rev. Plant Sci. 2006, 25, 159–198. [Google Scholar] [CrossRef]
- Sulieman, S.; Ha, C.V.; Schulze, J.; Tran, L.-S.P. Growth and Nodulation of Symbiotic Medicago Truncatula at Different Levels of Phosphorus Availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P. Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant Nutrient-Acquisition Strategies Change with Soil Age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root Developmental Adaptation to Phosphate Starvation: Better Safe than Sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus Acquisition and Use: Critical Adaptations by Plants for Securing a Nonrenewable Resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Raab, T.K.; Rao, I.M.; Terry, N. Effects of Phosphorus Nutrition on Photosynthesis in Glycine Max (L.) Merr. Planta 1990, 181, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, A.; Huss-Danell, K. Interaction Effects of Nitrogen and Phosphorus on Nodulation in Red Clover (Trifolium pratense L.). Acta Agric. Scand. Sect. B—Soil Plant Sci. 2000, 50, 135–142. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of Aluminium on Various Levels of Plant Cells and Organism: A Review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.S.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 Represents an R-Type Anion Channel Required for Stomatal Movement in Arabidopsis Guard Cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, M.R.G.; Hedrich, R. In the Light of Stomatal Opening: New Insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef] [PubMed]
- Hoekenga, O.A.; Maron, L.G.; Piñeros, M.A.; Cançado, G.M.A.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, Which Encodes a Malate Transporter, Is Identified as One of Several Genes Critical for Aluminum Tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A Wheat Gene Encoding an Aluminum-Activated Malate Transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Horst, W.J.; Wang, Y.; Eticha, D. The Role of the Root Apoplast in Aluminium-Induced Inhibition of Root Elongation and in Aluminium Resistance of Plants: A Review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Bennet, R.J.; Breen, C.M.; Bandu, V. Aluminium Toxicity and Regeneration of the Root Cap: Preliminary Evidence for a Golgi Apparatus Derived Morphogen in the Primary Root of Zea Mays. South Afr. J. Bot. 1985, 51, 363–370. [Google Scholar] [CrossRef]
- Rangel, A.F.; Rao, I.M.; Horst, W.J. Spatial Aluminium Sensitivity of Root Apices of Two Common Bean (Phaseolus vulgaris L.) Genotypes with Contrasting Aluminium Resistance. J. Exp. Bot. 2007, 58, 3895–3904. [Google Scholar] [CrossRef]
- Tolrà, R.; Vogel-Mikuš, K.; Hajiboland, R.; Kump, P.; Pongrac, P.; Kaulich, B.; Gianoncelli, A.; Babin, V.; Barceló, J.; Regvar, M.; et al. Localization of Aluminium in Tea (Camellia Sinensis) Leaves Using Low Energy X-Ray Fluorescence Spectro-Microscopy. J. Plant Res. 2011, 124, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Vitorello, V.A.; Capaldi, F.R.; Stefanuto, V.A. Recent Advances in Aluminum Toxicity and Resistance in Higher Plants. Braz. J. Plant Physiol. 2005, 17, 129–143. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium Tolerance in Plants and the Complexing Role of Organic Acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Broadley, M.; Robbrecht, E.; Smets, E. Aluminum Hyperaccumulation in Angiosperms: A Review of Its Phylogenetic Significance. Bot. Rev. 2002, 68, 235–269. [Google Scholar] [CrossRef]



| Chemical Characterization of the Soil | |||
|---|---|---|---|
| With Fertilization | Treatments | ||
| With Al3+ | Without Al3+ | Initial | |
| 4.5 | 5.0 | 4.33 | pH |
| 32 | 49 | 12 | V% |
| 5 | 1 | 11.0 | Al3 (mmolc/dm3) |
| 10.4 | 17.1 | 4.4 | Sum of bases de bases (mmolc/dm3) |
| 6 | 9 | 3.0 | Ca2+ (mmolc/dm3) |
| 1.4 | 1.1 | 0.4 | K+ (mmolc/dm3) |
| 3 | 7 | 1.00 | Mg2+ (mmolc/dm3) |
| 22 | 18 | 31.50 | Potential acidity (H+ + Al3+) (mmolc/dm3) |
| 7 | 7 | 7.0 | Organic matter (g/dm3) |
| 32.4 | 35.1 | 35.4 | Cation exchange capacity-CTC (mmolc/dm3) |
| 3 | 1 | 1.0 | P-resin (mg/dm3) |
| Physical Characterization of the Soil (g/Kg) | |||
|---|---|---|---|
| With Fertilization | Treatments | ||
| Without Al 3+ | Whit Al3+ | Initial | |
| 490 | 487 | 451 | Clay |
| 390 | 395 | 375 | Sand |
| 120 | 118 | 174 | Silt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, B.S.; Ferreira, T.C.; Olívio, M.L.G.; de Souza, L.A.; de Camargos, L.S. Physiological Responses of Crotalaria spp. to the Presence of High Aluminum Availability in the Soil. Plants 2024, 13, 2292. https://doi.org/10.3390/plants13162292
dos Santos BS, Ferreira TC, Olívio MLG, de Souza LA, de Camargos LS. Physiological Responses of Crotalaria spp. to the Presence of High Aluminum Availability in the Soil. Plants. 2024; 13(16):2292. https://doi.org/10.3390/plants13162292
Chicago/Turabian Styledos Santos, Beatriz Silvério, Tassia Caroline Ferreira, Maiara Luzia Grigoli Olívio, Lucas Anjos de Souza, and Liliane Santos de Camargos. 2024. "Physiological Responses of Crotalaria spp. to the Presence of High Aluminum Availability in the Soil" Plants 13, no. 16: 2292. https://doi.org/10.3390/plants13162292





