Identification and Evaluation of Sugarcane Cultivars for Antixenosis Resistance to the Leafhopper Yamatotettix flavovittatus Matsumura (Hemiptera: Cicadellidae)
Abstract
:1. Introduction
2. Results
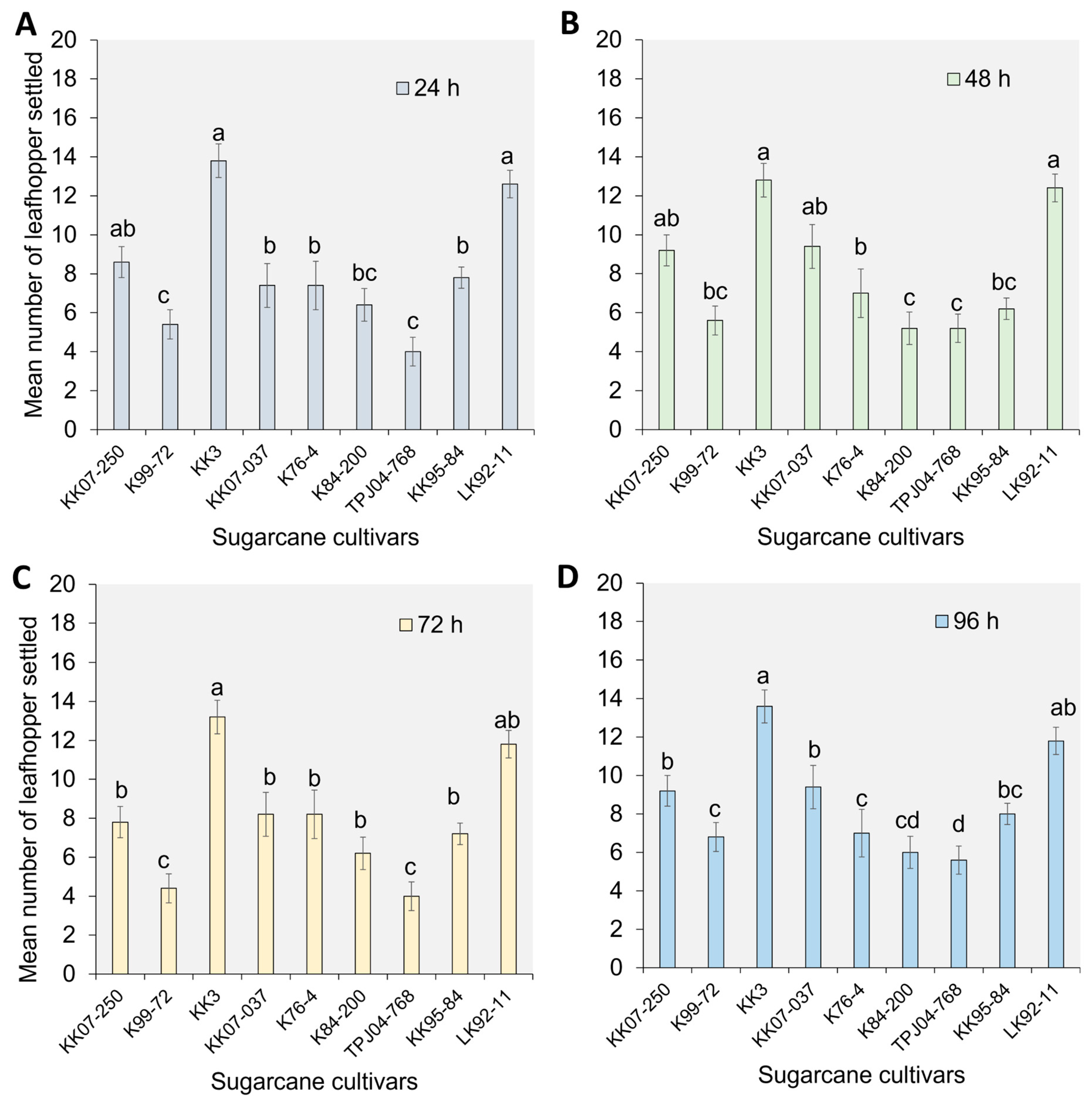
2.1. Settling Preference of Y. flavovittatus Leafhopper Adults
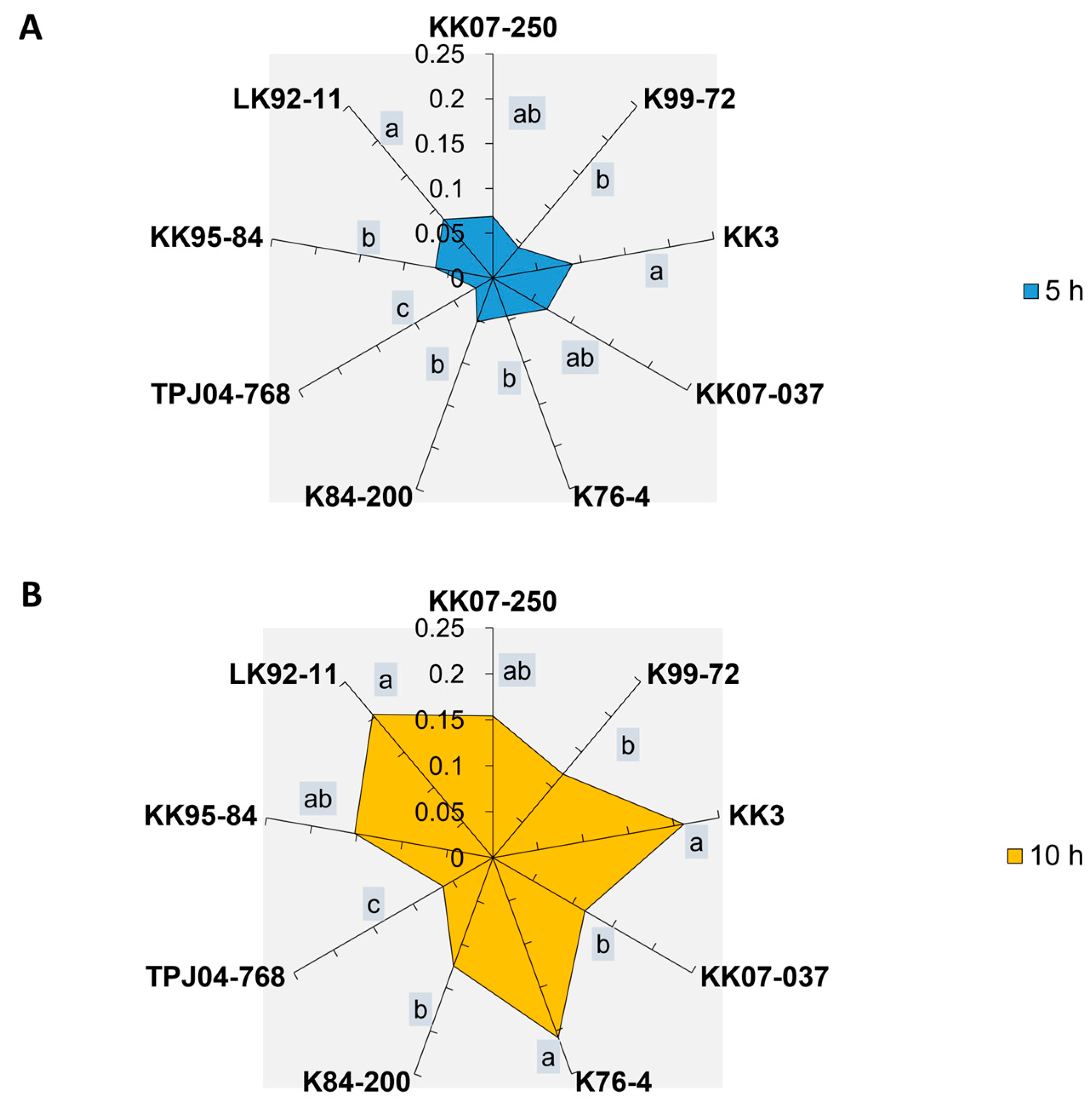
2.2. Honeydew Production
2.3. Measuring Adult Leafhopper Feeding Behavior by EPG Technique
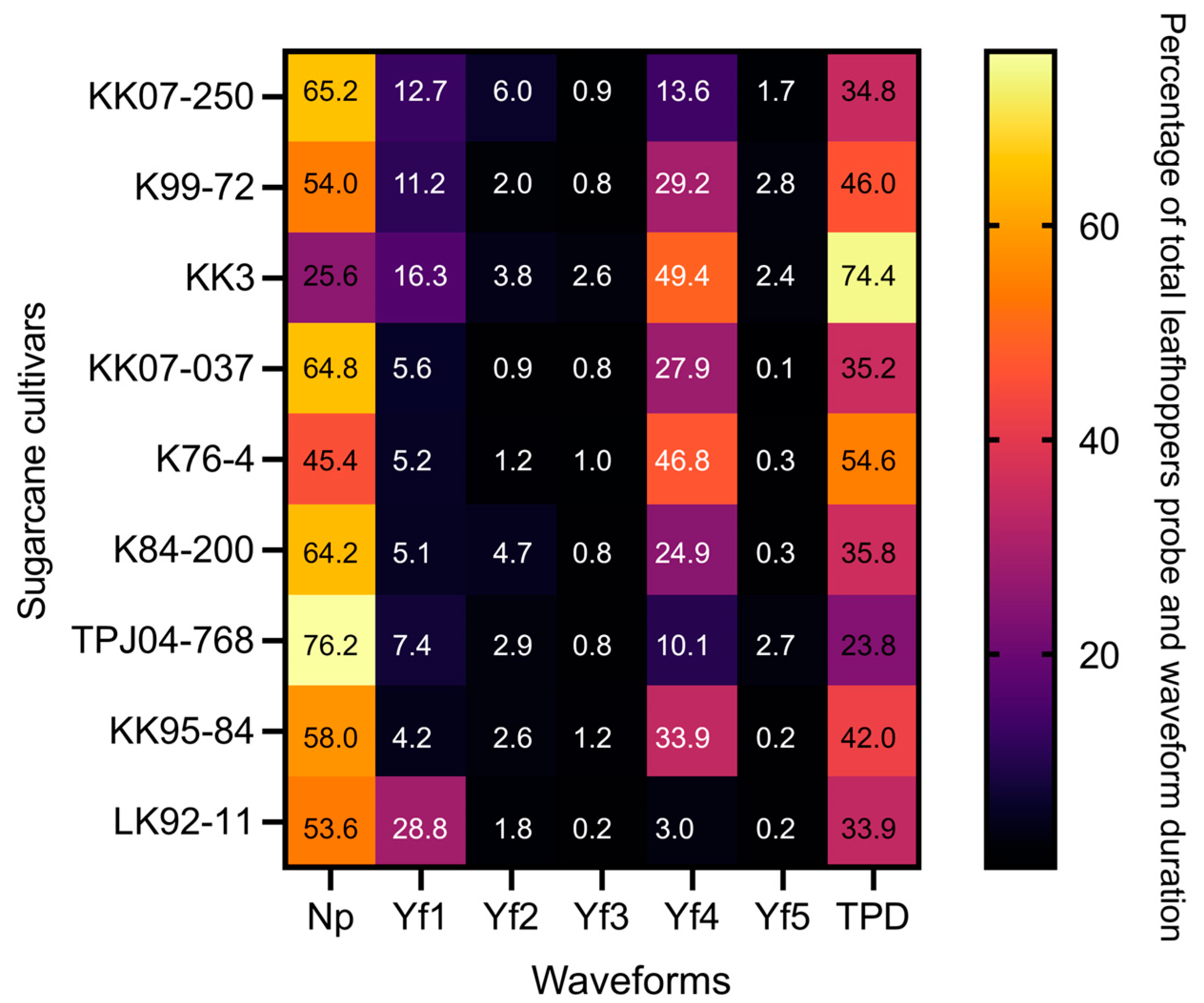
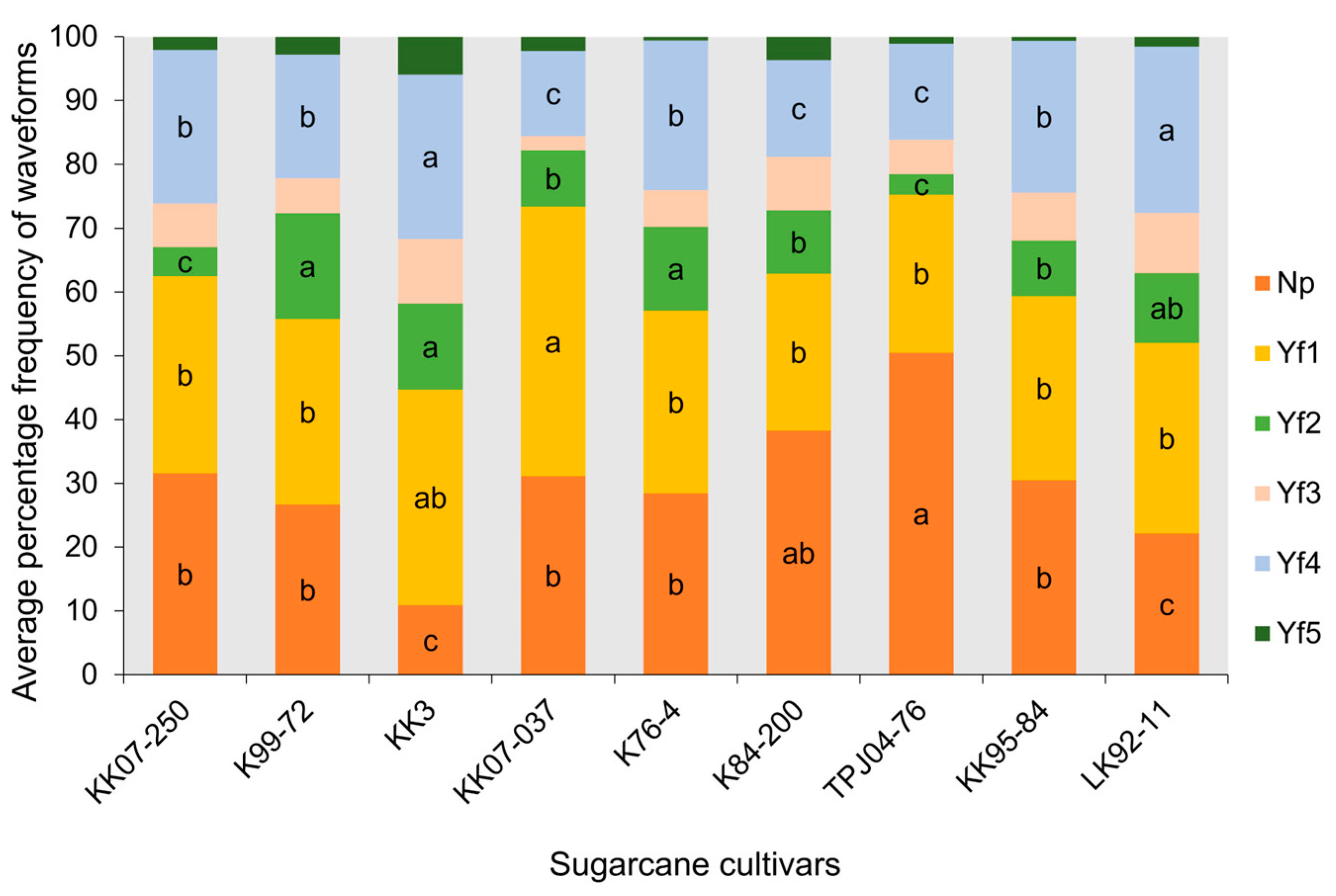
2.3.1. Comparison of the Frequency and Duration of EPG Waveforms
2.3.2. Total Probing Duration (TPD)
2.3.3. Total Waveform Duration (TWD)
2.3.4. Waveform Duration per Event per Insect (WDEI)

2.3.5. Number of Waveforms per Event Insect (NWEI)
2.4. Variation in Biophysical Factors among Sugarcane Cultivars
2.4.1. The Number of Silica Cells per 100 μm2
2.4.2. Silica Row per 100 μm2
2.4.3. Trichome Density
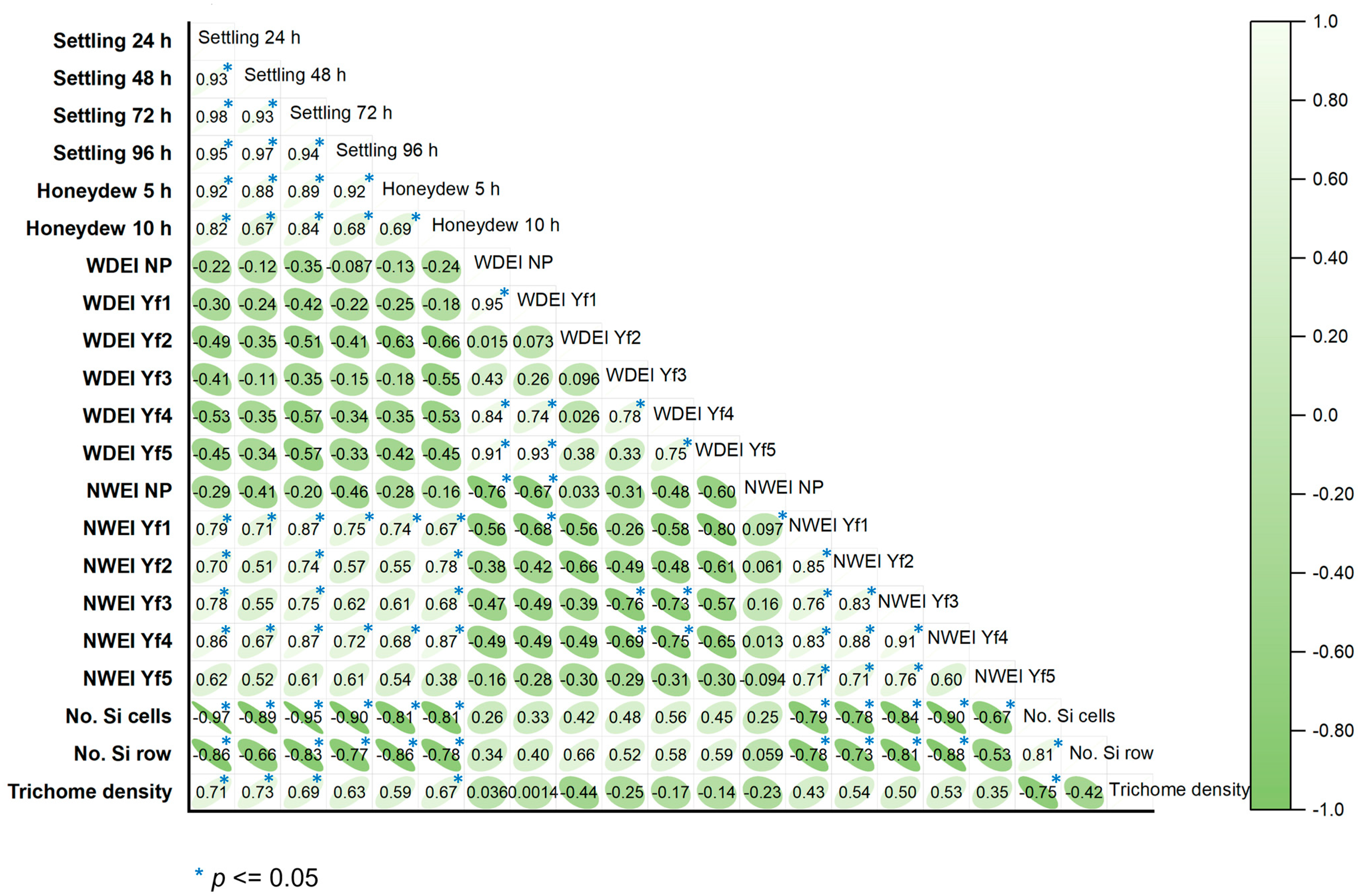
2.5. The Correlation between Settling Preference, Honeydew Production, Y. flavovittatus Feeding, and Biophysical Factors
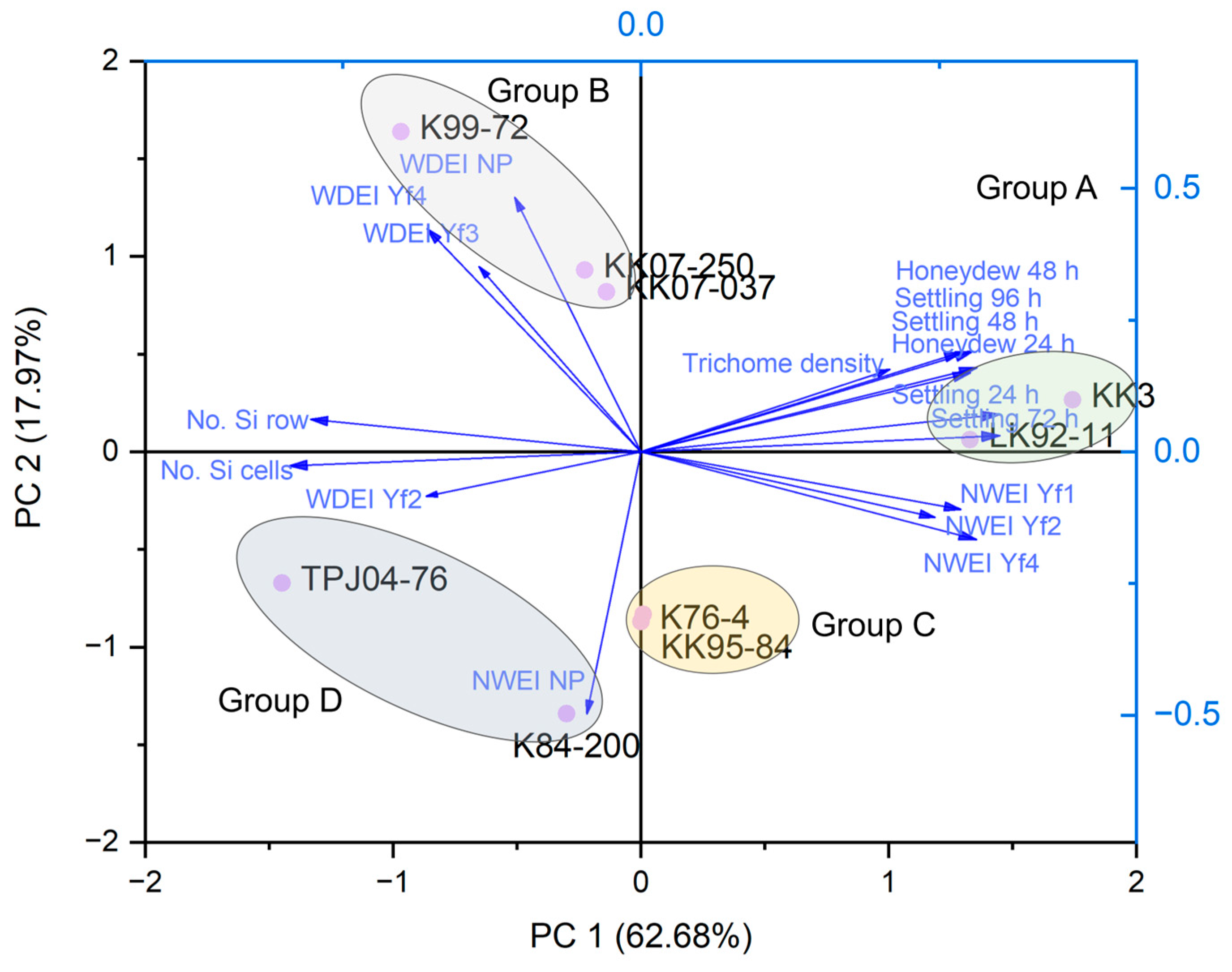
2.6. Principal Component Analysis
3. Discussion
3.1. The Settling Preference of Leafhopper
3.2. The Honeydew Production of Leafhoppers
3.3. Stylet Penetration Behavior of Y. flavovittatus on the Sugarcane Cultivars
3.4. The Biological Sugarcane Cultivars Factor
4. Materials and Methods
4.1. Plant Materials
4.2. Leafhoppers
4.3. Settling Preference of Leafhopper Adults (Choice Test)
4.4. Honeydew Production
4.5. Electrical Penetration Graph (EPG) Technique
4.6. Biophysical Factors
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanboonsong, Y.; Choosai, C.; Panyim, S.; Damark, S. Transovarial transmission of sugarcane white leaf phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumura). Insect. Mol. Biol. 2002, 11, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hanboonsong, Y.; Ritthison, W.; Choosai, C.; Sirithorn, P. Transmission of sugarcane white leaf phytoplasma by Yamatotettix flavovittatus, a new leafhopper vector. J. Econ. Entomol. 2006, 99, 1531–1537. [Google Scholar] [CrossRef]
- Roddee, J.; Wangkeeree, J.; Backus, E.A.; Hanboonsong, Y. Probing behavior of the leafhopper analyzed through DC electropenetrography and microscopy. J. Insect. Physiol. 2023, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Takeuchi, H.; Satoh, M.; Morimura, S.S.; Otuka, A.; Watanabe, T.; Thanh, D.V. Species-specific insecticide reistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and Southeast Asia. Pest Manag. Sci. 2008, 64, 1115–1121. [Google Scholar] [CrossRef]
- Abrol, D.P.; Shankar, U. History, overview and principles of ecologically based pest management. In Integrated Pest Management: Principles and Practice; CABI: Wallingford, UK, 2012; pp. 1–26. [Google Scholar]
- Thein, M.M.; Jamjanya, T.; Kobori, Y.; Hanboonsong, Y. Dispersal of the leafhoppers Matsumuratettix hiroglyphicus and Yamatotettix flavovittatus (Homoptera: Cicadellidae), vectors of sugarcane white leaf disease. Appl. Entomol. Zool. 2012, 47, 255–262. [Google Scholar] [CrossRef]
- Thein, M.M.; Jamjanya, T.; Hanboonsong, Y. Evaluation of colour traps to monitor insect vectors of sugarcane white leaf phytoplasma. Bull. Insectol. 2011, 64, 117–118. [Google Scholar]
- Wangkeeree, J.; Tewaruxsa, P.; Roddee, J.; Hanboonsong, Y. Wolbachia (Rickettsiales: Alphaproteobacteria) infection in the leahopper vector of sugarcane white leaf disease. J. Insect. Sci. 2020, 20, 20. [Google Scholar] [CrossRef]
- Wangkeeree, J.; Suwanchaisri, K.; Roddee, J.; Hanboonsong, Y. Effect of Wolbachia infection states on the life history and reproductive traits of the leafhopper Yamatotettix flavovittatus Matsumura. J. Invertebr. Pathol. 2020, 177, 107490. [Google Scholar] [CrossRef] [PubMed]
- Wangkeeree, J.; Sanit, P.; Roddee, J.; Hanboonsong, Y. Population dynamics of Wolbachia in the leafhopper vector Yamatotettix flavovittatus (Hemiptera: Cicadellidae). J. Insect. Sci. 2021, 21, 16. [Google Scholar] [CrossRef]
- Wangkeeree, J.; Sanit, P.; Roddee, J.; Hanboonsong, Y. Phylogeny and strain typing of Wolbachia from Yamatotettix flavovittatus Matsumura leafhoppers. Curr. Microbiol. 2021, 78, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.C.; Ortiz, R. Host plant resistance to insects: An eco-friendly approach for pest management and environmental conservation. J. Environ. Biol. 2002, 23, 111–135. [Google Scholar]
- Hu, B.; Wan, Y.; Li, X.; Zhang, F.; Yan, W.; Xie, J. Characterization and genetic analysis of rice with pubescent leaves and glabrous hulls (PLgh). Crop. Sci. 2013, 53, 1878–1886. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Liang, Y.; Miroslav, N.; Haijun, A.S. Silicon and insect pest resistance. In Silicon in Agriculture; Springer: Berlin, Germany, 2015; pp. 197–204. [Google Scholar]
- Kvedaras, O.L.; Keeping, M.G. Silicon impedes stalk penetration by the borer Eldana saccharina in sugarcane. Entomol. Exp. Appl. 2007, 125, 103–110. [Google Scholar] [CrossRef]
- Gebretsadik, K.G.; Zhang, Y.; Chen, J. Screening and evaluation for antixenosis resistance in wheat accessions and varieties to grain aphid, Sitobion miscanthi (Takahashi) (Hemiptera: Aphididae). Plants 2022, 18, 1094. [Google Scholar] [CrossRef]
- Roddee, J.; Kobori, Y.; Yorozuya, H.; Hanboonsong, Y. Characterization of direct current-electrical penetration graph wavforms and correlation with the probing behavior of Matsumuratettix hiroglyphicus (Hemiptera: Cicadellidae), the insect vector of sugarcane white leaf phytoplasma. J. Econ. Entomol. 2017, 110, 893–902. [Google Scholar] [CrossRef]
- Roddee, J.; Kobori, Y.; Hanboonsong, Y. Characteristics of sugarcane white leaf phytoplasma transmission by the leafhopper Matsumuratettix hiroglyphicus (Matsumura). Entomol. Exp. Appl. 2019, 167, 108–117. [Google Scholar] [CrossRef]
- Farook, U.B.; Khan, Z.H.; Ahad, I.; Aafreen, S.; Rafieq, I.; Yousuf, T.; Yousuf, W.; Jan, S.; Manzoor, A.; Wani, R. Screening for antixenosis resistance of winter wheat genotypes to cereal leaf beetles (Oulema melanopus L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3069–3073. [Google Scholar] [CrossRef]
- Anaii, M.M.; Yali, M.P. Resistance of nine wheat cultivars and lines to green bug, Schizaphis graminum (Rondani) in Iran. J. Agric. Sci. Technol. 2018, 20, 1173–1185. [Google Scholar]
- Achhami, B.B.; Gadi, V.P.; Sherman, J.D.; Peterson, R.K.D.; Weaver, D.K. Antixenosis, antibiosis, and potential yield compensatory response in barley cultivars exposed to wheat stem sawfly (Hymenoptera: Cephidae) under field conditions. J. Insect. Sci. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Zutter, N.D.; Audenaert, K.; Haesaert, G.; Smagghe, G. Preference of cereal aphids for different varieties of winter wheat. Arthropod. Plant Interact. 2012, 6, 345–350. [Google Scholar] [CrossRef]
- Hondelmann, P.; Paul, C.; Schreiner, M.; Meyhofer, R. Importance of antixenosis and antibiosis resistance to the cabbage whitefly (Aleyrodes proletella) in brussels sprout cultivars. Insects 2020, 11, 56. [Google Scholar] [CrossRef]
- Smith, C.M.; Clement, S.L. Molecular bases of plant resistance to arthropods. Annu. Rev. Entomol. 2012, 57, 309–328. [Google Scholar] [CrossRef]
- Smith, C.M.; Chuang, W.P. Plant resistance to aphid feeding: Behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag. Sci. 2014, 70, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.L.B.; Baldin, E.L.L.; Ribeiro, L.P.; Souza, C.M.; Soares, M.C.E.; Fanela, T.L.M.; Lourenção, A.L. Resistance sources and antixenotic factors in Brazilian bean genotypes against Bemisia tabaci. Neotrop. Entomol. 2021, 50, 129–144. [Google Scholar] [CrossRef]
- Ghaffer, A.B.M.B.; Pritchard, J.; Ford-Lloyd, B. Brow planthopper (N. lugens Stal) feeding behavior on rice germplasm as an indicator of resistance. PLoS ONE 2011, 6, e22137. [Google Scholar]
- Wari, D.; Kabir, M.A.; Mujiono, K.; Hojo, Y.; Shinya, T.; Tani, A.; Nakatani, H.; Galis, I. Honeydew-associated microbes elicit defense responses against brown planthopper in rice. J. Exp. Bot. 2019, 70, 1683–1696. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Mahmood, S.; Sarwar, G.; Ashraf, M.; Naeem, M.; Zafar, S. Physiological and biochemical changes in resistant and susceptible to leaf curl virus (CLCuV) cotton varieties at germination and early seedling stages: Changes in lipase, oil content, protein and soluble sugars. Int. J. Biol. Biot. 2004, 1, 217–222. [Google Scholar]
- Ni, X. Effect of wheat leaf epicuticular structure on host selection and probing rhythm of Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol. 2014, 90, 1400–1407. [Google Scholar] [CrossRef]
- George, J.; Lapointe, S.L. Host-plant resistance associated with Poncirus trifoliata influence oviposition, development and adult emergence of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2019, 75, 279–285. [Google Scholar] [CrossRef]
- Ninkovic, V.; Glinwood, R.; Unlu, A.G.; Ganji, S. Effects of methyl salicylate on host plant acceptance and feeding by the aphid Rhopalosiphum padi. Front. Plant Sci. 2021, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Dhandapani, N.; Sathiah, N.; Murugan, M. Influence of organic manures and fertilizers on the incidence of sucking pests of sunflower, Helianthus annus L. J. Plant Prot. Sci. 2006, 14, 41–44. [Google Scholar]
- Hu, X.; Zhao, H.; Hu, Z.; Li, D.; Zhang, Y. EPG comparison of Sitobion avenae (Fab.) feeding behavior on three wheat varieties. Agric. Sci. China 2008, 7, 180–186. [Google Scholar] [CrossRef]
- Backus, E.A.; Cervantes, F.A.; Guedes, R.N.C.; Li, A.Y.; Wayadande, A.C. AC–DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann. Entomol. Soc. Am. 2019, 112, 236–248. [Google Scholar] [CrossRef]
- Singh, B.; Simon, A.; Halsey, K.; Kurup, S.; Clark, S.; Aradottir, G.I. Characterization of bird cherry-oat aphid (Rhopalosiphum padi L.) behavior and aphid host preference in relation to partially resistant and susceptible wheat landraces. Ann. Appl. Biol. 2020, 177, 184–194. [Google Scholar] [CrossRef]
- Wu, B.; Chun, E.; Xie, R.; Knox, G.W.; Gu, M.; Qin, H. Real-time feeding behavior monitoring by electrical penetration graph rapidly reveals host plant susceptibility to crapemyrtle bark scale (Hemiptera: Eriococcidae). Insects 2022, 13, 495. [Google Scholar] [CrossRef]
- Velusamy, R.; Heinrichs, E.A. Electronic monitoring of feeding behavior of Nilaparvata lagens (Homoptera: Delphacidae) on resistant and susceptible rice cultivars. Environ. Entomol. 1986, 15, 678–682. [Google Scholar] [CrossRef]
- Baldin, E.L.L.; Stamm, M.D.; Bentivenha, J.P.F.; Koch, K.G.; Tiffany, M.; Hunt, T.E. Feeding behavior of Aphis glycines (Hemiptera: Aphididae) on soybeans exhibiting antibiosis, antixenosis, and tolerance resistance. BioOne 2018, 101, 223–228. [Google Scholar]
- Stout, M.J. Reevaluating the conceptual framework for applied research on host-plant resistance. Insect. Sci. 2013, 20, 263–272. [Google Scholar] [CrossRef]
- Onstad, D.W.; Knolhoff, L. Arthropod resistance to crops. In Insect Resistance Management: Biology, Economics and Prediction, 2nd ed.; Academic Press: London, UK, 2014; pp. 293–326. [Google Scholar]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Baldin, E.L.L.; Cruz, P.L.; Morando, R.; Silva, I.F.; Bentivenha, J.P.F.; Tozin, L.R.S.; Rodrigues, T.M. Characterization of antixenosis in soybean genotypes to Bemisia tabaci (Hemiptera: Aleyrodidae) biotype B. J. Econ. Entomol. 2017, 110, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Rahman Shah, M.M.; Zhang, Z.; Hu, J.; Gaber, A.; Hossain, A. Impact of leaf trichomes of tomatoes and weeds on the host selection and developmental bioassays of Bemisia tabaci Q and A cryptic species. Heliyon 2023, 12, e20077. [Google Scholar] [CrossRef]
- Oriani, M.A.D.; Vendramim, J.D. Influence of trichomes on attractiveness and ovipositional preference of Bemisia tabaci (Genn.) B biotype (Hemiptera: Aleroydae) on tomato genotypes. Neotrop. Entomol. 2010, 39, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Leetanasaksakul, K.; Roytrakul, S.; Phaonakrop, N.; Kittisenachai, S.; Thaisakun, S.; Srithuanok, N.; Sriroth, K.; Soulard, L. Discovery of potential protein biomarkers associated with sugarcane white leaf disease susceptibility using a comparative proteomic approach. Peer J. 2022, 10, e12740. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electronic recording of penetration behavior by aphids. Entomol. Exp. Appl. 1978, 24, 721–730. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In World Crop Pests, Vol. 2b. Aphids, Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1988; pp. 95–108. [Google Scholar]
- Tjallingii, W.F.; Hogen Esch, T.H. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Roddee, J.; Backus, E.A.; Wangkeeree, J.; Hanboonsong, Y. Alteration in the stylet probing behavior and host preference of the vector Matsumuratettix hiroglyphicus (Hemiptera: Cicadellidae) after infection with sugarcane white leaf phytoplasma. J. Econ. Entomol. 2021, 114, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Backus, E.A.; Cline, A.R.; Ellerseick, M.R.; Serrano, M.S. Lygus hesperus (Hemiptera: Miridae) feeding on cotton: New methods and parameters for analysis of nonsequential electrical penetration graph data. Ann. Entomol. Soc. Am. 2007, 100, 296–310. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, R.; Liu, J.; Liu, L.; Li, R.; Men, L.; Zhang, Z. Effects of environmental factors on the spatial distribution pattern and diversity of insect communities along altitude gradients in guandi mountain, China. Insects 2023, 14, 224. [Google Scholar] [CrossRef]
- Moberly, J.G.; Bernards, M.T.; Waynant, K.V. Key features and updates for Origin 2018. J. Cheminform. 2018, 10, 5. [Google Scholar] [CrossRef]
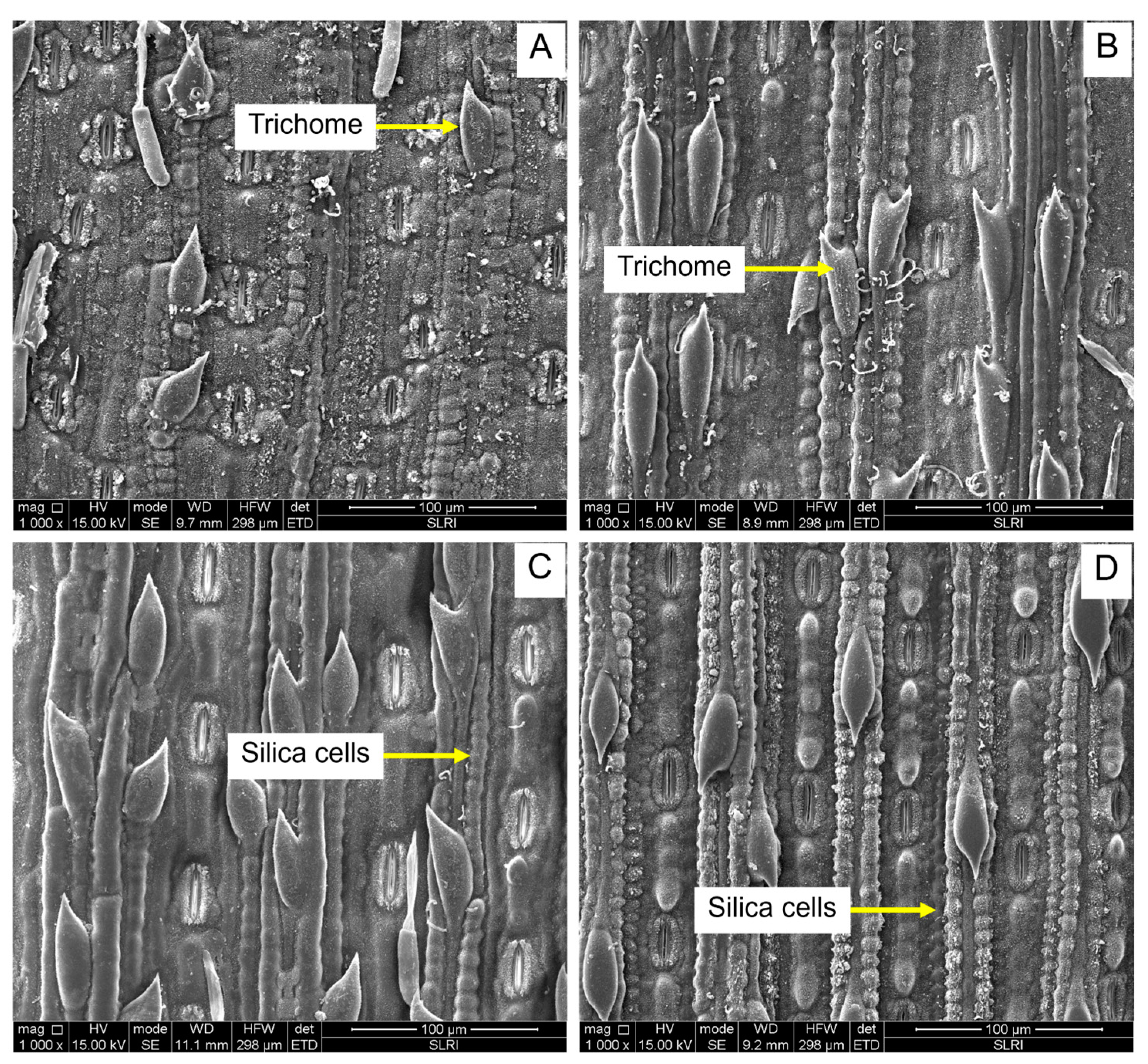
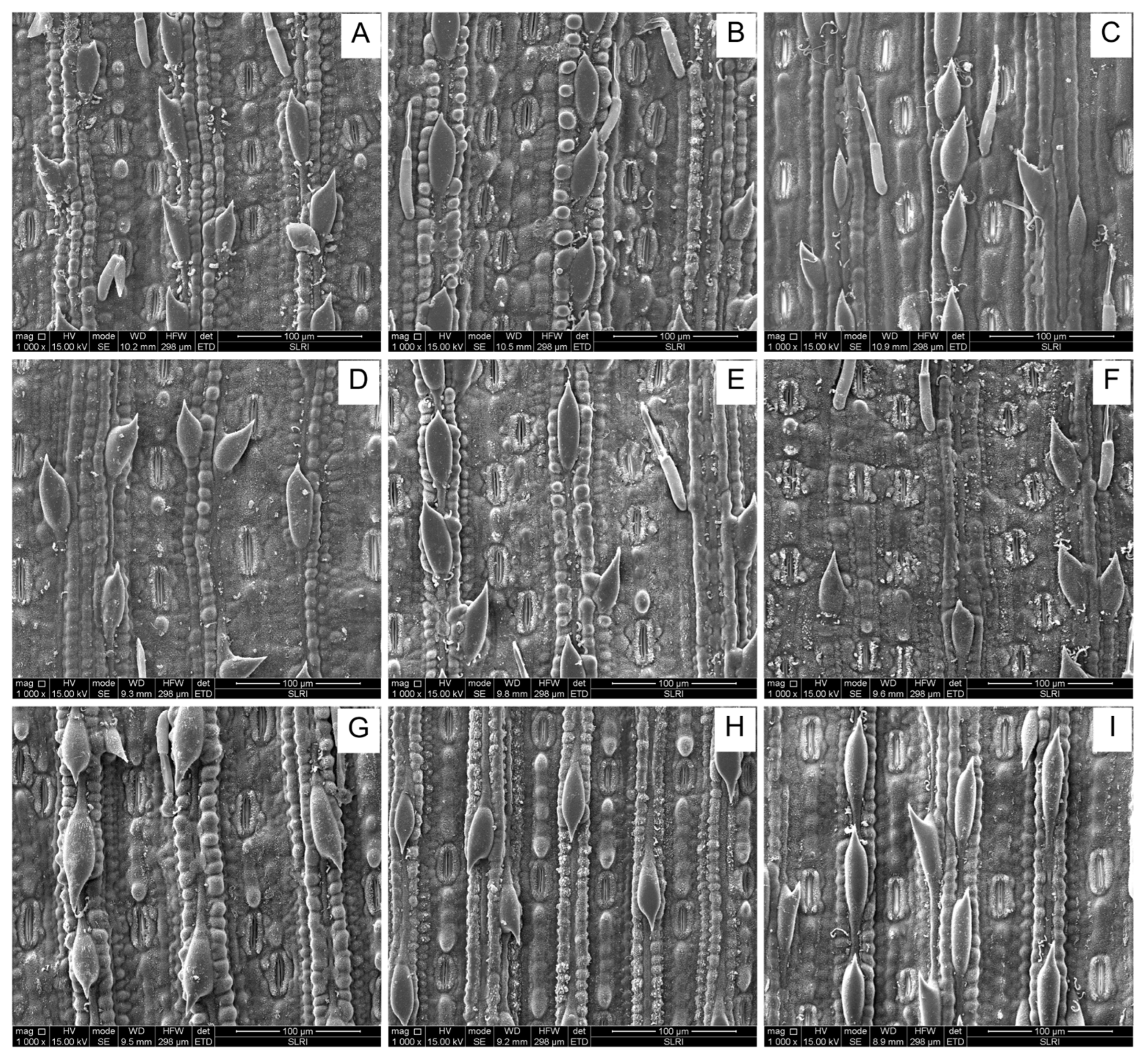
| Sugarcane Cultivars | No. Silica Cells per 100 µm2 | No. Silica Rows per 100 µm2 | Trichome Density per 100 µm2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KK07-250 | 109.2 | ± | 8.40 | bc 1/ | 8.4 | ± | 0.93 | ab | 8.6 | ± | 0.93 | ab |
| K99-72 | 132.4 | ± | 6.52 | ab | 9.0 | ± | 1.94 | a | 8.4 | ± | 0.92 | b |
| KK3 | 18.5 | ± | 3.79 | d | 5.4 | ± | 0.92 | c | 9.6 | ± | 1.63 | a |
| KK07-037 | 119.6 | ± | 3.76 | b | 8.4 | ± | 0.58 | ab | 7.8 | ± | 0.58 | c |
| K76-4 | 99.6 | ± | 3.87 | c | 8.2 | ± | 0.37 | b | 8.8 | ± | 0.37 | ab |
| K84-200 | 118.8 | ± | 5.00 | b | 8.0 | ± | 0.80 | b | 8.2 | ± | 0.80 | b |
| TPJ04-768 | 143.0 | ± | 11.65 | a | 10.4 | ± | 0.73 | a | 6.2 | ± | 0.73 | c |
| KK95-84 | 115.2 | ± | 4.22 | b | 7.2 | ± | 0.92 | c | 5.6 | ± | 0.81 | c |
| LK92-11 | 27.5 | ± | 1.58 | d | 6.0 | ± | 0.81 | c | 11.8 | ± | 1.63 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roddee, J.; Wangkeeree, J.; Hanboonsong, Y. Identification and Evaluation of Sugarcane Cultivars for Antixenosis Resistance to the Leafhopper Yamatotettix flavovittatus Matsumura (Hemiptera: Cicadellidae). Plants 2024, 13, 2299. https://doi.org/10.3390/plants13162299
Roddee J, Wangkeeree J, Hanboonsong Y. Identification and Evaluation of Sugarcane Cultivars for Antixenosis Resistance to the Leafhopper Yamatotettix flavovittatus Matsumura (Hemiptera: Cicadellidae). Plants. 2024; 13(16):2299. https://doi.org/10.3390/plants13162299
Chicago/Turabian StyleRoddee, Jariya, Jureemart Wangkeeree, and Yupa Hanboonsong. 2024. "Identification and Evaluation of Sugarcane Cultivars for Antixenosis Resistance to the Leafhopper Yamatotettix flavovittatus Matsumura (Hemiptera: Cicadellidae)" Plants 13, no. 16: 2299. https://doi.org/10.3390/plants13162299





