Study on Betaine and Growth Characteristics of Lycium chinense Mill. in Different Cultivation Environments in South Korea
Abstract
:1. Introduction
2. Results
2.1. Soil Physicochemical Properties and Meteorological Variables
2.2. Growth Characteristics of L. chinense Fruit
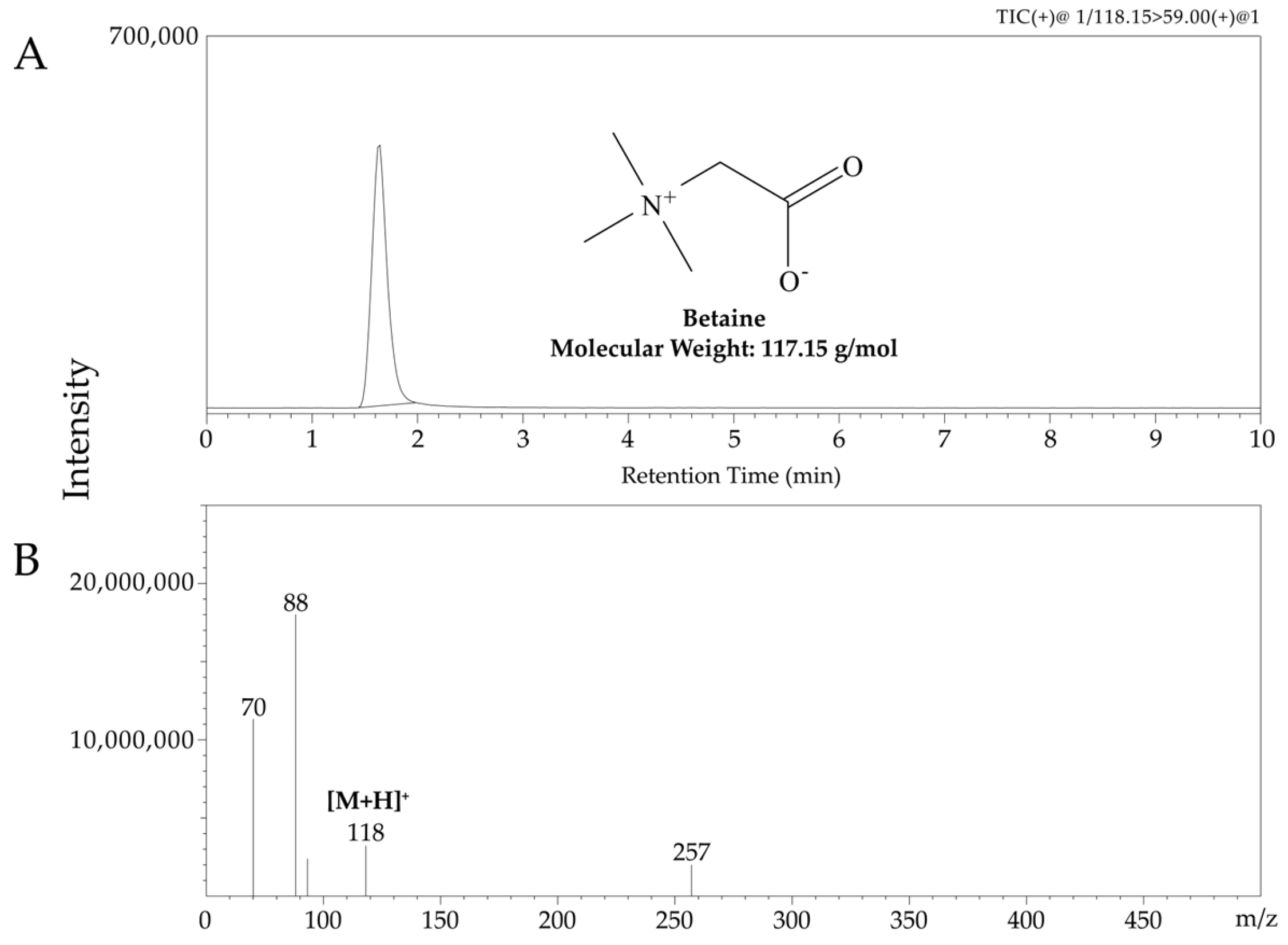
2.3. Validation and Quantification of Betaine
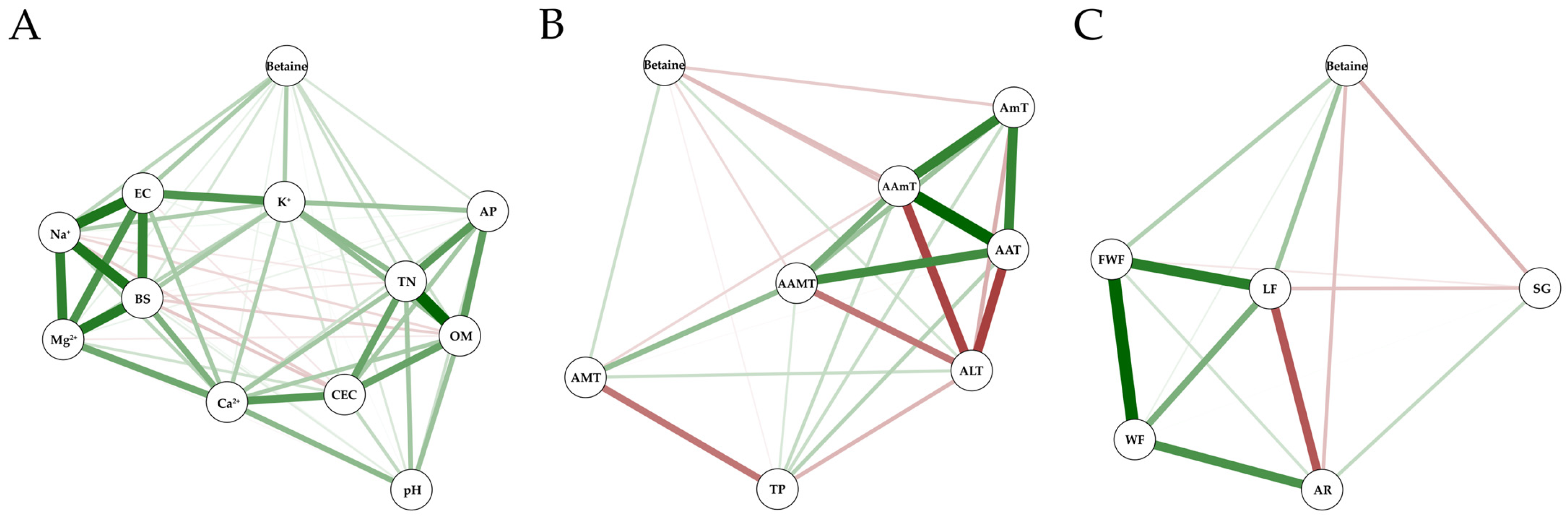
2.4. Correlations among Betaine, Environments, and Fruit Growth
3. Discussion
4. Materials and Methods
4.1. Sampling of Plant Material and Chemicals
4.2. Sample and Standard Preparation
4.3. UHPLC-MS/MS Analysis
4.4. Soil Analysis and Collecting Meteorological Data
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bucheli, P.; Gao, Q.; Redgwell, R. Chapter 14—Biomolecular and Clinical Aspects of Chinese Wolfberry. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Watchtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 289–292. [Google Scholar]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects–A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Lasekan, O. Exotic berries as a functional food. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2014, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Jia, H.; Li, X.; Bai, Z.; Liu, Z.; Sun, L.; Zhu, Z.; Bucheli, P.; Ballèvre, O.; Wang, J.; et al. A milk-based wolfberry preparation prevents prenatal stress-induced cognitive impairment of offspring rats, and inhibits oxidative damage and mitochondrial dysfunction in vitro. Neurochem. Res. 2010, 35, 702–711. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Li, Y.; Feng, X.; Song, L.; Gao, H.; Cao, B. Fruit morphological and nutritional quality features of goji berry (Lycium barbarum L.) during fruit development. Sci. Hortic. 2023, 308, 111555. [Google Scholar] [CrossRef]
- Amagase, H.; Nance, D.M. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) juice, GoChi™. J. Integr. Complement. Med. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Chang, R.C.C.; So, K.F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell. Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef]
- Lee, H.C.; Lee, B.C.; Kim, S.D.; Paik, S.W.; Lee, S.S.; Lee, K.S.; Kim, S.M. Changes in composition of Gugija (Lycii fructus) species according to harvest time. Korean J. Med. Crop Sci. 2008, 16, 306–312. (In Korean) [Google Scholar]
- Timalsina, D.; Devkota, H.P. Chapter 23—Lycium spp. (Lycium barbarum L., Lycium chinense Mill.). In Himalayan Fruits and Berries; Tarun, B., Indra, D.B., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 225–233. [Google Scholar]
- Ministry of Food and Drug Safety. The Korean Pharmacopoeia, 12th ed.; The KFDA notification No. 2022–10: Subparagraph 4 of Article 2; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2022; p. 75. (In Korean)
- The Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of People’s Republic of China (C. P.); Ministry of Health of the People’s Republic of China: Beijing, China, 2010; pp. 232–233.
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 1098–1106. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in inflammation: Mechanistic aspects and applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Niknahad, H.; Sadeghi, A.; Mohammadi, H.; Ghanbarinejad, V.; Ommati, M.M.; Hosseini, A.; Azarpira, N.; Khodaei, F.; Farshad, O.; et al. Betaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injury. Biomed. Pharmacother. 2018, 103, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Q.; Zheng, Z.Y.; Xu, X.; Hu, Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from lycium barbarum (Goji) fruit european cultivars at different maturation steps: Extraction, hplc-dad analyses, and biological evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef]
- Mäkelä, P.S.A.; Jokinen, K.; Himanen, K. Roles of endogenous glycinebetaine in plant abiotic stress responses. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 153–173. [Google Scholar]
- National Institute of Forest Science. Forest Soil Acidification Status in Korea; National Institute of Forest Science: Seoul, Republic of Korea, 2021; p. 23. (In Korean)
- Pinto, M.; Soares, C.; Pinto, A.S.; Fidalgo, F. Phytotoxic effects of bulk and nano-sized Ni on Lychium barbarum L. grown in vitro − Oxidative damage and antioxidant response. Chemosphere 2019, 218, 507–516. [Google Scholar] [CrossRef]
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction methods affect the structure of Goji (Lychium barbarum) polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef]
- Li, K.; Lu, H.; Nkoh, J.N.; Hong, Z.; Xu, R. Aluminum mobilization as influenced by soil organic matter during soil and mineral acidification: A constant pH study. Geoderma 2022, 418, 115853. [Google Scholar] [CrossRef]
- King, A.E.; Ali, G.A.; Gillespie, A.W.; Wagner-Riddle, C. Soil Organic Matter as Catalyst of Crop Resource Capture. Front. Environ. Sci. 2020, 8, 50. [Google Scholar] [CrossRef]
- Clivot, H.; Mary, B.; Valé, M.; Cohan, J.; Champolivier, L.; Piraux, F.; Laurent, F.; Justes, E. Quantifying in situ and modeling net nitrogen mineralization from soil organic matter in arable cropping systems. Soil Biol. Biochem. 2017, 111, 44–59. [Google Scholar] [CrossRef]
- Korea National Arboretum. Nature. Available online: http://www.nature.go.kr/kbi/plant/pilbk/selectPlantPilbkDtl.do?plantPilbkNo=27131 (accessed on 5 June 2024). (In Korean)
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, B.; Li, M.; Wei, Q.; Han, Z. Multivariate analysis between meteorological factor and fruit quality of Fuji apple at different locations in China. J. Integr. Agric. 2018, 17, 1338–1347. [Google Scholar] [CrossRef]
- Fischer, G.; Ramírez, F.; Casieraa-Posada, F. Ecophysiological aspects of fruit crops in the era of climate change. A review. Agron. Colomb. 2016, 34, 190–199. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, T.; Wang, Z.; Li, G.; Zhao, W.; Lv, X.; Zhuang, L. Succession of endophytic fungi and arbuscular mycorrhizal fungi associated with the growth of plant and their correlation with secondary metabolites in the roots of plants. BMC Plant Biol. 2021, 21, 165. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Soto, E.M.; Figueroa-Soto, C.G. Biosynthesis and degradation of glycine betaine and its potential to control plant growth and development. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 123–140. [Google Scholar]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clue from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 2017, 7, 2035. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine: And effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. Genetic engineering of glycinebetaine synthesis in plants: Current status and implications for enhancement of stress tolerance. J. Exp. Bot. 2000, 51, 81–88. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline. Validation of Analytical Procedures Q2 (R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Rural Development Administration. Analysis Manual of Comprehensive Testing Laboratory; Rural Development Administration: Jeonju, Republic of Korea, 2023; pp. 21–85. (In Korean)



| Cultivation Sites (n = 3) | LF | WF | AR | FWF | SG |
|---|---|---|---|---|---|
| (mm) | (mm) | (α:1) | (g) | (Brix°) | |
| 1 | 26.09 ± 0.28 ab | 10.12 ± 0.43 ab | 0.39 ± 0.01 def | 1.44 ± 0.15 abcd | 14.02 ± 0.44 defg |
| 2 | 19.37 ± 0.65 fgh | 7.68 ± 0.19 defg | 0.40 ± 0.02 bcdef | 0.81 ± 0.08 ghi | 16.14 ± 0.40 abcde |
| 3 | 17.29 ± 0.34 hi | 7.55 ± 0.30 defg | 0.44 ± 0.02 abcdef | 0.82 ± 0.06 fghi | 18.86 ± 0.50 abc |
| 4 | 23.22 ± 0.30 bcde | 8.99 ± 0.40 bcdefg | 0.39 ± 0.02 def | 1.24 ± 0.07 abcdef | 15.20 ± 1.41 bcdefg |
| 5 | 22.09 ± 0.59 def | 11.38 ± 0.11 a | 0.52 ± 0.01 abc | 1.55 ± 0.05 ab | 16.29 ± 0.76 abcde |
| 6 | 21.03 ± 0.29 efg | 8.66 ± 0.05 bcdefg | 0.41 ± 0.01 abcdef | 0.95 ± 0.00 efghi | 15.69 ± 0.94 abcdef |
| 7 | 19.41 ± 0.77 fgh | 8.67 ± 0.51 bcdefg | 0.45 ± 0.01 abcdef | 0.89 ± 0.11 fghi | 15.23 ± 0.82 bcdefg |
| 8 | 24.98 ± 1.01 abcd | 10.29 ± 0.05 ab | 0.42 ± 0.02 abcdef | 1.62 ± 0.06 a | 14.13 ± 0.82 defg |
| 9 | 25.32 ± 0.84 abc | 8.47 ± 0.16 bcdefg | 0.34 ± 0.02 f | 1.12 ± 0.03 cdefghi | 13.80 ± 0.76 defg |
| 10 | 22.13 ± 0.13 def | 8.80 ± 0.30 bcdefg | 0.40 ± 0.01 bcdef | 0.96 ± 0.11 efghi | 11.10 ± 1.02 g |
| 11 | 19.16 ± 0.16 fgh | 10.00 ± 0.44 ab | 0.52 ± 0.03 ab | 1.37 ± 0.06 abcde | 13.69 ± 0.48 defg |
| 12 | 22.05 ± 0.47 def | 9.52 ± 0.41 abcde | 0.43 ± 0.01 abcdef | 1.09 ± 0.11 defghi | 17.41 ± 1.24 abcd |
| 13 | 22.12 ± 0.57 def | 8.87 ± 0.16 bcdefg | 0.40 ± 0.00 abcdef | 0.97 ± 0.06 efghi | 16.03 ± 0.62 abcde |
| 14 | 22.44 ± 0.91 cde | 10.08 ± 0.32 ab | 0.45 ± 0.03 abcdef | 1.18 ± 0.05 bcdefg | 18.80 ± 0.75 abc |
| 15 | 15.62 ± 0.22 i | 7.88 ± 0.13 cdefg | 0.51 ± 0.01 abcde | 0.79 ± 0.03 hi | 19.62 ± 1.09 a |
| 16 | 22.71 ± 0.39 cde | 7.46 ± 0.16 fg | 0.33 ± 0.01 f | 1.01 ± 0.03 efghi | 12.89 ± 1.12 efg |
| 17 | 22.03 ± 0.32 def | 9.56 ± 0.45 abcd | 0.43 ± 0.01 abcdef | 1.21 ± 0.11 abcdefg | 12.58 ± 0.28 efg |
| 18 | 18.15 ± 0.32 ghi | 9.52 ± 0.41 abcde | 0.53 ± 0.03 a | 1.20 ± 0.04 bcdefgh | 16.18 ± 0.67 abcde |
| 19 | 26.49 ± 0.51 a | 8.74 ± 0.24 bcdefg | 0.33 ± 0.00 f | 1.51 ± 0.10 abc | 17.20 ± 0.13 abcd |
| 20 | 19.31 ± 0.10 fgh | 7.39 ± 0.39 fg | 0.38 ± 0.02 ef | 0.75 ± 0.04 i | 18.91 ± 0.44 ab |
| 21 | 16.89 ± 0.50 hi | 7.09 ± 0.88 g | 0.42 ± 0.06 abcdef | 0.73 ± 0.05 i | 11.71 ± 0.87 fg |
| 22 | 19.19 ± 0.53 fgh | 7.48 ± 0.29 efg | 0.39 ± 0.03 cdef | 0.87 ± 0.04 fghi | 14.96 ± 0.05 bcdefg |
| 23 | 19.38 ± 0.15 fgh | 9.88 ± 0.59 abc | 0.51 ± 0.03 abcd | 0.96 ± 0.07 efghi | 14.74 ± 0.40 cdefg |
| 24 | 22.79 ± 1.12 cde | 9.36 ± 0.54 abcdef | 0.42 ± 0.04 abcdef | 1.13 ± 0.06 bcdefghi | 15.77 ± 0.54 abcdef |
| 25 | 23.87 ± 0.11 abcde | 11.07 ± 0.23 a | 0.46 ± 0.01 abcde | 1.46 ± 0.12 abcd | 16.12 ± 0.45 abcde |
| Parameters | Value |
|---|---|
| Linearity range (µg/mL) | 0.3125–5 |
| Regression equation | Y = 1,053,004.8258 X + 88,006.7500 |
| Regression coefficient (r2) | 0.9991 |
| LOD, limit of detection (µg/mL) | 0.01 |
| LOQ, limit of quantification (µg/mL) | 0.02 |
| Precision, Intra-day a (%RSD) | 0.10–0.51 |
| Precision, Inter-day b (%RSD) | 0.08–0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Lee, D.H.; Jeong, D.H.; Jang, J.H.; Son, Y.; Lee, S.-Y.; Kim, H.-J. Study on Betaine and Growth Characteristics of Lycium chinense Mill. in Different Cultivation Environments in South Korea. Plants 2024, 13, 2316. https://doi.org/10.3390/plants13162316
Cho H, Lee DH, Jeong DH, Jang JH, Son Y, Lee S-Y, Kim H-J. Study on Betaine and Growth Characteristics of Lycium chinense Mill. in Different Cultivation Environments in South Korea. Plants. 2024; 13(16):2316. https://doi.org/10.3390/plants13162316
Chicago/Turabian StyleCho, Hyejung, Dong Hwan Lee, Dae Hui Jeong, Jun Hyuk Jang, Yonghwan Son, Sun-Young Lee, and Hyun-Jun Kim. 2024. "Study on Betaine and Growth Characteristics of Lycium chinense Mill. in Different Cultivation Environments in South Korea" Plants 13, no. 16: 2316. https://doi.org/10.3390/plants13162316





