Characterization of New Wheat-Thinopyrum intermedium Derivative Lines with Superior Genes for Stripe Rust and Powdery Mildew Resistance
Abstract
1. Introduction
2. Results
2.1. Comparative Analysis of Karyotype between TAI7045 and 78784
2.2. Transmission of Thinopyrum Chromosomes and Identification of Stable Wheat-Th. intermedium-Derived Lines
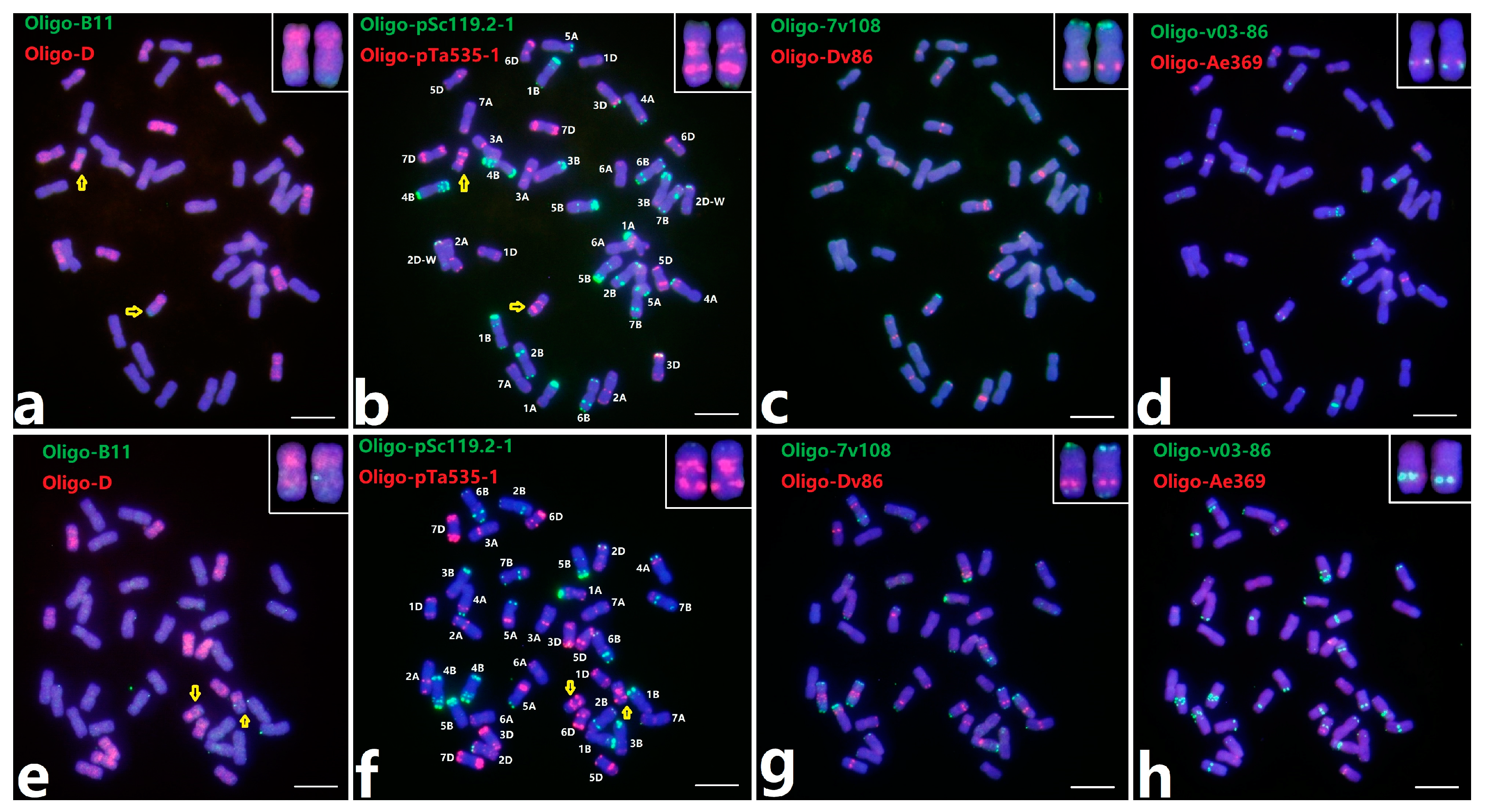
2.3. ND-FISH of Two Distinct Wheat-Th. intermedium Translocation Lines by Multiple Oligo Probes
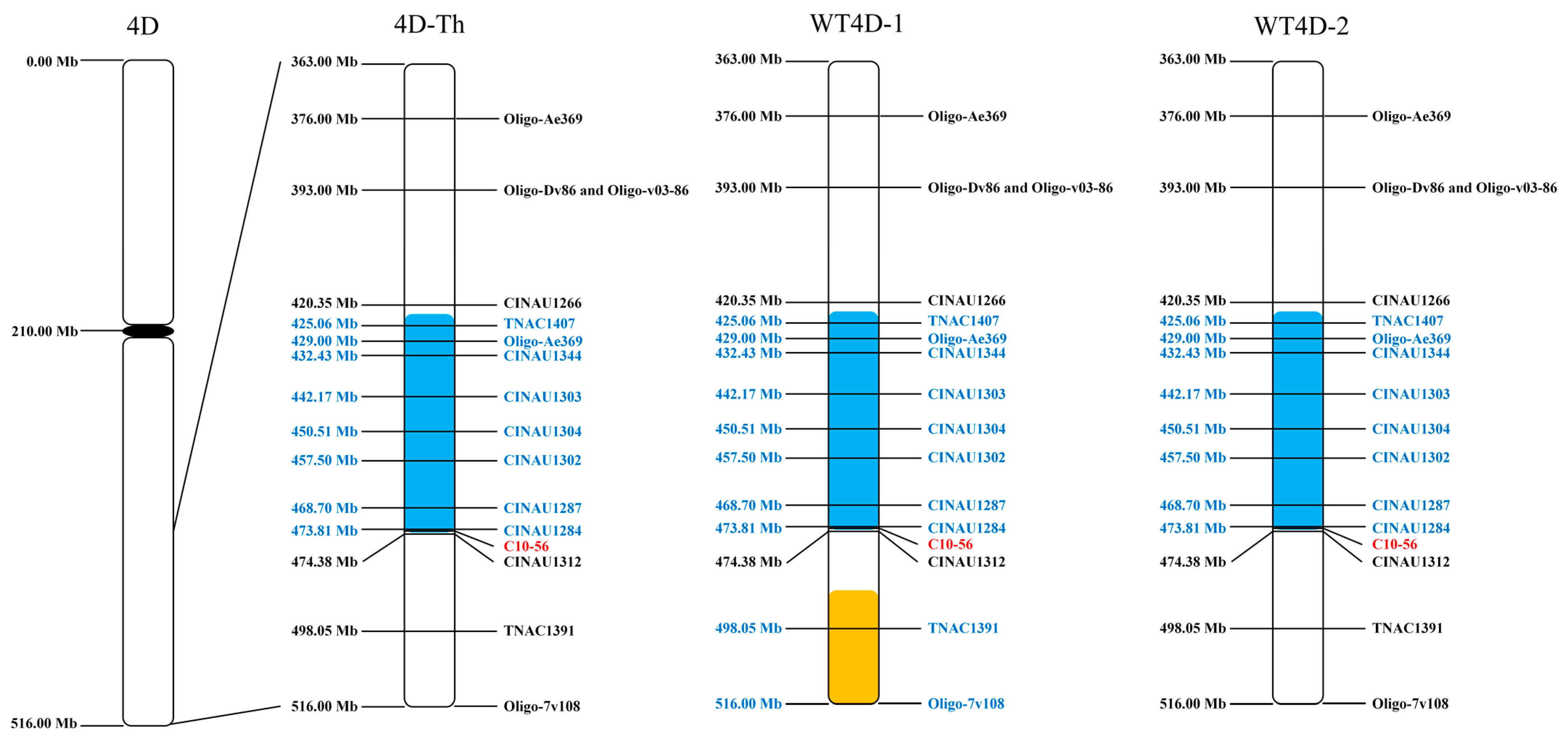
2.4. Molecular Marker Analysis for Structure of Chromosomes WT4D-1 and WT4D-2
2.5. Powdery Mildew and Stripe Rust Reactions for the Wheat-Th. intermedium Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Oligonucleotide Probes Development
4.3. ND-FISH and Sequential ND-FISH
4.4. Molecular Marker Analysis
4.5. Powdery Mildew and Stripe Rust Response Observations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef]
- Morgounov, A.; Tufan, H.A.; Sharma, R.; Akin, B.; Bagci, A.; Braun, H.J.; Kaya, Y.; Keser, M.; Payne, T.S.; Sonder, K.; et al. Global incidence of wheat rusts and powdery mildew during 1969–2010 and durability of resistance of winter wheat variety bezostaya 1. Eur. J. Plant Pathol. 2012, 132, 323–340. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wellings, C.; Chen, X.M.; Kang, Z.S.; Liu, T.G. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J. Catalogue of gene symbols for wheat. Ann. Wheat Newsl. 2018, 64 (Suppl. S19), 73–93. [Google Scholar]
- Tan, C.; Li, G.; Cowger, C.; Carver, B.F.; Xu, X. Characterization of Pm63, a powdery mildew resistance gene in Iranian landrace PI 628024. Theor. Appl. Genet. 2019, 132, 1137–1144. [Google Scholar] [CrossRef]
- Li, G.R.; Tang, L.R.; Yin, Y.; Zhang, A.H.; Yu, Z.H.; Yang, E.N.; Tang, Z.X.; Fu, S.L.; Yang, Z.J. Molecular dissection of Secale africanum chromosome 6Rafr in wheat enabled localization of genes for resistance to powdery mildew and stripe rust. BMC Plant Biol. 2020, 20, 134. [Google Scholar] [CrossRef]
- Baker, L.; Grewal, S.; Yang, C.Y.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.; Burridge, A.J.; Przewieslik-Allen, A.M.; Wilkinson, P.A.; King, I.P.; et al. Exploiting the genome of Thinopyrum elongatum to expand the gene pool of hexaploid wheat. Theor. Appl. Genet. 2020, 133, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, R.; Wang, X.L.; Gong, W.P.; Cheng, D.G.; Cao, X.Y.; Liu, A.F.; Li, H.S.; Liu, J.J. Research progress of wheat wild hybridization, disease resistance genes transfer and utilization. Sci. Agric. Sin. 2020, 53, 1287–1308. [Google Scholar]
- Wan, A.M.; Zhao, Z.H.; Chen, X.M.; He, Z.H.; Jin, S.L.; Jia, Q.Z.; Yao, G.; Yang, J.X.; Wang, B.T.; Li, G.B.; et al. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis. 2004, 88, 896–904. [Google Scholar] [CrossRef]
- Zhang, H.T.; Guan, H.Y.; Li, J.T.; Zhu, J.; Xie, C.J.; Zhou, Y.L.; Duan, X.Y.; Yang, T.; Sun, Q.X.; Liu, Z.Y. Genetic and comparative genomics mapping reveals that a powdery mildew resistance gene Ml3D232 originating from wild emmer co-segregates with an NBS-LRR analog in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Tsvelev, N.N.; Fedorov, A.A. Grasses of the Soviet Union; Oxonian Press Pvt. Ltd.: New Delhi, India, 1983; pp. 196–298. [Google Scholar]
- Bao, Y.G.; Wu, X.; Zhang, C.; Li, X.F.; He, F.; Qi, X.L.; Wang, H.G. Chromosomal constitutions and reactions to powdery mildew and stripe rust of four novel wheat-Thinopyrum intermedium partial amphiploids. J. Genet. Genom. 2014, 41, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, C.H.; Bao, Y.G.; Wang, H.G.; Li, X.F. Molecular cytogenetic characterization of a novel wheat-Thinopyrum intermedium introgression line tolerant to phosphorus deficiency. Crop J. 2020, 9, 816–822. [Google Scholar] [CrossRef]
- Chen, Q.; Conner, R.L.; Laroche, A.; Ji, W.; Armstrong, K.C.; Fedak, G. Genomic in situ hybridization analysis of Thinopyrum chromatin in a wheat-Th. intermedium partial amphiploid and six derived chromosome addition lines. Genome 1999, 42, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Lang, T.; Li, B.; Yu, Z.H.; Wang, H.J.; Li, G.; Yang, E.N.; Yang, Z.J. Introduction of Thinopyrum intermedium ssp. trichophorum chromosomes to wheat by trigeneric hybridization involving Triticum, Secale and Thinopyrum genera. Planta 2017, 245, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Fedak, G.; Chen, Q.; Conner, R.L.; Laroche, A.; Petroski, R.; Armstrong, K.W. Characterization of wheat-Thinopyrum partial amphiploids by meiotic analysis and genomic in situ hybridization. Genome 2000, 43, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.Y.; Zhang, X.J.; Li, X.; Jia, J.Q.; Yang, H.Z.; Zhan, H.X.; Qiao, L.Y.; Guo, H.J.; Chang, Z.J. Mapping of powdery mildew resistance gene pmCH89 in a putative wheat-Thinopyrum intermedium introgression line. Int. J. Mol. Sci. 2015, 16, 17231–17244. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.T.; Zhang, N.; Boshoff, W.H.P.; Li, H.W.; Li, B.; Li, Z.S.; Zheng, Q. Identification and introgression of a novel leaf rust resistance gene from Thinopyrum intermedium chromosome 7JS into wheat. Theor. Appl. Genet. 2023, 136, 231. [Google Scholar] [CrossRef]
- Luo, P.G.; Luo, H.Y.; Chang, Z.J.; Zhang, H.Y.; Zhang, M.; Ren, Z.L. Characterization and chromosomal location of Pm40 in common wheat: A new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009, 118, 1059–1064. [Google Scholar] [CrossRef]
- He, R.L.; Chang, Z.J.; Yang, Z.J.; Zhan, H.X.; Zhang, X.J.; Liu, J.X. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor. Appl. Genet. 2009, 118, 1173–1180. [Google Scholar] [CrossRef]
- Liu, J.; Chang, Z.J.; Zhang, X.J.; Yang, Z.J.; Li, X.; Jia, J.Q.; Zhan, H.X.; Guo, H.J.; Wang, J.M. Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013, 126, 265–274. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, C.Y.; Feng, X.B.; Zhao, J.X.; Deng, P.C.; Wang, Y.J.; Zhang, H.; Liu, X.L.; Li, T.D.; Chen, C.H.; et al. Molecular cytogenetics and development of St-chromosome-specific molecular markers of novel stripe rust resistant wheat-Thinopyrum intermedium and wheat-Thinopyrum ponticum substitution lines. BMC Plant Biol. 2022, 22, 111. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, J.B.; Ge, Y.D.; Guan, H.X.; Li, G.R.; Zhang, S.W.; Wang, X.L.; Li, X.; Chang, Z.J.; Zhang, P.; et al. Molecular cytogenetic characterization of a new wheat-Thinopyrum intermedium homoeologous group-6 chromosome disomic substitution line with resistance to leaf rust and stripe rust. Front. Plant Sci. 2022, 13, 1000281. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Chen, Q.H.; Jiang, W.X.; Zhang, A.H.; Yang, E.N.; Yang, Z.J. Molecular and cytogenetic identification of wheat-Thinopyrum intermedium double substitution line-derived progenies for stripe rust resistance. Plants 2022, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Wang, H.J.; Xu, Y.F.; Li, Y.S.; Lang, T.; Yang, Z.J.; Li, G.R. Characterization of chromosomal rearrangement in new wheat-Thinopyrum intermedium addition lines carrying Thinopyrum-specific grain hardness genes. Agronomy 2019, 9, 18. [Google Scholar] [CrossRef]
- Yu, Z.H.; Wang, H.J.; Yang, E.N.; Li, G.R.; Yang, Z.J. Precise identification of chromosome constitution and rearrangements in wheat–Thinopyrum intermedium derivatives by ND-FISH and Oligo-FISH painting. Plants 2022, 11, 2109. [Google Scholar] [CrossRef]
- Ishikawa, G.; Nakamura, T.; Ashida, T.; Saito, M.; Nasuda, S.; Endo, T.R.; Wu, J.Z.; Matsumoto, T. Localization of anchor loci representing five hundred annotated rice genes to wheat chromosomes using PLUG markers. Theor. Appl. Genet. 2009, 118, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Dai, K.L.; Xiao, J.; Yuan, C.X.; Zhao, R.H.; Doležel, J.; Wu, Y.F.; Cao, A.Z.; Chen, P.D.; Zhang, S.Z.; et al. Development of intron targeting (IT) markers specific for chromosome arm 4VS of Haynaldia villosa by chromosome sorting and next-generation sequencing. BMC Genom. 2017, 18, 167. [Google Scholar] [CrossRef]
- Qiao, L.Y.; Liu, S.J.; Li, J.B.; Li, S.J.; Yu, Z.H.; Liu, C.; Li, X.; Liu, J.; Ren, Y.K.; Zhang, P.; et al. Development of sequence-tagged site marker set for identification of J, JS, and St sub-genomes of Thinopyrum intermedium in wheat background. Front. Plant Sci. 2021, 12, 685216. [Google Scholar] [CrossRef]
- Chen, Q.; Conner, R.L.; Li, H.J.; Sun, S.C.; Ahmad, F.; Laroche, A.; Graf, R.J. Molecular cytogenetic discrimination and reaction to wheat streak mosaic virus and the wheat curl mite in Zhong series of wheat-Thinopyrum intermedium partial amphiploids. Genome 2003, 46, 135–145. [Google Scholar] [CrossRef]
- Sepsi, A.; Molnár, I.; Szalay, D.; Molnár-Láng, M. Characterization of a leaf rust-resistant wheat-Thinopyrum ponticum partial amphiploid BE-1, using sequential multicolor GISH and FISH. Theor. Appl. Genet. 2008, 116, 825–834. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Xu, Z.Y.; Zou, M.Q.; Dawei, Z.; Zhensan, P.; Shui, H. The establishment of two sets of alien addition lines of wheat-wheatgrass. Sci. China Ser. B 1988, 32, 695–705. [Google Scholar]
- Han, F.P.; Fedak, G.; Benabdelmouna, A.; Armstrong, K.; Ouellet, T. Characterization of six wheat x Thinopyrum intermedium derivatives by GISH, RFLP, and multicolor GISH. Genome 2003, 46, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; La, S.X.; Li, B.; Yu, Z.H.; Chen, Q.H.; Li, J.B.; Yang, E.N.; Li, G.R.; Yang, Z.J. Precise identification of wheat-Thinopyrum intermedium translocation chromosomes carrying resistance to wheat stripe rust in line Z4 and its derived progenies. Genome 2018, 61, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.J.; Li, G.R.; Zhan, H.X.; Liu, C.; Yang, Z.J. New St-chromosome-specific molecular markers for identifying wheat-Thinopyrum intermedium derivative lines. J. Genet. 2012, 91, e69–e74. [Google Scholar] [CrossRef]
- Nie, L.M.; Yang, Y.N.; Zhang, J.; Fu, T.H. Disomic chromosome addition from Thinopyrum intermedium to bread wheat appears to confer stripe rust resistance. Euphytica 2019, 215, 1–8. [Google Scholar] [CrossRef]
- Rey, E.; Abrouk, M.; Keeble-Gagnère, G.; Karafiátová, M.; Vrána, J.; Balzergue, S.; Soubigou-Taconnat, L.; Brunaud, V.; Martin-Magniette, M.L.; Endo, T.R.; et al. Transcriptome reprogramming due to the introduction of a barley telosome into bread wheat affects more barley genes than wheat. Plant Biotechnol. J. 2018, 16, 1767–1777. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Cao, Q.; Zhang, J.J.; Wang, S.W.; Chen, C.H.; Wang, C.Y.; Zhang, H.; Wang, Y.J.; Ji, W.Q. Cytogenetic analysis and molecular marker development for a new wheat-Thinopyrum ponticum 1JS (1D) disomic substitution line with resistance to stripe rust and powdery mildew. Front. Plant Sci. 2020, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xing, P.Y.; Qi, X.L.; Bao, Y.G.; Wang, H.G.; Wang, R.R.; Li, X.F. Characterization of chromosome constitution in three wheat-Thinopyrum intermedium amphiploids revealed frequent rearrangement of alien and wheat chromosomes. BMC Plant Biol. 2021, 21, 129. [Google Scholar] [CrossRef]
- Mahelka, V.; Kopecký, D.; Paštová, L. On the genome constitution and evolution of intermediate wheatgrass (Thinopyrum intermedium: Poaceae, Triticeae). BMC Evol. Biol. 2011, 11, 127. [Google Scholar] [CrossRef]
- Lammer, D.; Cai, X.W.; Arterburn, M.; Chatelain, J.; Murray, T.; Jones, S. A single chromosome addition from Thinopyrum elongatum confers a polycarpic, perennial habit to annual wheat. J. Exp. Bot. 2004, 55, 1715–1720. [Google Scholar] [CrossRef]
- Li, Z.S.; Mu, S.M.; Zhou, H.P.; Wu, J. The establishment and application of blue-grained monosomics in wheat chromosome engineering. Cereal Res. Commun. 1986, 14, 133–137. [Google Scholar]
- Yang, G.T.; Deng, P.C.; Ji, W.Q.; Fu, S.L.; Li, H.W.; Li, B.; Li, Z.S.; Zheng, Q. Physical mapping of a new powdery mildew resistance locus from Thinopyrum ponticum chromosome 4AgS. Front. Plant Sci. 2023, 14, 1131205. [Google Scholar] [CrossRef]
- Li, H.J.; Arterburn, M.; Jones, S.S.; Murray, T.D. Resistance to eyespot of wheat, caused by Tapesia yallundae, derived from Thinopyrum intermedium homoeologous group 4 chromosome. Theor. Appl. Genet. 2005, 111, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Zhang, X.Y. Characterization of the translocated chromosome using fluorescence in situ hybridization and random amplified polymorphic DNA on two Triticum aestivum-Thinopyrum intermedium translocation lines resistant to wheat streak mosaic or barley yellow dwarf virus. Chromosome Res. 1996, 4, 583–587. [Google Scholar] [CrossRef]
- Ali, N.; Heslop-Harrison, J.P.; Ahmad, H.; Graybosch, R.A.; Hein, G.L.; Schwarzacher, T. Introgression of chromosome segments from multiple alien species in wheat breeding lines with wheat streak mosaic virus resistance. Heredity 2016, 117, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Zhang, H.; Yang, Y.L.; Zhang, J.W.; Zhu, W.; Xu, L.L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.; et al. Development and identification of a novel wheat-Thinopyrum scirpeum 4E (4D) chromosomal substitution line with stripe rust and powdery mildew resistance. Plant Dis. 2022, 106, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Sears, E.R. Nullisomic-tetrasomic combinations in hexaploid wheat. In Chromosome Manipulations and Plant Genetics; Riley, R., Lewis, K.R., Eds.; Oliver and Boyd: Edinburgh, UK, 1966; pp. 29–45. [Google Scholar]
- Zhang, X.; Wang, H.Y.; Sun, H.J.; Li, Y.B.; Feng, Y.L.; Jiao, C.Z.; Li, M.L.; Song, X.Y.; Wang, T.; Wang, Z.K.; et al. A chromosome-scale genome assembly of Dasypyrum villosum provides insights into its application as a broad-spectrum disease resistance resource for wheat improvement. Mol. Plant 2023, 16, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhang, Y.X.; Niu, Y.Q.; Sha, Y.; Wang, Z.H.; Zhang, Z.B.; Yang, J.; Liu, B.; Li, L.F. Post-hybridization introgression and natural selection promoted genomic divergence of Aegilops speltoides and the four S*-genome diploid species. Plant J. 2023, 115, 1500–1513. [Google Scholar] [CrossRef]
- Lang, T.; Li, G.R.; Wang, H.J.; Yu, Z.H.; Chen, Q.H.; Yang, E.N.; Fu, S.L.; Tang, Z.X.; Yang, Z.J. Physical location of tandem repeats in the wheat genome and application for chromosome identification. Planta 2019, 249, 663–675. [Google Scholar] [CrossRef]
- Tang, Z.X.; Yang, Z.J.; Fu, S.L. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Xi, W.; Tang, Z.X.; Tang, S.Y.; Yang, Z.J.; Luo, J.; Fu, S.L. New ND-FISH-positive Oligo probes for identifying Thinopyrum Chromosomes in wheat backgrounds. Int. J. Mol. Sci. 2019, 20, 2031. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Tang, Z.X.; Qiu, L.; Yang, Z.J.; Li, G.R.; Lang, T.; Zhu, W.Q.; Zhang, J.H.; Fu, S.L. Developing new oligo probes to distinguish specific chromosomal segments and the A, B, D genomes of wheat (Triticum aestivum L.) using ND-FISH. Front. Plant Sci. 2018, 9, 1104. [Google Scholar] [CrossRef]
- Fu, S.L.; Chen, L.; Wang, Y.Y.; Li, M.; Yang, Z.J.; Qiu, L.; Yan, B.J.; Ren, Z.L.; Tang, Z.X. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 2015, 5, 10552. [Google Scholar] [CrossRef]
- Han, F.P.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Yu, Z.H.; Li, G.R.; Yang, Z.J. Diversified chromosome rearrangements detected in a wheat–Dasypyrum breviaristatum substitution line induced by gamma-ray irradiation. Plants 2019, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, G.R.; Sehgal, S.K.; Jia, J.Q.; Yang, Z.J.; Friebe, B.; Gill, B.S. Genome relationships in the genus Dasypyrum: Evidence from molecular phylogenetic analysis and in situ hybridization. Plant Syst. Evol. 2010, 288, 149–156. [Google Scholar] [CrossRef]
- Mohler, V.; Stadlmeier, M. Dynamic QTL for adult plant resistance to powdery mildew in common wheat (Triticum aestivum L.). J. Appl. Genet. 2019, 60, 291–300. [Google Scholar] [CrossRef]
- Bariana, H.S.; McIntosh, R.A. Cytogenetic studies in wheat. 15. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome 1993, 36, 476–482. [Google Scholar] [CrossRef] [PubMed]




| Material Name | The Added Alien Chromosome or the Wheat-Th. intermedium Recombinant Chromosome | ITs for Pst | ITs for Bgt |
|---|---|---|---|
| MY11 | / | 4 | 4 |
| TAI7045 | 1St-JS, 2St, 3JS, 4J, 5J.St, 6JS.J, 7JS | 0 | 0 |
| WT75-1 | 1St-JS | 4 | nt |
| WT75-2 | 2St | ;1 | 0 |
| WT75-4 | 4J | 3 | 3 |
| WT75-5 | 5J.St | 4 | 4 |
| WT75-6 | 6JS.J | 3 | nt |
| WT75-7 | 7JS | ; | 3 |
| WT4D-1 | 4DS.4DL-4StL-4DL-4JL | 0 | 0; |
| 78784 | 1St-JS, 2St.JS, 3St, 4St, 5St, 6JS.J, 7JS | 0 | 0 |
| WT78-1 | 1St-JS | ; | nt |
| WT78-2 | 2St.JS | ;1 | 1 |
| WT78-3 | 3St | 4 | 4 |
| WT78-4 | 4St | 0 | 0 |
| WT78-5 | 5St | 4 | nt |
| WT78-6 | 6JS.J | 3 | nt |
| WT78-7 | 7JS | ; | 3 |
| WT4D-2 | 4DS.4DL-4StL-4DL | 0 | 0 |
| Name of Probes | Nucleotide Sequences of Probes (5′-3′) | Length of Oligo Probes (bp) | Reference |
|---|---|---|---|
| Oligo-Dv86 | GTCGTCGCTACCGCGACGACGTCCGCCTCGACTCGCGTTACCCTAAGAC | 49 | / |
| Oligo-7v108 | TATTAACGTGGATAATCGAAATACTGAATTTTAGTATT | 38 | / |
| Oligo-v03-86 | CGAGGCGGACGTCGTCGCGGTAGCGACGACGGACGCCGAGACGAGCACGT | 50 | / |
| Oligo-Ae369 | GAAAGAATCCTTTGAAGCATCTGGTCGTCACAAACGTTTTGACTACT | 47 | / |
| Oligo-pSc119.2-1 | CCGTTTTGTGGACTATTACTCACCGCTTTGGGGTCCCATAGCTAT | 45 | [53] |
| Oligo-pTa535-1 | GACGAGAACTCATCTGTTACATGGGCACTTCAATGTTTTTTAAACTTATTTGAACTCCA | 59 | [53] |
| Oligo-B11 | TCCGCTCACCTTGATGACAACATCAGGTGGAATTCCGTTCGAGGG | 45 | [54] |
| Oligo-D | TACGGGTGCCAAACGAGTGTCTGAAAGACTCCTCGAGAGGAAAATGCGAA | 50 | [55] |
| Oligo-pTa71-2 | GGGCAAAACCACGTACGTGGCACACGCCGCGTA | 33 | [53] |
| Oligo-pSc200 | CTCACTTGCTTTGAGAGTCTCGATCAATTCGGACTCTAGGTTGATTTTTGTATTTTCT | 58 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Li, G.; Zheng, Z.; Wang, H.; Yang, Z. Characterization of New Wheat-Thinopyrum intermedium Derivative Lines with Superior Genes for Stripe Rust and Powdery Mildew Resistance. Plants 2024, 13, 2333. https://doi.org/10.3390/plants13162333
Yu Z, Li G, Zheng Z, Wang H, Yang Z. Characterization of New Wheat-Thinopyrum intermedium Derivative Lines with Superior Genes for Stripe Rust and Powdery Mildew Resistance. Plants. 2024; 13(16):2333. https://doi.org/10.3390/plants13162333
Chicago/Turabian StyleYu, Zhihui, Guangrong Li, Zhiqiang Zheng, Hongjin Wang, and Zujun Yang. 2024. "Characterization of New Wheat-Thinopyrum intermedium Derivative Lines with Superior Genes for Stripe Rust and Powdery Mildew Resistance" Plants 13, no. 16: 2333. https://doi.org/10.3390/plants13162333
APA StyleYu, Z., Li, G., Zheng, Z., Wang, H., & Yang, Z. (2024). Characterization of New Wheat-Thinopyrum intermedium Derivative Lines with Superior Genes for Stripe Rust and Powdery Mildew Resistance. Plants, 13(16), 2333. https://doi.org/10.3390/plants13162333







