The Potential Habitat Response of Cyclobalanopsis gilva to Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection for Cyclobalanopsis gilva Distribution Points
2.2. Source and Filter of Bioclimatic Variables
2.3. Model Optimization
2.4. Model Establishment and Evaluation
2.5. Habitat Suitability Level Analysis and Area Statistics
2.6. Spatial Distribution Pattern Changes in Suitable Habitat
3. Results and Analysis
3.1. Model Optimization and Accuracy Evaluation
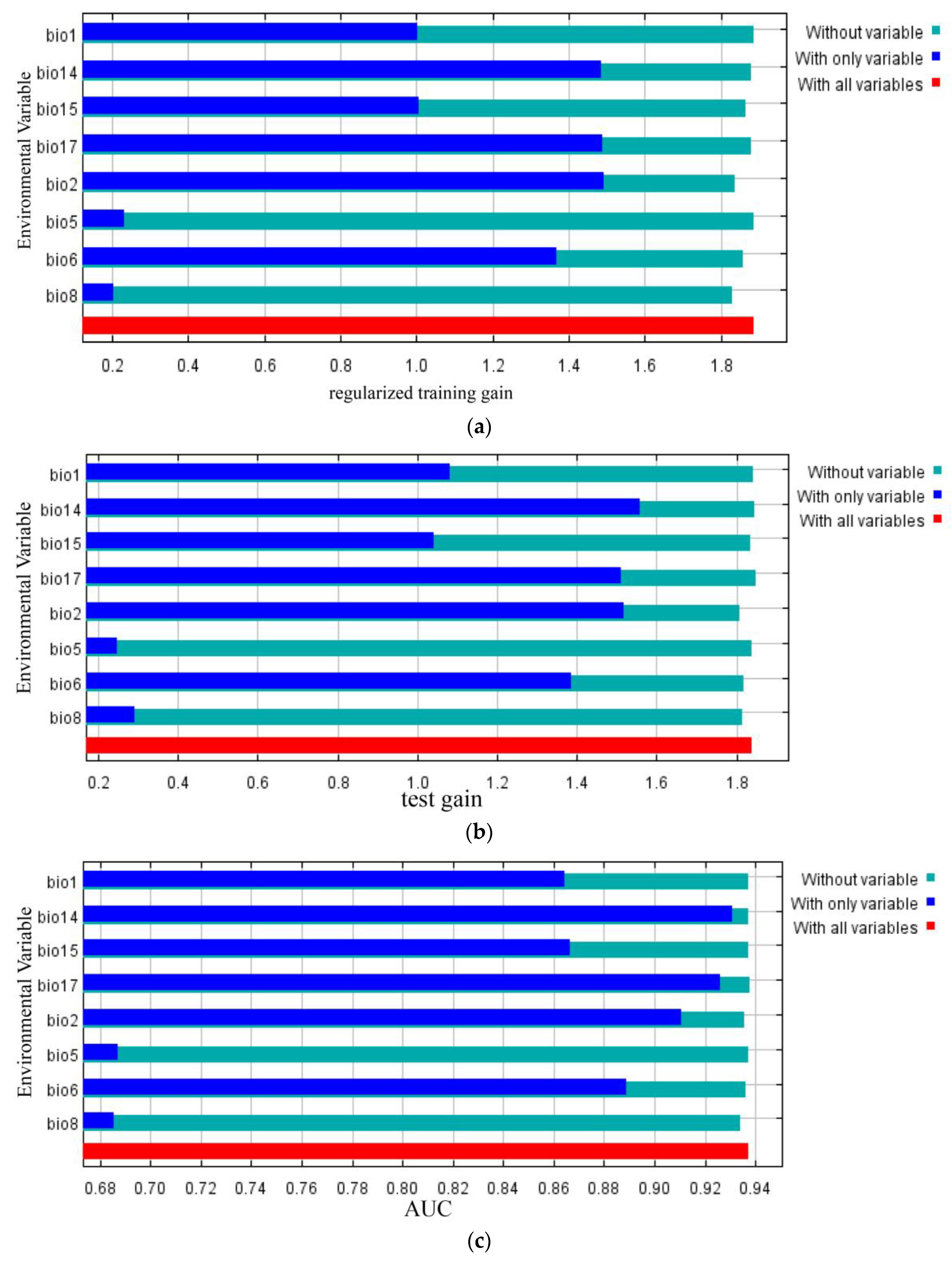
3.2. Importance of Bioclimatic Variables
3.3. Modern Potential Distribution
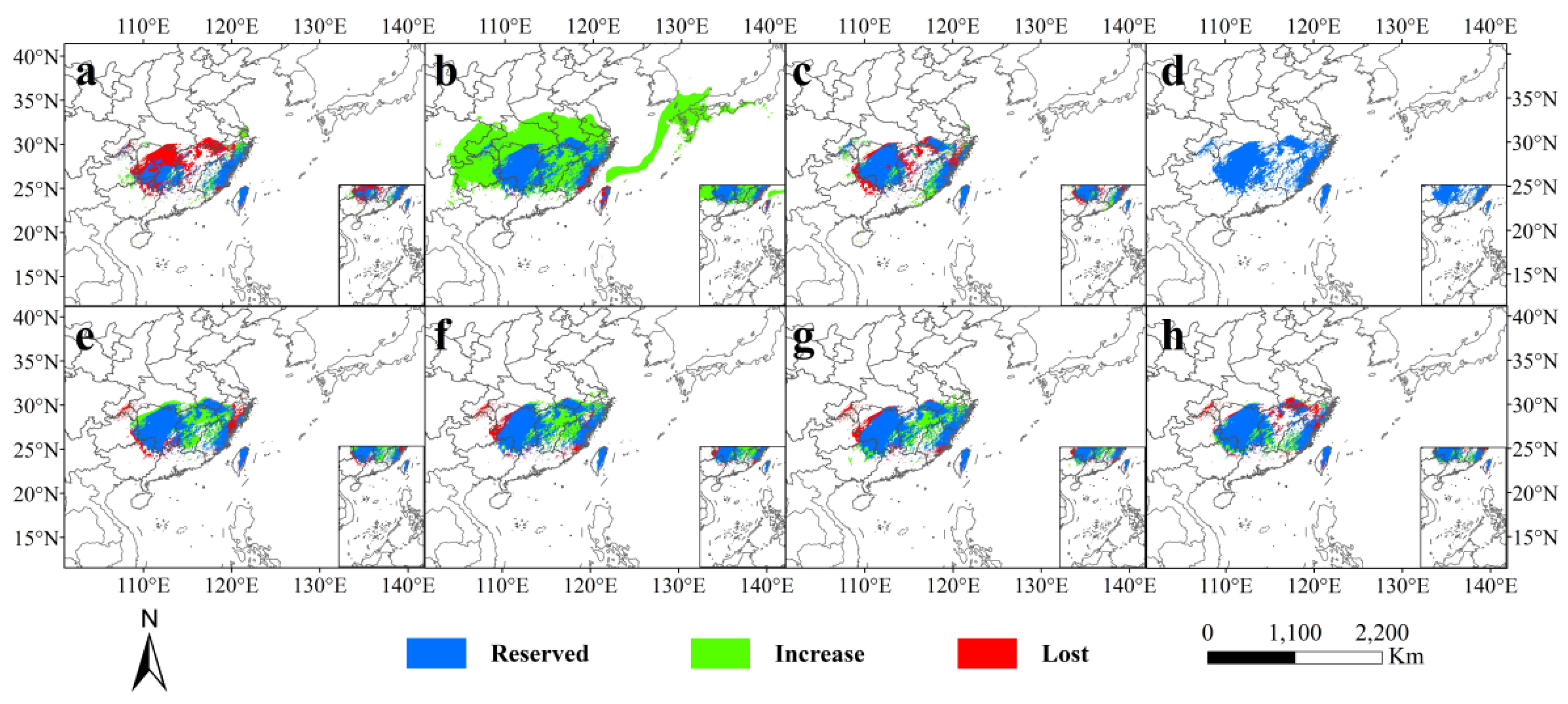
3.4. Past and Future Potential Distribution
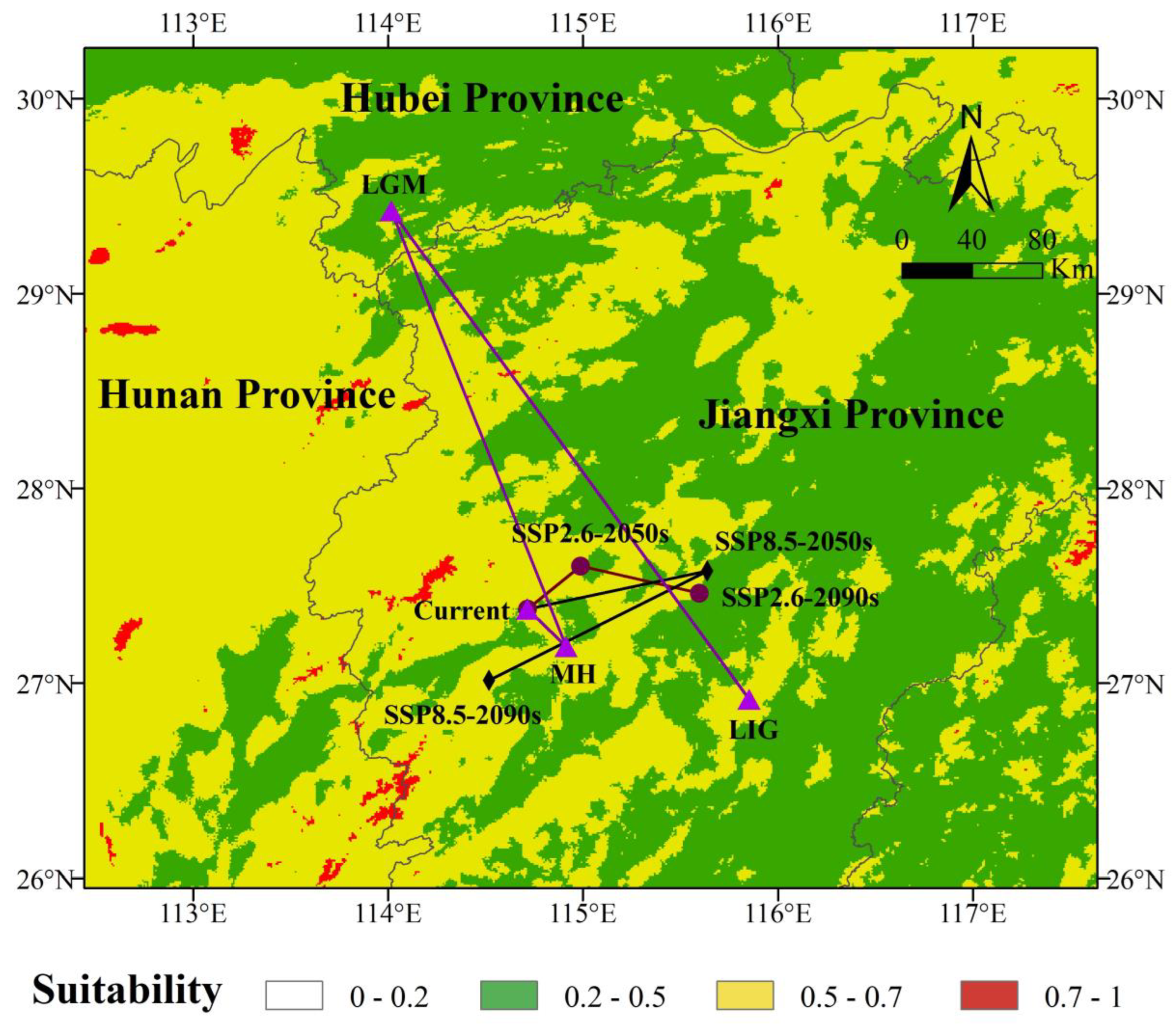
3.5. Centroid Migration Routes
4. Discussion
4.1. Overall Assessment of Prediction Results
4.2. Constraints of Climatic Factors on the Geographic Distribution of Cyclobalanopsis gilva
4.3. Patterns of Potential Distribution Changes
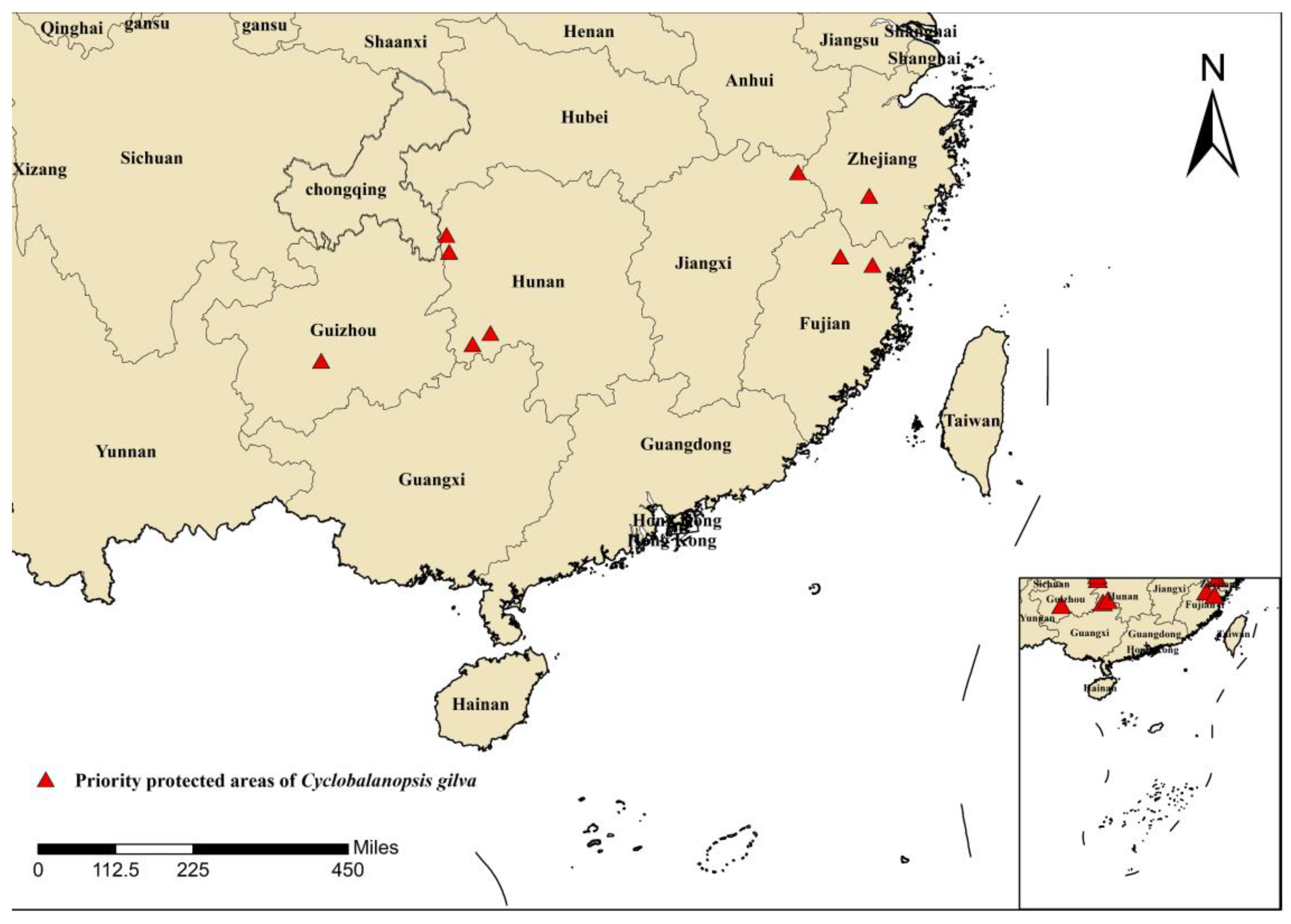
4.4. Recommendations for the Protection of Cyclobalanopsis gilva
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.L.; Li, S.F.; Huang, W.Y.; Xiang, H.L.L.; Jin, J.H.; Oskolski, A.A. Glacial expansion of cold-tolerant species in low latitudes: Megafossil evidence and species distribution modelling. Natl. Sci. Rev. 2023, 10, nwad038. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Zhang, Z.X.; Zhang, Y.K. Effects of late quaternary and contemporary climates on ecoregional plant diversity across different biomes. J. Anim. Plant Sci. 2021, 31, 480–487. [Google Scholar] [CrossRef]
- Xu, W.B.; Guo, W.Y.; Serra-Diaz, J.M.; Schrodt, F.; Eiserhardt, W.L.; Enquist, B.J.; Maitner, B.S.; Merow, C.; Violle, C.; Anand, M.; et al. Global beta-diversity of angiosperm trees is shaped by Quaternary climate change. Sci. Adv. 2023, 9, e68337. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Tian, E.; Jim, C.Y.; Liu, D.; Hu, Z. Effects of climate-change scenarios on the distribution patterns of Castanea henryi. Ecol. Evol. 2022, 12, e9597. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Duffy, K.; Gouhier, T.C.; Ganguly, A.R. Climate-mediated shifts in temperature fluctuations promote extinction risk. Nat. Clim. Change 2022, 12, 1037–1044. [Google Scholar] [CrossRef]
- Du, Z.; He, Y.; Wang, H.; Wang, C.; Duan, Y. Potential geographical distribution and habitat shift of the genus Ammopiptanthus in China under current and future climate change based on the MaxEnt model. J. Arid. Environ. 2021, 184, 104328. [Google Scholar] [CrossRef]
- Sun, J.; Feng, L.; Wang, T.; Tian, X.; He, X.; Xia, H.; Wang, W. Predicting the Potential Habitat of Three Endangered Species of Carpinus Genus under Climate Change and Human Activity. Forests 2021, 12, 1216. [Google Scholar] [CrossRef]
- Franklin, J. Species distribution modelling supports the study of past, present and future biogeographies. J. Biogeogr. 2023, 50, 1533–1545. [Google Scholar] [CrossRef]
- Huang, Z.H.; Li, Z.X.; Yao, L.W.; Yuan, Y.H.; Hong, Z.Y.; Huang, S.Y.; Wang, Y.; Ye, J.H.; Zhang, L.Y.; Ding, J.L. Geographical distribution and potential distribution prediction of thirteen species of Citrus L. in China. Environ. Sci. Pollut. Res. 2024, 31, 6558–6571. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R. Potential geographical distribution of the edangred plant Isoetes under human activities using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Saqib, Z.; Malik, R.N.; Husain, S.Z. Modelling potential distribution of Taxus wallichiana in Palas Valley, Pakistan. Pak. J. Bot. 2006, 38, 539–542. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Merow, C.; Silander, J.A.J. A comparison of Maxlike and Maxent for modelling species distributions. Methods Ecol. Evol. 2014, 5, 215–225. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; DiTommaso, A.; Diez, J.M.; Zhao, Y.; Wang, F.G. Predicting the Potential Distribution of Three Allergenic Invasive Ambrosia (Ragweed) Species in Asia. J. Environ. Inform. 2022, 39, 49–66. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.J.; Zhu, Y.Z.; Cao, Y.T.; Yang, X.M.; Geng, Y.; Zhang, F.J.; Sun, R.Q.; Jia, R.W.; Yan, C.L.; et al. Impacts of Climate Changes on Geographic Distribution of Primula filchnerae, an Endangered Herb in China. Plants 2023, 12, 3561. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Nasser, M.G.; Abou-Shaara, H.F.; Radwan, M.H. Modeling the Potential Global Distribution of Honeybee Pest, Galleria mellonella under Changing Climate. Insects 2022, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, V.A.; Reeves, P.A.; Reilley, A.; de Aguiar, A.V.; Stefenon, V.M.; Richards, C.M. Genetic diversity and biogeographic determinants of population structure in Araucaria angustifolia (Bert.) O. Ktze. Conserv. Genet. 2020, 21, 217–229. [Google Scholar] [CrossRef]
- Qi, C.J.; Tang, G.G. Dendrology (Southern Edition), 2nd ed.; China Forestry Publishing House: Beijing, China, 2005; p. 235. [Google Scholar]
- Zhang, Y. The effects of container size and seedling age on the afforestation Cyclobalanopsis gilva. Fujian For. 2019, 6, 35–38. [Google Scholar]
- Ye, X.X.; Xiao, J.J.; Zhou, H.M. Container Seedling Techniques of Cyclobalanopsis gilva. J. Fujian For. Sci. Technol. 2013, 40, 147–149. [Google Scholar]
- Zaynab, M.; Pan, D.; Fatima, M.; Sharif, Y.; Chen, S.; Chen, W. Proteomics analysis of Cyclobalanopsis gilva provides new insights of low seed germination. Biochimie 2021, 180, 68–78. [Google Scholar] [CrossRef]
- Xie, J. Survival analysis on Cyclobalanopsis gilva population. J. For. Environ. 2011, 31, 254–256. [Google Scholar]
- Wu, X.; Zhang, D.; Chu, X.; Wang, X.; Zhou, Z. Effect of Substrate Ratio and Slow-release Fertilizer Dose on the Growth of Containerized Cyclobalanopsis gilva Seedlings. For. Res. 2014, 27, 794–800. [Google Scholar]
- Ouyang, Z.; Li, Z.; Ouyang, S.; Cheng, Y.; Zhou, Z.; Wu, J. Prediction of the potential distribution of Cyclobalanopsis gilva in China based on the Maxent and ArcGIS model. J. Cent. South. Univ. For. Technol. 2023, 43, 19–26. [Google Scholar]
- Verbruggen, H.; Tyberghein, L.; Belton, G.S.; Mineur, F.; Jueterbock, A.; Hoarau, G.; Gurgel, C.F.D.; De Clerck, O. Improving Transferability of Introduced Species’ Distribution Models: New Tools to Forecast the Spread of a Highly Invasive Seaweed. PLoS ONE 2013, 8, e68337. [Google Scholar] [CrossRef]
- Randin, C.F.; Engler, R.; Normand, S.; Zappa, M.; Zimmermann, N.E.; Pearman, P.B.; Vittoz, P.; Thuiller, W.; Guisan, A. Climate change and plant distribution: Local models predict high-elevation persistence. Glob. Change Biol. 2009, 15, 1557–1569. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Yang, Y.; Wang, T.; Wu, C.; Zhang, X. Prediction of Historical, Current, and Future Configuration of Tibetan Medicinal Herb Gymnadenia orchidis Based on the Optimized MaxEnt in the Qinghai-Tibet Plateau. Plants 2024, 13, 645. [Google Scholar] [CrossRef]
- Record, S.; Strecker, A.; Tuanmu, M.N.; Beaudrot, L.; Zarnetske, P.; Belmaker, J.; Gerstner, B. Does scale matter? A systematic review of incorporating biological realism when predicting changes in species distributions. PLoS ONE 2018, 13, e0194650. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Li, Y.; Zhu, W.; Chen, Y. Maxent Modelling Predicts a Shift in Suitable Habitats of a Subtropical Evergreen Tree (Cyclobalanopsis glauca (Thunberg) Oersted) under Climate Change Scenarios in China. Forests 2022, 13, 126. [Google Scholar] [CrossRef]
- Crivellaro, A.; Piermattei, A.; Dolezal, J.; Dupree, P.; Büntgen, U. Biogeographic implication of temperature-induced plant cell wall lignification. Commun. Biol. 2022, 5, 767. [Google Scholar] [CrossRef]
- Huang, E.H.; Chen, Y.X.; Fang, M.; Zheng, Y.; Yu, S.X. Environmental drivers of plant distributions at global and regional scales. Glob. Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, B.; Ou, G. Displacement distribution and climate explanation on humid and semi-humid evergreen broadleaved forests using niche model of Cyclobalanopsis glauca and C. glaucoides in China. Guihaia 2022, 42, 460–469. [Google Scholar]
- Xia, H.M.; Li, A.N.; Feng, G.; Li, Y.; Qin, Y.C.; Lei, G.B.; Cui, Y.P. The Effects of Asymmetric Diurnal Warming on Vegetation Growth of the Tibetan Plateau over the Past Three Decades. Sustainability 2018, 10, 1103. [Google Scholar] [CrossRef]
- Atkin, O.K.; Turnbull, M.H.; Zaragoza-Castells, J.; Fyllas, N.M.; Lloyd, J.; Meir, P.; Griffin, K.L. Light inhibition of leaf respiration as soil fertility declines along a post-glacial chronosequence in New Zealand: An analysis using the Kok method. Plant Soil 2013, 367, 163–182. [Google Scholar] [CrossRef]
- Du, Y.; Lu, R.; Sun, H.; Cui, E.; Yan, L.; Xia, J. Plant photosynthetic overcompensation under nocturnal warming: Lack of evidence in subtropical evergreen trees. Ann. Bot. 2022, 130, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Piao, S.; Sun, Y.; Rogers, B.M.; Li, X.; Lian, X.; Liu, Z.; Chen, A.; Penuelas, J. Future reversal of warming-enhanced vegetation productivity in the Northern Hemisphere. Nat. Clim. Change 2022, 12, 581–586. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Yang, M.; Li, C.; Li, J.; Li, A. Effect of drought stress on light response characteristics of photosynthesis of Cyclobalanopsis gilva seedlings. Hubei Agric. Sci. 2020, 59, 86. [Google Scholar]
- Yin, H.-M.; Yang, M.-H.; Li, P.-L.; Yu, X.-L.; Xiong, H.; Xu, Q.-Y.; Zou, F.-L.; Chen, Y.; Dai, W.-H.; Jiang, Y.; et al. Seasonality of Photosynthetic Physiology and Leaf Anatomy in Three Different Quercus L. Section Cyclobalanopsis Seedlings of Quercus chungii, Quercus gilva, and Quercus glauca in the Subtropical Region of South China. Forests 2022, 13, 2067. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Jiang, X.; Qin, Z.; Farooq, T.H.; Cao, F.; Li, H. A chromosome-scale genome assembly of Quercus gilva: Insights into the evolution of Quercus section Cyclobalanopsis (Fagaceae). Front. Plant Sci. 2022, 13, 1012277. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, X.-L.; Deng, M.; Westwood, M.; Song, Y.-G.; Zheng, S.-S. Conservation genetics of rare trees restricted to subtropical montane cloud forests in southern China: A case study from Quercus arbutifolia (Fagaceae). Tree Genet. Genomes 2016, 12, 90. [Google Scholar] [CrossRef]
- Dai, L.; Hao, Q.; Xue, J.; Mao, L. Last Glacial Maximum, early Holocene and modern environments of the northern South China Sea region: Insight from SEM analysis of Oak (Quercus) pollen. Sci. Total Environ. 2019, 691, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Shu, J.; Chen, W. Changes of vegetation in southern China. Sci. China Earth Sci. 2019, 62, 1316–1328. [Google Scholar] [CrossRef]
- Xe, T.; Shi, X.; Liu, S.; Qiao, S.; Yao, Z.; Fang, X.; Wu, Y.; Shan, X.; Liu, J.; Yang, G.; et al. Late Quaternary sedimentary evolution of the outer shelf of the East China Sea. Quat. Int. 2018, 493, 59–69. [Google Scholar]
- Zheng, Z.; Huang, K.Y.; Deng, Y.; Cao, L.L.; Yu, S.H.; Suc, J.P.; Berne, S.; Guichard, F. A ~200 ka pollen record from Okinawa Trough: Paleoenvironment reconstruction of glacial-interglacial cycles. Sci. China Earth Sci. 2013, 56, 1731–1747. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, C.; Huang, K.; Zhang, X.; Kershaw, P.; Cheng, J.; Li, J.; Yue, Y.; Wan, Q.; Zhang, Y.; et al. Holocene warming and evergreen/deciduous forest replacement across eastern China. Quat. Sci. Rev. 2023, 307, 108057. [Google Scholar] [CrossRef]
- Hayashi, R.; Inoue, J.; Makino, M.; Takahara, H. Vegetation history during the last 17,000 years around Sonenuma Swamp in the eastern shore area of Lake Biwa, western Japan: With special reference to changes in species composition of Quercus subgenus Lepidobalanus trees based on SEM pollen morphology. Quat. Int. 2012, 254, 99–106. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Peng, B.; Wu, B.; Yu, J.; Dai, L. Palynological evidence for palaeoenviromental change and human activity in Ningde of Fujian Province during Holocene. Mar. Geol. Quat. Geol. 2021, 41, 170–181. [Google Scholar] [CrossRef]
- Prior, S.A.; Runion, G.B.; Marble, S.C.; Rogers, H.H.; Gilliam, C.H.; Torbert, H.A. A Review of Elevated Atmospheric CO2 Effects on Plant Growth and Water Relations: Implications for Horticulture. Hortscience 2011, 46, 158–162. [Google Scholar] [CrossRef]
- Zhang, M.-G.; Zhou, Z.-K.; Chen, W.-Y.; Cannon, C.H.; Raes, N.; Slik, J.W.F. Major declines of woody plant species ranges under climate change in Yunnan, China. Divers. Distrib. 2014, 20, 405–415. [Google Scholar] [CrossRef]


| Model Evaluation | Feature Combination | Regularization Multiplier | Delta. AICc | Avg. diff. AUC | Avg. Test. or10pct |
|---|---|---|---|---|---|
| default | LQHPT | 1 | 80.56426871 | 0.023329891 | 0.152252568 |
| optimize | LQ | 1.8 | 0 | 0.011517256 | 0.141835901 |
| Code | Bioclimatic Variables | PC (%) | PI (%) | TRGW | TRGO | TGW | TGO | AUCW | AUCO |
|---|---|---|---|---|---|---|---|---|---|
| Bio2 | Mean Diurnal Range (mean of monthly (max temp–min temp)) | 40.0 | 19.7 | 1.8357 | 1.4913 | 1.8069 | 1.5168 | 0.9356 | 0.9109 |
| Bio6 | Min Temperature of Coldest Month | 28.1 | 54.1 | 1.8591 | 1.3691 | 1.8187 | 1.3867 | 0.9362 | 0.8890 |
| Bio17 | Precipitation of Driest Quarter | 16.9 | 1.7 | 1.8782 | 1.4877 | 1.8479 | 1.5112 | 0.9377 | 0.9263 |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | 5.6 | 12.8 | 1.8658 | 1.0065 | 1.8349 | 1.0400 | 0.9372 | 0.8665 |
| Bio14 | Precipitation of Driest Month | 4.9 | 2.1 | 1.8812 | 1.4860 | 1.8448 | 1.5578 | 0.9374 | 0.9312 |
| Bio8 | Mean Temperature of Wettest Quarter | 3.3 | 8.7 | 1.8311 | 0.2048 | 1.8132 | 0.2921 | 0.9339 | 0.6855 |
| Bio1 | Annual Mean Temperature | 1.0 | 0.4 | 1.8856 | 1.0031 | 1.8401 | 1.0828 | 0.9371 | 0.8646 |
| Bio5 | Max Temperature of Warmest Month | 0.2 | 0.5 | 1.8854 | 0.2342 | 1.8398 | 0.2478 | 0.9372 | 0.6870 |
| Period | Generally Suitable Habitats | Moderately Suitable Habitats | Highly Suitable Habitats | Total |
|---|---|---|---|---|
| Last Interglacial | 118.21 | 24.6 | 3.87 | 146.68 |
| Last Glacial Maximum | 180.54 | 118.66 | 46.52 | 345.72 |
| Middle Holocene | 107.68 | 37.07 | 2.92 | 147.67 |
| Current | 99.5 | 40.41 | 3.14 | 143.05 |
| 2050 s-SSP126 | 115.35 | 50.41 | 1.27 | 167.03 |
| 2050 s-SSP585 | 112.3 | 50.11 | 1.6 | 164.01 |
| 2090 s-SSP126 | 110.65 | 48.29 | 1.66 | 160.6 |
| 2090 s-SSP585 | 113.04 | 40.75 | 1.02 | 154.81 |
| Period | Area (km2) | Change (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Increase | Reserved | Lost | Change | Increase Rate | Reserved Rate | Lost Rate | Change Rate | |
| Last Interglacial | 6.12 | 22.22 | 21.27 | −15.16 | 14.05% | 51.03% | 49.85% | −34.81% |
| Last Glacial Maximum | 124.42 | 40.64 | 2.88 | 121.54 | 285.70% | 93.33% | 6.61% | 279.10% |
| Middle Holocene | 7.47 | 32.38 | 11.18 | −3.71 | 17.16% | 74.37% | 25.68% | −8.52% |
| 2050 s-SSP126 | 14.90 | 36.66 | 6.70 | 8.19 | 34.21% | 84.17% | 15.39% | 18.82% |
| 2050 s-SSP585 | 14.24 | 37.49 | 5.97 | 8.28 | 32.70% | 86.10% | 13.70% | 19.01% |
| 2090 s-SSP126 | 12.57 | 37.19 | 6.26 | 6.31 | 28.86% | 85.40% | 14.36% | 14.49% |
| 2090 s-SSP585 | 6.81 | 35.02 | 8.40 | −1.59 | 15.64% | 80.41% | 19.29% | −3.65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, Y.; Zhao, J.; Weng, H.; Ye, X.; Liu, S.; Zhao, Z.; Ahmad, S.; Zhan, C. The Potential Habitat Response of Cyclobalanopsis gilva to Climate Change. Plants 2024, 13, 2336. https://doi.org/10.3390/plants13162336
Liu B, Li Y, Zhao J, Weng H, Ye X, Liu S, Zhao Z, Ahmad S, Zhan C. The Potential Habitat Response of Cyclobalanopsis gilva to Climate Change. Plants. 2024; 13(16):2336. https://doi.org/10.3390/plants13162336
Chicago/Turabian StyleLiu, Bao, Yinglin Li, Jintao Zhao, Huiying Weng, Xingzhuang Ye, Shouqun Liu, Zixin Zhao, Sagheer Ahmad, and Chaoyu Zhan. 2024. "The Potential Habitat Response of Cyclobalanopsis gilva to Climate Change" Plants 13, no. 16: 2336. https://doi.org/10.3390/plants13162336






