X-ray Microanalysis of Elemental Composition of Vitis sylvestris Pollen Grains
Abstract
:1. Introduction
2. Materials and Methods
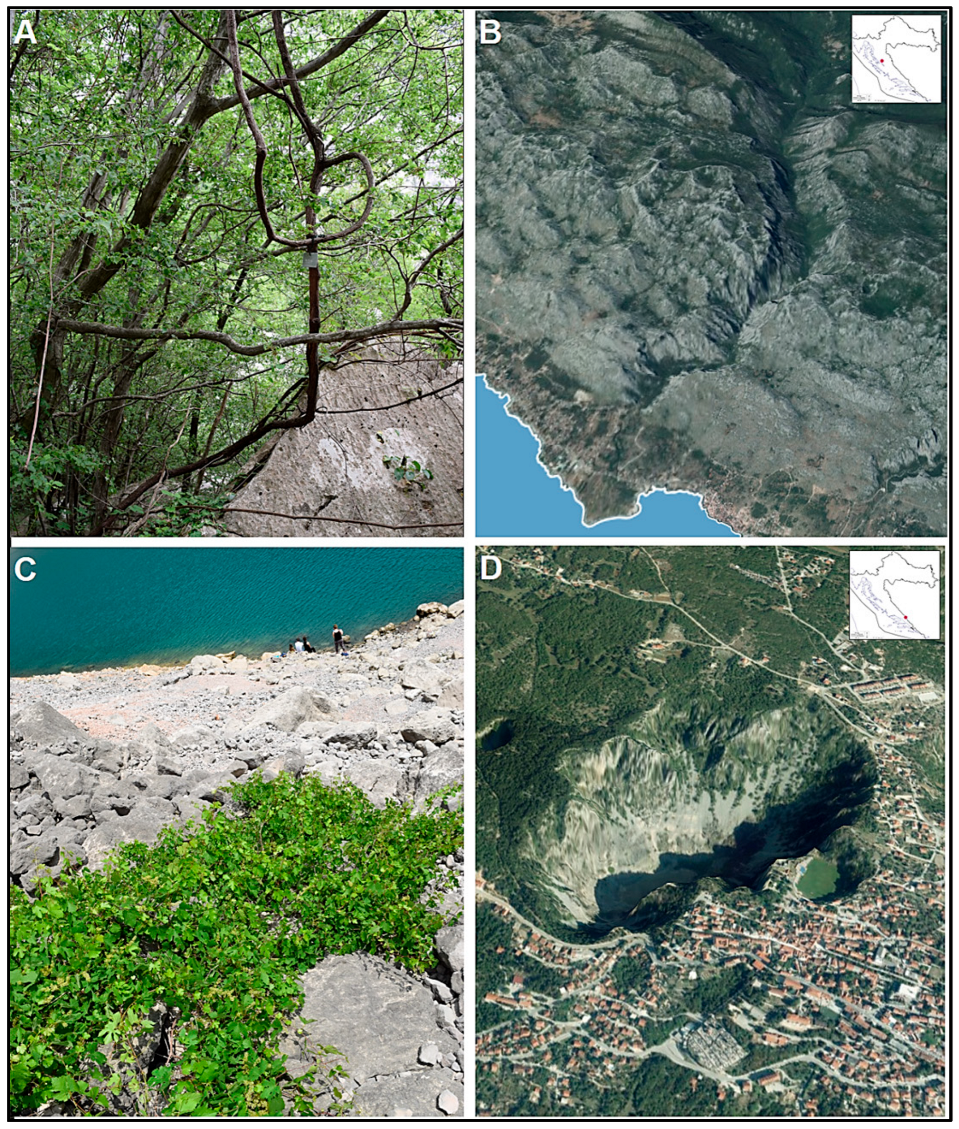
2.1. Sampling Sites and Plant Material
2.2. Scanning Electron Microscopy and Elemental Composition of Pollen
2.3. Statistical Analysis
3. Results
3.1. Elemental Composition of the Pollen of Vitis vinifera L.
3.2. Principal Component Analysis (PCA) Based on Elemental Pollen Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clément, C. Sugars and flowering in the grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar] [CrossRef]
- Kidman, C.M.; Dry, P.R.; McCarthy, M.G.; Collins, C. Effect of rootstock on nutrition, pollination and fertilisation in ‘Shiraz’ (Vitis vinifera L.). Vitis 2014, 53, 139–145. [Google Scholar] [CrossRef]
- Alva, O.; Roa-Roco, R.N.; Pérez-Díaz, R.; Yáñez, M.; Tapia, J.; Moreno, Y.; Ruiz-Lara, S.; González, E. Pollen morphology and boron concentration in floral tissues as factors triggering natural and GA-induced parthenocarpic fruit development in grapevine. PLoS ONE 2015, 10, e0139503. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, M.L.; Dry, P.R.; Sedgley, M. Effects of sodium molybdate foliar sprays on molybdenum concentration in the vegetative and reproductive structures and on yield components of Vitis vinifera cv. Merlot. Aust. J. Grape Wine Res. 2010, 16, 477–490. [Google Scholar] [CrossRef]
- Longbottom, M.L. The Reproductive Biology of Grapevines: Factors That Affect Flowering and Fruitset. Ph.D. Dissertation, University of Adelaide, Adelaide, Australia, 2007. [Google Scholar]
- Blackmore, S.; Wortley, A.H.; Skvarla, J.J.; Rowley, J.R. Pollen wall development in flowering plants. New Phytol. 2007, 174, 483–498. [Google Scholar] [CrossRef]
- Gallardo, A.; Ocete, R.; López, M.A.; Lara, M.; Rivera, D. Assessment of pollen dimorphism in populations of Vitis vinifera L. subsp. sylvestris (Gmelin) Hegi in Spain. Vitis 2009, 48, 59–62. [Google Scholar]
- Mercuri, A.M.; Torri, P.; Florenzano, A.; Clò, E.; Mariotti Lippi, M.; Sgarbi, E.; Bignami, C. Sharing the agrarian knowledge with archaeology: First evidence of the dimorphism of Vitis pollen from the Middle Bronze Age of N Italy (Terramara Santa Rosa di Poviglio). Sustainability 2021, 13, 2287. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Cao, J. Pollen wall development: The associated enzymes and metabolic pathways. Plant Biol. 2013, 15, 249–263. [Google Scholar] [CrossRef]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R. Illustrated Pollen Terminology, 2nd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Büyükkartal, H.N.; Çölgeçen, H.; Marasal, B. Development of anther wall throughout microsporogenesis in Vitis vinifera L. cv. Çavus. Int. J. Agric. Biol. 2005, 7, 616–620. [Google Scholar]
- Abd Mutalib, M.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J. Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) spectroscopy. In Membrane Characterization; Hilal, N., Fauzi Ismail, A., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; pp. 161–179. [Google Scholar]
- Shaban, S.E.; Ibrahiem, N.M.; El-mongy, S.A.; Elshereafy, E.E. Validation of scanning electron microscope (SEM), energy dispersive X-ray (EDX) and gamma spectrometry to verify source nuclear material for safeguards purposes. J. Radioanal. Nucl. Chem. 2013, 296, 1219–1224. [Google Scholar] [CrossRef]
- Clò, E.; Furia, E.; Florenzano, A.; Mercuri, A.M. Flora-vegetation history and land use in Medieval Tuscany: The palynological evidence of a local biodiversity heritage. Quat. Int. 2023, in press. [Google Scholar] [CrossRef]
- Crang, R.E.; May, G. Evidence for silicon as a prevalent elemental component in pollen wall structure. Canad. J. Bot. 1974, 52, 2171–2174. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [CrossRef] [PubMed]
- Bucsek, M.J.; Nyéki, J.; Szabó, Z.; Kádár, A. Quantitation of Mineral Elements of Different Fruit Pollen Grains. In Microbeam and Nanobeam Analysis; Benoit, D., Bresse, J.F., Van’t dack, L., Werner, H., Wernisch, J., Eds.; Mikrochim. Acta Supplement; Springer: Vienna, Austria, 1996; p. 13. [Google Scholar] [CrossRef]
- Duque, L.; Guimarães, F.; Ribeiro, H.; Sousa, R.; Abreu, I. Elemental characterization of the airborne pollen surface using Electron Probe Microanalysis (EPMA). Atmos. Environ. 2013, 75, 296–302. [Google Scholar] [CrossRef]
- Acma, F.M.; Mendez, N.P. Pollen morphology and pollen elemental composition of selected philippine native gingers in tribe Alpinieae (Alpinioideae: Zingiberaceae). Biol. Forum 2018, 10, 1–10. [Google Scholar]
- Rane, P.; Thakre, M.; Verma, M.K.; Kumar, C.; Prakash, J.; Srivastava, V.; Shashank, P.R.; Murukan, N.; Chawla, G.; Kumar Mandal, P.; et al. Studies on pollen micro-morphology, pollen storage method, and cross-compatibility among the grape (Vitis spp.) genotypes. Front. Plant Sci. 2024, 15, 1353808. [Google Scholar] [CrossRef]
- Trouvé, G.; Michelin, L.; Kehrli, D.; Josien, L.; Rigolet, S.; Lebeau, B.; Gieré, R. The multi-analytical characterization of calcium oxalate phytolith crystals from grapevine after treatment with calcination. Crystals 2023, 13, 967. [Google Scholar] [CrossRef]
- Zdunić, G.; Maul, E.; Eiras Dias, J.E.J.; Munoz Organero, G.; Carka, F.; Maletić, E.; Savvides, S.; Jahnke, G.G.; Nagy, Z.A.; Nikolić, D.; et al. Guiding principles for identification, evaluation and conservation of Vitis vinifera subsp. sylvestris. Vitis 2017, 56, 127–131. [Google Scholar] [CrossRef]
- Lukšić, K.; Zdunić, G.; Mucalo, A.; Marinov, L.; Ranković-Vasić, Z.; Ivanović, J.; Nikolić, D. Microstructure of Croatian wild grapevine (Vitis vinifera subsp. sylvestris Gmel Hegi) pollen grains revealed by scanning electron microscopy. Plants 2022, 11, 1479. [Google Scholar] [CrossRef]
- Butorac, L.; Hančević, K.; Lukšić, K.; Škvorc, Ž.; Leko, M.; Maul, E.; Zdunić, G. Assessment of wild grapevine (Vitis vinifera ssp. sylvestris) chlorotypes and accompanying woody species in the Eastern Adriatic region. PLoS ONE 2018, 13, e0199495. [Google Scholar] [CrossRef]
- Nikolić, D.; Milatović, D. Pollen morphology of some sweet cherry cultivars observed by scanning electron microscopy. Acta Hortic. 2016, 1139, 369–374. [Google Scholar] [CrossRef]
- Nikolić, D.; Milatović, D. Scanning electron microscopy study of pollen morphology of five sour cherry cultivars. Acta Hortic. 2017, 1161, 395–400. [Google Scholar] [CrossRef]
- Radović, A.; Nikolić, D.; Milatović, D.; Djurović, D.; Trajković, J. Investigation of pollen morphological characteristics in some quince (Cydonia oblonga Mill.) cultivars. Turk. J. Agric. For. 2016, 40, 441–449. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Lê, S.; Mazet, J. FactoMineR: Factor Analysis and Data Mining with R. R Package Version 1.04. 2007. Available online: https://CRAN.R-project.org/package=FactoMineR (accessed on 23 May 2024).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 May 2024).
- Pereira, M.R.; Ribeiro, H.; Cunha, M.; Abreu, I. Comparison of pollen quality in Vitis vinifera L. cultivars. Sci. Hortic. 2018, 227, 112–116. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater photosynthesis of submerged plants–recent advances and methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Kinzel, H. Calcium in the vacuoles and cell walls of plant tissue. Flora 1989, 182, 99–125. [Google Scholar] [CrossRef]
- Zienkiewicz, K.; Rejón, J.D.; Suárez, C.; Castro, A.J.; de Dios Alché, J.; Rodríguez García, M.I. Whole-Organ analysis of calcium behaviour in the developing pistil of olive (Olea europaea L.) as a tool for the determination of key events in sexual plant reproduction. BMC Plant Biol. 2011, 11, 1–12. [Google Scholar] [CrossRef]
- Das, D.; Mondal, S.; Mandal, S. Studies on pollen viability and in vitro pollen germination of Nymphoides indica (L.) Kuntz. IJRAR 2018, 5, 461–467. [Google Scholar]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Zheng, R.H.; Su, S.D.; Xiao, H.; Tian, H.Q. Calcium: A Critical factor in pollen germination and tube elongation. Int. J. Mol. Sci. 2019, 20, 420. [Google Scholar] [CrossRef]
- Tian, H.Q.; Kuang, A.; Musgrave, M.E.; Russell, S.D. Calcium distribution in fertile and sterile anthers of a photoperiod-sensitive genic male-sterile rice. Planta 1998, 204, 183–192. [Google Scholar] [CrossRef]
- Hai-Yan, K.; Gui-Xia, J. Calcium distribution during pollen development of Larix principis-rupprechtii. J. Integr. Plant Biol. 2004, 46, 69–76. [Google Scholar]
- Melcrová, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T.; Mishra, B.; Myneni, S.C.; Bellenger, J.P.; Kraepiel, A.M. Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nat. Geosci. 2009, 2, 625–629. [Google Scholar] [CrossRef]
- Bittner, F. Molybdenum metabolism in plants and crosstalk to iron. Front. Plant Sci. 2014, 5, 76931. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Bhantana, P.; Imran, M.; Saleem, M.H.; Moussa, M.G.; Khan, Z.; Khan, I.; Alam, M.; Abbas, M.; Binyamin, R.; et al. Molybdenum potential vital role in plants metabolism for optimizing the growth and development. Ann. Environ. Sci. Toxicol. 2020, 4, 32–44. [Google Scholar] [CrossRef]
- Redlich, C.; Quadbeck, P.; Thieme, M.; Kieback, B. Molybdenum–A biodegradable implant material for structural applications? Acta Biomater. 2020, 104, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Schauer, A.; Redlich, C.; Scheibler, J.; Poehle, G.; Barthel, P.; Maennel, A.; Adams, V.; Weissgaerber, T.; Linke, A.; Quadbeck, P. Biocompatibility and degradation behavior of molybdenum in an in vivo rat model. Materials 2021, 14, 7776. [Google Scholar] [CrossRef]
- Fernandes, C.; Taurino, I. Biodegradable molybdenum (mo) and tungsten (w) devices: One step closer towards fully-transient biomedical implants. Sensors 2022, 22, 3062. [Google Scholar] [CrossRef] [PubMed]
- Usherwood, N.R. The role of potassium in crop quality. In Potassium in Agriculture, 1st ed.; Munson, R.D., Ed.; American Society of Agronomy: Madison, WI, USA, 1985; pp. 489–513. [Google Scholar] [CrossRef]
- Cuq, S.; Lemetter, V.; Kleiber, D.; Levasseur-Garcia, C. Assessing macro-(P, K, Ca, Mg) and micronutrient (Mn, Fe, Cu, Zn, B) concentration in vine leaves and grape berries of Vitis vinifera by using near-infrared spectroscopy and chemometrics. Comput. Electron. Agric. 2020, 179, 105841. [Google Scholar] [CrossRef]
- Benito, A.; Muñoz-Organero, G.; De Andrés, M.T.; Ocete, R.; García-Muñoz, S.; López, M.Á.; Arroyo-García, R.; Cabello, F. Ex situ ampelographical characterisation of wild Vitis vinifera from fifty-one Spanish populations. Aust. J. Grape Wine Res. 2016, 23, 143–152. [Google Scholar] [CrossRef]
- Marrano, A.; Micheletti, D.; Lorenzi, S.; Neale, D.; Grando, M.S. Genomic signatures of different adaptations to environmental stimuli between wild and cultivated Vitis vinifera L. Hortic. Res. 2018, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Yun, S.J. Developmental regulation of K accumulation in pollen, anthers, and papillae: Are anther dehiscence, papillae hydration, and pollen swelling leading to pollination and fertilization in barley (Hordeum vulgare L.) regulated by changes in K concentration? J. Exp. Bot. 2006, 57, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Vasilevskaya, N. Pollution of the environment and pollen: A Review. Stresses 2022, 2, 515–530. [Google Scholar] [CrossRef]
- Exley, C.; Rotheray, E.; Goulson, D. Bumblebee pupae contain high levels of aluminium. PLoS ONE 2015, 10, e0127665. [Google Scholar] [CrossRef]
- EFSA. Alvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F.; et al. Peer review of the pesticide risk assessment of the active substance aluminium ammonium sulfate. EFSA 2022, 20, e07319. [Google Scholar] [CrossRef]
- Baldi, B.G.; Franceschi, V.R.; Loewus, F.A. Localization of phosphorus and cation reserves in Lilium longiflorum pollen. Plant Physiol. 1987, 83, 1018–1021. [Google Scholar] [CrossRef]
- Katifori, E.; Alben, S.; Cerda, E.; Nelson, D.R.; Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl. Acad. Sci. USA 2010, 107, 7635–7639. [Google Scholar] [CrossRef]




| Genotype | Flower | Carbon | Oxygen | Magnesium | Phosphorus | Potassium | Calcium | Molybdenum | Aluminum |
|---|---|---|---|---|---|---|---|---|---|
| Pak10 | F | 71.67 ± 3.23 ab | 20.76 ± 5.35 | 0.38 ± 0.02 a | 1.30 ± 0.18 | 0.96 ± 0.25 ab | 1.08 ± 0.31 bc | 3.85 ± 1.51 a | nd |
| Pak12 | F | 73.58 ± 3.91 ab | 19.96 ± 5.27 | 0.31 ± 0.06 abc | 1.39 ± 0.43 | 0.97 ± 0.36 ab | 1.01 ± 0.15 abc | 2.78 ± 0.49 ab | nd |
| Pak11 | M | 77.05 ± 1.85 ab | 16.93 ± 2.47 | 0.17 ± 0.03 d | 1.05 ± 0.14 | 0.77 ± 0.13a | 0.99 ± 0.05 abc | 3.04 ± 0.40 ab | nd |
| Pak13 | M | 76.49 ± 2.44 ab | 18.43 ± 3.68 | 0.28 ± 0.04 abcd | 0.89 ± 0.28 | 0.89 ± 0.24 ab | 0.58 ± 0.17 a | 2.43 ± 0.58 ab | nd |
| Pak19 | M | 75.45 ± 1.75 ab | 17.79 ± 3.13 | 0.34 ± 0.04 ab | 1.00 ± 0.22 | 1.05 ± 0.25 ab | 0.74 ± 0.09 ab | 3.64 ± 0.80 ab | nd |
| Pak21 | M | 77.29 ± 0.39 ab | 15.57 ± 1.39 | 0.36 ± 0.07 ab | 1.28 ± 0.41 | 0.92 ± 0.22 ab | 0.66 ± 0.13 ab | 3.92 ± 0.93 a | nd |
| Pak32 | M | 75.51 ± 3.64 ab | 18.42 ± 4.18 | 0.25 ± 0.04 bcd | 1.13 ± 0.10 | 0.92 ± 0.13 ab | 0.83 ± 0.12 abc | 2.94 ± 0.20 ab | nd |
| Im11 | M | 77.82 ± 0.69 a | 15.79 ± 1.19 | 0.33 ± 0.07 ab | 1.15 ± 0.11 | 1.04 ± 0.14 ab | 1.03 ± 0.06 abc | 2.83 ± 0.35 ab | nd |
| Im18 | M | 77.63 ± 2.40 a | 18.20 ± 2.26 | 0.19 ± 0.01 cd | 0.72 ± 0.07 | 0.56 ± 0.04 a | 0.82 ± 0.03 abc | 1.89 ± 0.18 b | nd |
| Im19 | M | 70.57 ± 2.17 b | 22.07 ± 2.33 | 0.30 ± 0.04 abc | 1.33 ± 0.33 | 0.89 ± 0.23 ab | 1.29 ± 0.28 c | 3.55 ± 0.44 ab | nd |
| cv. PMC | H | 75.72 ± 1.03 ab | 17.87 ± 1.63 | 0.40 ± 0.04 a | 1.25 ± 0.13 | 1.53 ± 0.25 b | 0.90 ± 0.20 abc | 2.08 ± 0.10 ab | 0.26 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukšić, K.; Mucalo, A.; Marinov, L.; Ozretić Zoković, M.; Ranković-Vasić, Z.; Nikolić, D.; Zdunić, G. X-ray Microanalysis of Elemental Composition of Vitis sylvestris Pollen Grains. Plants 2024, 13, 2338. https://doi.org/10.3390/plants13162338
Lukšić K, Mucalo A, Marinov L, Ozretić Zoković M, Ranković-Vasić Z, Nikolić D, Zdunić G. X-ray Microanalysis of Elemental Composition of Vitis sylvestris Pollen Grains. Plants. 2024; 13(16):2338. https://doi.org/10.3390/plants13162338
Chicago/Turabian StyleLukšić, Katarina, Ana Mucalo, Luka Marinov, Maja Ozretić Zoković, Zorica Ranković-Vasić, Dragan Nikolić, and Goran Zdunić. 2024. "X-ray Microanalysis of Elemental Composition of Vitis sylvestris Pollen Grains" Plants 13, no. 16: 2338. https://doi.org/10.3390/plants13162338






