Effect of CO2 Elevation on Tomato Gas Exchange, Root Morphology and Water Use Efficiency under Two N-Fertigation Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Treatments
2.3. Measurements
2.3.1. Leaf Gas Exchange
2.3.2. Leaf Water Relation and ABA
2.3.3. Stomatal Aperture and Density
2.3.4. Root Morphology
2.3.5. Leaf Area and Plant Water Use Efficiency
2.3.6. Statistics Analysis
3. Results
3.1. Gas Exchange and WUE at Leaf Level
3.2. Plant Water Relation and Stomatal Morphology
3.3. Root Morphology
3.4. Leaf Area and Specific Leaf Area
3.5. Dry Matter and WUE at Plant Level
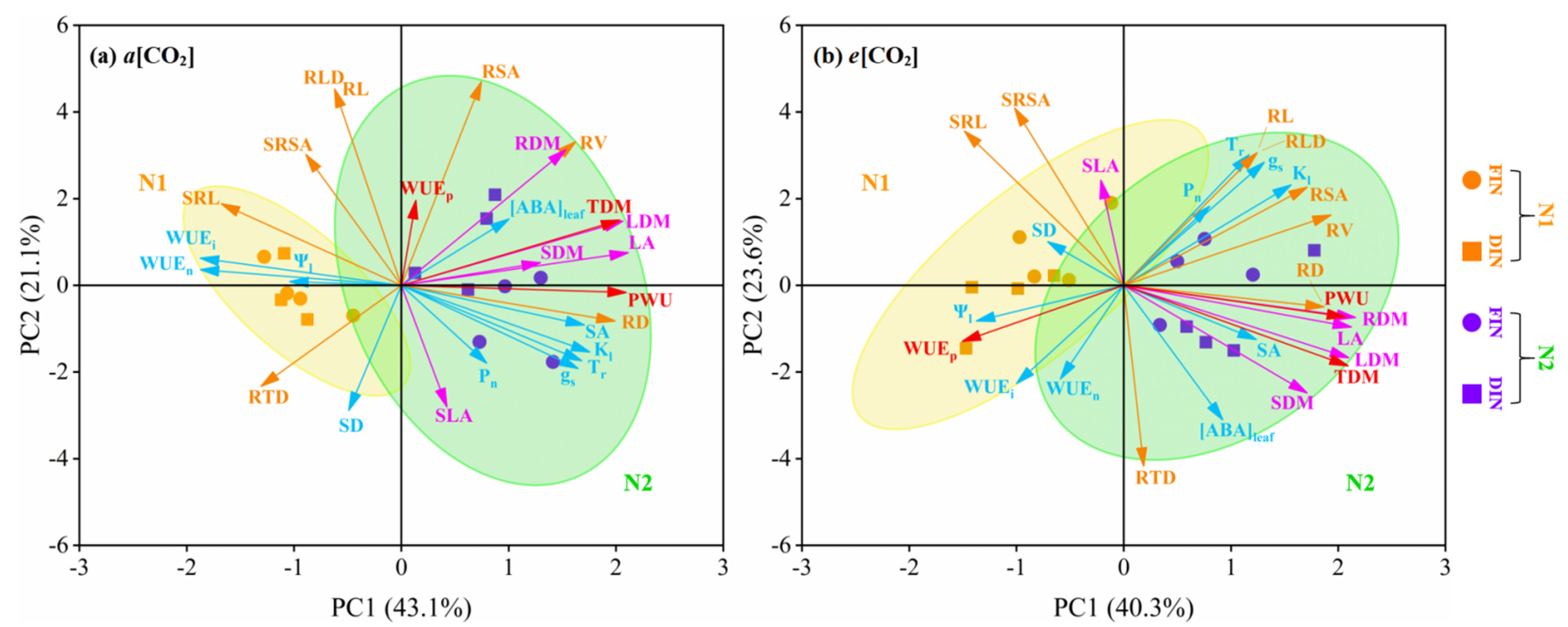
3.6. Principal Component Analysis (PCA) of Tomato Physiology, Growth and WUE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Wang, Y.; Janz, B.; Engedal, T.; Neergaard, A.D. Effect of irrigation regimes and nitrogen rates on water use efficiency and nitrogen uptake in maize. Agric. Water Manag. 2017, 179, 271–276. [Google Scholar] [CrossRef]
- Liu, J.; Hu, T.; Fang, L.; Peng, X.; Liu, F. CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric. For. Meteorol. 2019, 268, 181–188. [Google Scholar] [CrossRef]
- Avila, R.T.; Cardoso, A.A.; De Almeida, W.L.; Costa, L.C.; Machado, K.L.G.; Barbosa, M.L.; de Souza, R.P.B.; Oliveira, L.A.; Batista, D.S.; Martins, S.C.V.; et al. Coffee plants respond to drought and elevated [CO2] through changes in stomatal function, plant hydraulic conductance, and aquaporin expression. Environ. Exp. Bot. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Yan, F.; Li, X.; Liu, F. ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ. Exp. Bot. 2017, 139, 99–104. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered root traits due to elevated CO2: A meta-analysis. Glob. Ecol. Biogeogr. 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Cohen, I.; Halpern, M.; Yermiyahu, U.; Bar-Tal, A.; Gendler, T.; Rachmilevitch, S. CO2 and nitrogen interaction alters root anatomy, morphology, nitrogen partitioning and photosynthetic acclimation of tomato plants. Planta 2019, 250, 1423–1432. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Wei, Z.; Liu, J.; Hu, X.; Liu, F. Effects of elevated CO2 and nitrogen supply on leaf gas exchange, plant water relations and nutrient uptake of tomato plants exposed to progressive soil drying. Sci. Hortic. 2022, 292, 110643. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Kang, S.; Jensen, C.R. Alternate partial root-zone drying irrigation improves nitrogen nutrition in maize (Zea mays L.) leaves. Environ. Exp. Bot. 2012, 75, 36–40. [Google Scholar] [CrossRef]
- Zhou, Z.; Plauborg, F.; Thomsen, A.G.; Andersen, M.N. A RVI/LAI-reference curve to detect N stress and guide N fertigation using combined information from spectral reflectance and leaf area measurements in potato. Eur. J. Agron. 2017, 87, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Liu, F. Alternate partial root-zone N-fertigation increases water use efficiency and N uptake of barley at elevated CO2. Agric. Water Manag. 2021, 258, 107168. [Google Scholar] [CrossRef]
- Azad, N.; Behmanesh, J.; Rezaverdinejad, V.; Abbasi, F.; Navabian, M. Developing an optimization model in drip fertigation management to consider environmental issues and supply plant requirements. Agric. Water Manag. 2018, 208, 344–356. [Google Scholar] [CrossRef]
- Alandia, G.; Jacobsen, S.E.; Kyvsgaard, N.C.; Condori, B.; Liu, F. Nitrogen sustains seed yield of quinoa under intermediate drought. J. Agron. Crop Sci. 2016, 202, 281–291. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Andersen, M.N.; Jensen, C.R. Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Funct. Plant Biol. 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Ashraf, U.; Salim, M.N.; Sher, A.; Sabir, S.U.R.; Khan, A.; Pan, S.G.; Tang, X.R. Maize growth, yield formation and water-nitrogen usage in response to varied irrigation and nitrogen supply under semi-arid climate. Turk. J. Field Crops 2016, 21, 88–95. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.H.; Gruber, B.D.; von Wirén, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Zhang, S.; Ge, C.; Shen, Q.; Ma, H.; Zhang, X.; Dong, H.; Zhao, X.; Pang, C. Nitrogen modulates cotton root morphology by affecting abscisic acid (ABA) and salicylic acid (SA) content. Arch. Agron. Soil Sci. 2021, 67, 1722–1738. [Google Scholar] [CrossRef]
- Mohd-Radzman, N.A.; Djordjevic, M.A.; Imin, N. Nitrogen modulation of legume root architecture signaling pathways involves phytohormones and small regulatory molecules. Front Plant Sci. 2013, 4, 385. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.; Buttar, G.S.; Brar, A.S. Drip irrigation and fertigation improve economics, water and energy productivity of spring sunflower (Helianthus annuus L.) in Indian Punjab. Agric. Water Manag. 2017, 185, 58–64. [Google Scholar] [CrossRef]
- Jayakumar, M.; Janapriya, S.; Surendran, U. Effect of drip fertigation and polythene mulching on growth and productivity of coconut (Cocos nucifera L.), water, nutrient use efficiency and economic benefits. Agric. Water Manag. 2017, 182, 87–93. [Google Scholar] [CrossRef]
- Wang, C.; Wu, S.; Tankari, M.; Zhang, X.; Li, L.; Gong, D.; Hao, W.; Zhang, Y.; Mei, X.; Wang, Y.; et al. Stomatal aperture rather than nitrogen nutrition determined water use efficiency of tomato plants under nitrogen fertigation. Agric. Water Manag. 2018, 209, 94–101. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Nicola, S.; Tibaldi, G.; Fontana, E.; Crops, A.V.; Plants, A. Tomato Production Systems and Their Application to the Tropics. Acta Hortic. 2009, 821, 27–34. [Google Scholar] [CrossRef]
- Asch, F. Determination of Abscisic Acid by Indirect Enzyme Linked Immuno Sorbent Assay (ELISA); Technical Report: Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Science, The Royal Veterinary and Agricultural University: Taastrup, Denmark, 2000. [Google Scholar]
- Yang, X.; Bornø, M.L.; Wei, Z.; Liu, F. Combined effect of partial root drying and elevated atmospheric CO2 on the physiology and fruit quality of two genotypes of tomato plants with contrasting endogenous ABA levels. Agric. Water Manag. 2021, 254, 106987. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.J.; Iba, K.; Schroeder, J.I. CO2 sensing and CO2 regulation of stomatal conductance: Advances and open questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.A.; Walker, H.J.; Liang, Y.K.; McLachlan, D.H.; Casson, S.; Isner, J.C.; Wilson, I.; et al. Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef]
- Fang, L.; Abdelhakim, L.O.A.; Hegelund, J.N.; Li, S.; Liu, J.; Peng, X.; Li, X.; Wei, Z.; Liu, F. ABA-mediated regulation of leaf and root hydraulic conductance in tomato grown at elevated CO2 is associated with altered gene expression of aquaporins. Hortic. Res. 2019, 6, 104. [Google Scholar] [CrossRef]
- Wei, Z.; Du, T.; Li, X.; Fang, L.; Liu, F. Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric. Water Manag. 2018, 202, 174–182. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Li, G.; Liu, F. Elevated CO2 modulates carbon assimilation and leaf water use efficiency of Nicotiana tabacum L. (tobacco) under patchy soil nutrient deficiency. Ind. Crops Prod. 2021, 166, 113500. [Google Scholar] [CrossRef]
- Li, Q.; Wei, M.; Li, Y.; Feng, G.; Wang, Y.; Li, S.; Zhang, D. Effects of soil moisture on water transport, photosynthetic carbon gain and water use efficiency in tomato are influenced by evaporative demand. Agric. Water Manag. 2019, 226, 105818. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, F.; Cui, X.; Liu, F. Plasticity in stomatal size and density of potato leaves under different irrigation and phosphorus regimes. J. Plant Physiol. 2014, 171, 1248–1255. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Y.; Song, F.; Liu, F. Differential responses of stomatal morphology to partial root-zone drying and deficit irrigation in potato leaves under varied nitrogen rates. Sci. Hortic. 2012, 145, 76–83. [Google Scholar] [CrossRef]
- Wang, N.; Gao, G.; Wang, Y.; Wang, D.; Wang, Z.; Gu, J. Coordinated responses of leaf and absorptive root traits under elevated CO2 concentration in temperate woody and herbaceous species. Environ. Exp. Bot. 2020, 179, 104199. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, G.; Cui, B.; Liu, C.; Wan, H.; Hou, J.; Chen, Y.; Zhang, J.; Liu, J.; Wei, Z. CO2 elevation and N fertilizer supply modulate leaf physiology, crop growth and water use efficiency of maize in response to progressive soil drought. J. Agron. Crop Sci. 2024, 210, e12692. [Google Scholar] [CrossRef]
- He, Y.; Li, G.; Xi, B.; Zhao, H.; Jia, L. Fine root plasticity of young Populus tomentosa plantations under drip irrigation and nitrogen fertigation in the North China Plain. Agric. Water Manag. 2022, 261, 107341. [Google Scholar] [CrossRef]
- Mueller, K.E.; LeCain, D.R.; McCormack, M.L.; Pendall, E.; Carlson, M.; Blumenthal, D.M. Root responses to elevated CO2, warming and irrigation in a semi-arid grassland: Integrating biomass, length and life span in a 5-year field experiment. J. Ecol. 2018, 106, 2176–2189. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, W.; Wang, Q.; Cao, Y.; Xu, F.; Dodd, I.C.; Xu, W. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ. 2022, 45, 871–883. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Abdelhakim, L.O.A.; Fang, L.; Wei, Z.; Liu, F. Carbon dioxide elevation combined with sufficient irrigation and nitrogen fertilization improves fruit quality of tomato grown in glasshouse. Arch. Agron. Soil Sci. 2021, 67, 1134–1149. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
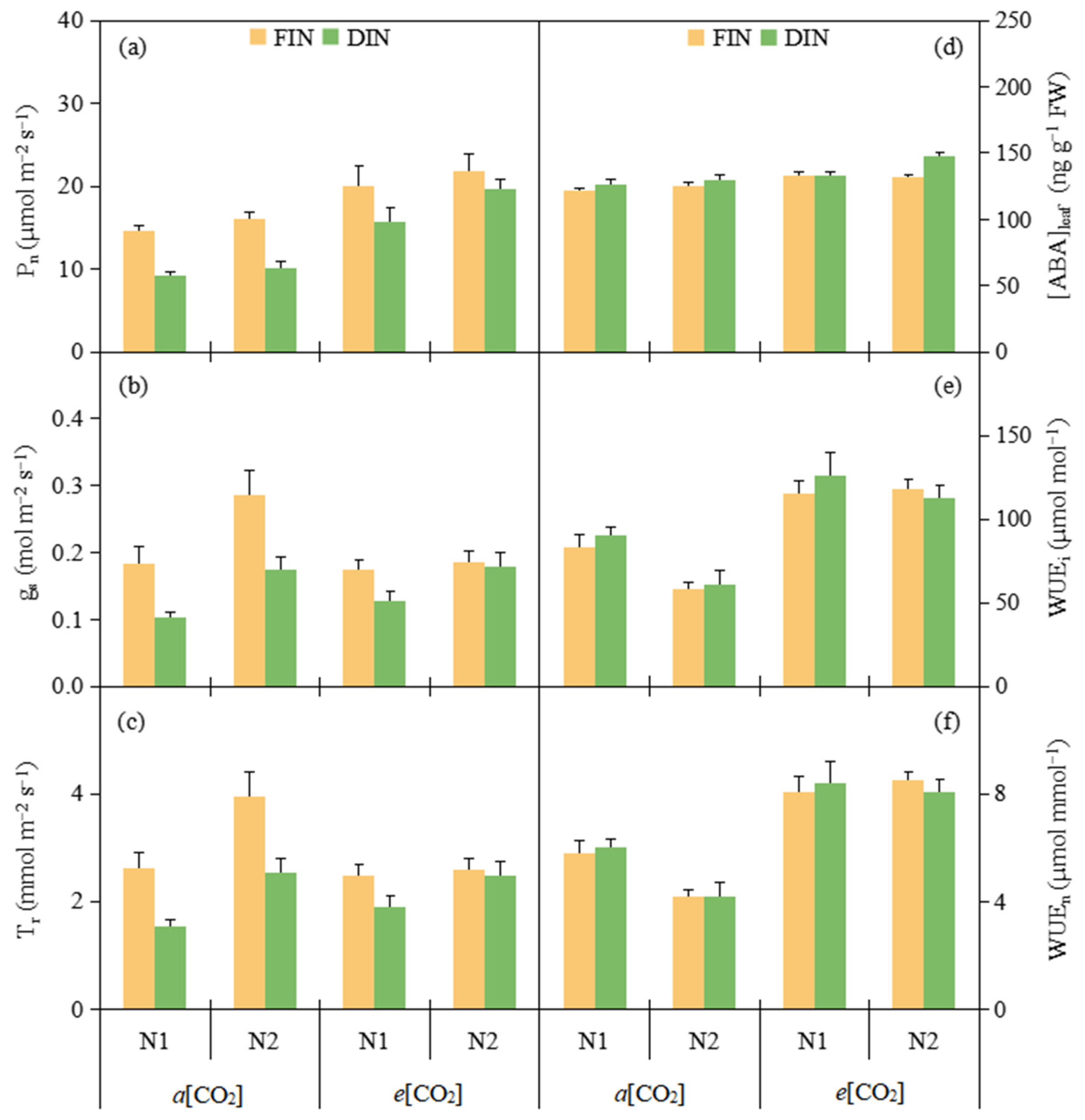
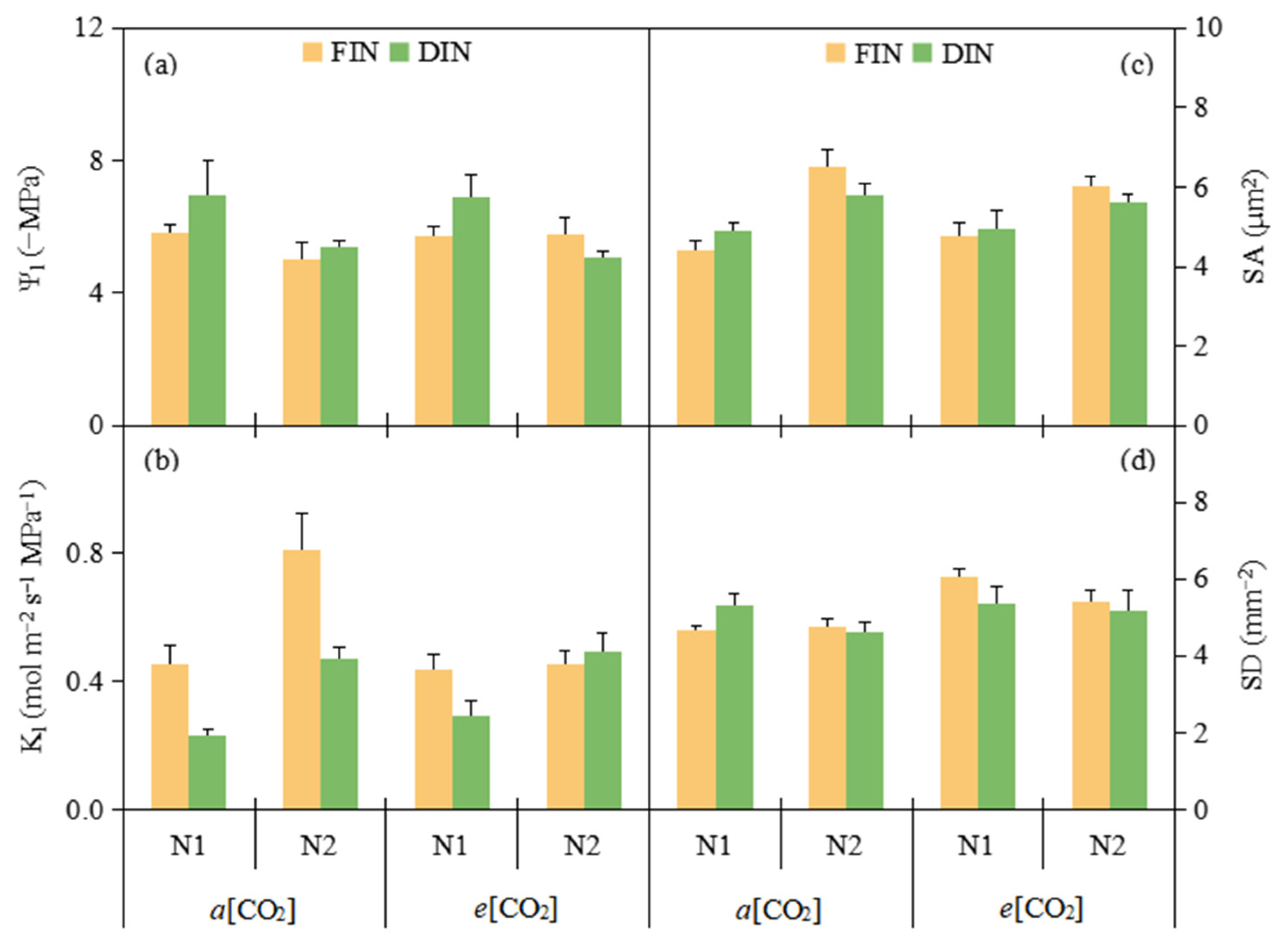
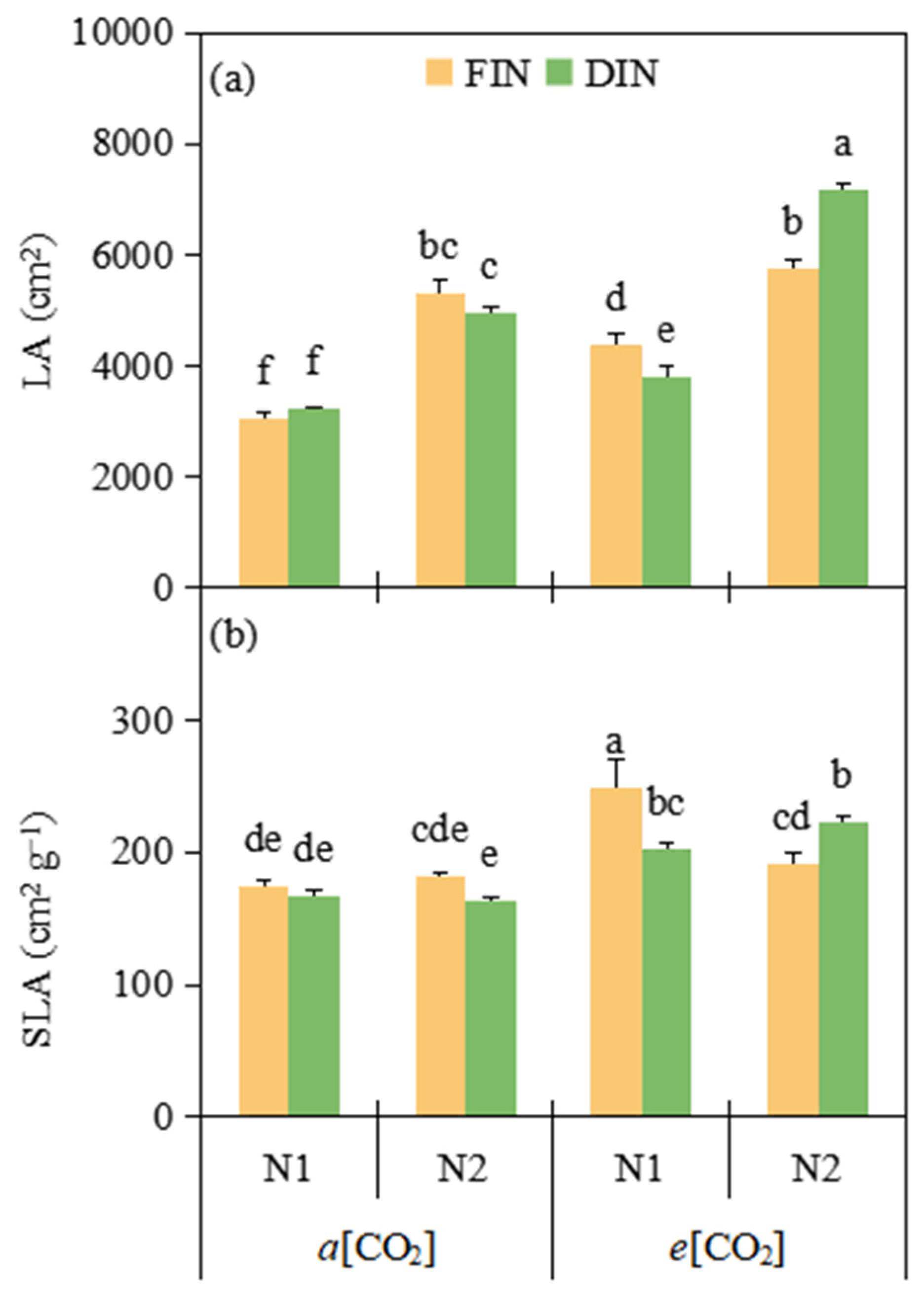
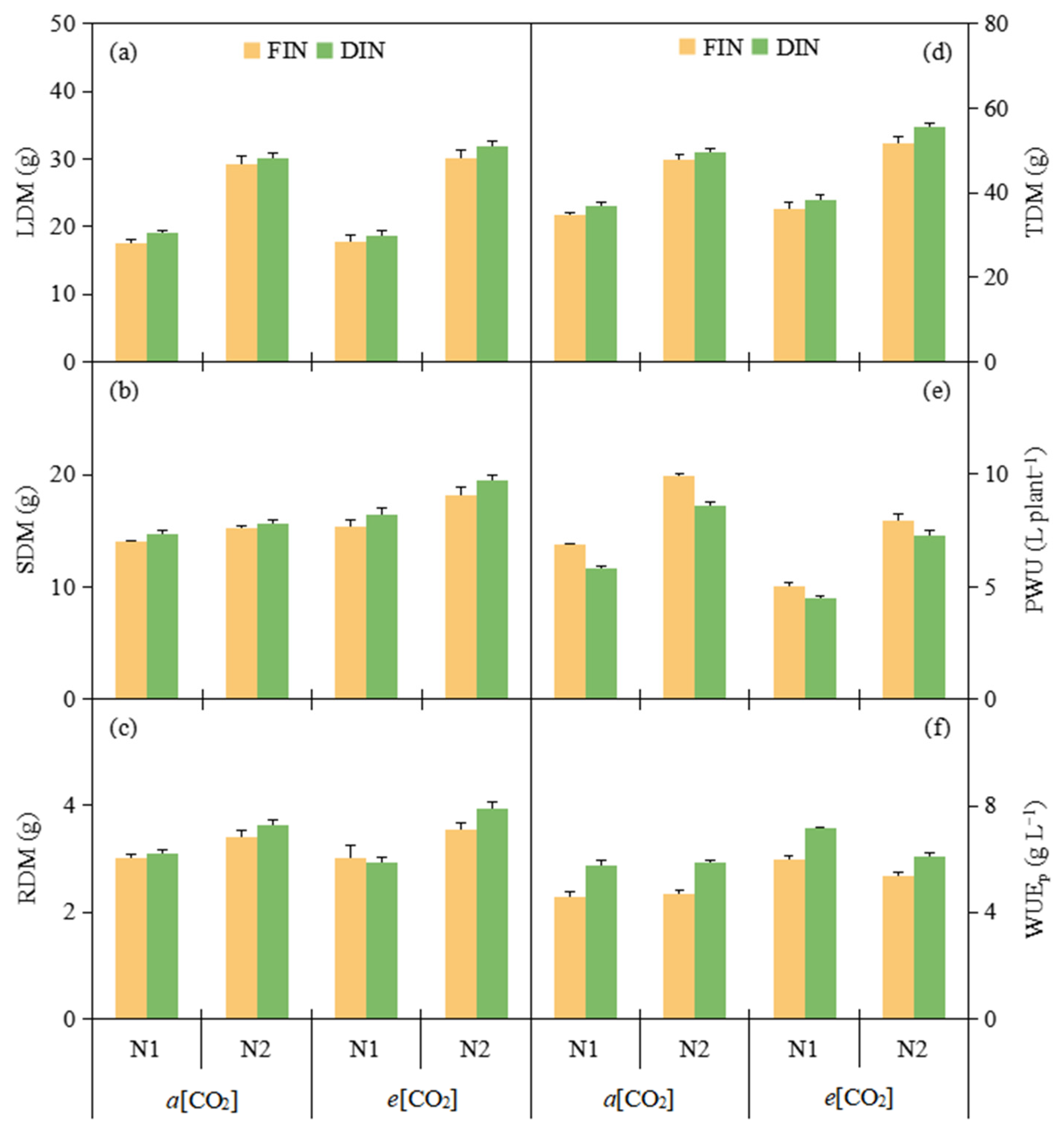
| Factors | Pn | gs | Tr | [ABA]leaf | WUEi | WUEn | Ψl | Kl | SA | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| [CO2] | *** | ns | ns | *** | *** | *** | ns | ns | ns | ** |
| [FN] | *** | *** | *** | * | ns | ns | ns | *** | ns | ns |
| [N] | * | ** | ** | * | ** | * | * | *** | *** | ns |
| [CO2] × [FN] | ns | * | * | ns | ns | ns | ns | * | ns | ns |
| [CO2] × [N] | ns | ns | * | ns | ns | * | ns | * | ns | ns |
| [FN] × [N] | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| [CO2] × [FN] × [N] | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| [CO2] | [FN] | [N] | RL (m) | RD (µm) | RSA (cm2) | RV (cm3) | SRL (m g−1) | SRSA (cm2 g−1) | RLD (cm cm−3) | RTD (mg cm−3) |
|---|---|---|---|---|---|---|---|---|---|---|
| a[CO2] | FIN | N1 | 223.53 ± 8.10 | 310.56 ± 5.12 | 2149.66 ± 57.74 | 16.56 ± 0.33 | 74.04 ± 2.52 a | 711.98 ± 15.64 a | 4.97 ± 0.18 | 182.40 ± 1.98 |
| N2 | 191.88 ± 14.66 | 371.53 ± 13.45 | 2169.06 ± 127.97 | 19.70 ± 0.84 | 56.21 ± 3.18 d | 636.01 ± 23.28 b | 4.26 ± 0.33 | 173.19 ± 4.12 | ||
| DIN | N1 | 211.36 ± 9.49 | 319.42 ± 4.67 | 2089.22 ± 72.19 | 16.53 ± 0.42 | 68.36 ± 3.37 abc | 675.92 ± 28.73 ab | 4.70 ± 0.21 | 187.80 ± 7.46 | |
| N2 | 223.91 ± 10.31 | 355.86 ± 6.19 | 2434.21 ± 138.69 | 21.29 ± 1.52 | 61.79 ± 1.62 cd | 670.94 ± 20.93 ab | 4.98 ± 0.23 | 171.62 ± 7.58 | ||
| e[CO2] | FIN | N1 | 219.20 ± 15.75 | 312.70 ± 4.29 | 2101.56 ± 176.43 | 16.17 ± 1.55 | 72.68 ± 1.92 ab | 695.18 ± 21.19 ab | 4.87 ± 0.35 | 188.24 ± 7.23 |
| N2 | 231.50 ± 11.10 | 326.29 ± 4.52 | 2332.94 ± 118.96 | 18.90 ± 1.12 | 65.09 ± 2.35 bc | 655.54 ± 22.41 ab | 5.14 ± 0.25 | 189.28 ± 7.39 | ||
| DIN | N1 | 206.29 ± 7.89 | 305.95 ± 2.52 | 1946.95 ± 76.25 | 14.70 ± 0.61 | 70.22 ± 1.65 ab | 662.68 ± 15.28 ab | 4.58 ± 0.18 | 200.28 ± 5.27 | |
| N2 | 217.64 ± 14.69 | 331.67 ± 5.85 | 2240.50 ± 140.74 | 18.47 ± 1.15 | 55.08 ± 2.49 d | 566.91 ± 20.46 c | 4.84 ± 0.33 | 214.68 ± 6.99 | ||
| ANOVA factor | ||||||||||
| [CO2] | ns | *** | ns | ns | ns | ns | ns | *** | ||
| [FN] | ns | ns | ns | ns | ns | ns | ns | * | ||
| [N] | ns | *** | * | *** | *** | ** | ns | ns | ||
| [CO2] × [FN] | ns | ns | ns | ns | ns | ns | ns | ns | ||
| [CO2] × [N] | ns | ** | ns | ns | ns | ns | ns | * | ||
| [FN] × [N] | ns | ns | ns | ns | ns | ns | ns | ns | ||
| [CO2] × [FN] × [N] | ns | ns | ns | ns | * | * | ns | ns | ||
| Factors | LA | SLA | LDM | SDM | RDM | TDM | PWU | WUEp |
|---|---|---|---|---|---|---|---|---|
| [CO2] | *** | *** | ns | *** | ns | ** | *** | *** |
| [FN] | ns | ns | * | * | ns | ** | *** | *** |
| [N] | *** | ns | *** | *** | *** | *** | *** | ** |
| [CO2] × [FN] | * | ns | ns | ns | ns | ns | * | ns |
| [CO2] × [N] | ns | ns | ns | * | ns | * | ns | *** |
| [FN] × [N] | ** | * | ns | ns | ns | ns | ns | ns |
| [CO2] × [FN] × [N] | *** | ** | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhao, W.; Liu, C.; Xu, C.; Wei, G.; Cui, B.; Hou, J.; Wan, H.; Chen, Y.; Zhang, J.; et al. Effect of CO2 Elevation on Tomato Gas Exchange, Root Morphology and Water Use Efficiency under Two N-Fertigation Levels. Plants 2024, 13, 2373. https://doi.org/10.3390/plants13172373
Zhang M, Zhao W, Liu C, Xu C, Wei G, Cui B, Hou J, Wan H, Chen Y, Zhang J, et al. Effect of CO2 Elevation on Tomato Gas Exchange, Root Morphology and Water Use Efficiency under Two N-Fertigation Levels. Plants. 2024; 13(17):2373. https://doi.org/10.3390/plants13172373
Chicago/Turabian StyleZhang, Manyi, Wentong Zhao, Chunshuo Liu, Changtong Xu, Guiyu Wei, Bingjing Cui, Jingxiang Hou, Heng Wan, Yiting Chen, Jiarui Zhang, and et al. 2024. "Effect of CO2 Elevation on Tomato Gas Exchange, Root Morphology and Water Use Efficiency under Two N-Fertigation Levels" Plants 13, no. 17: 2373. https://doi.org/10.3390/plants13172373
APA StyleZhang, M., Zhao, W., Liu, C., Xu, C., Wei, G., Cui, B., Hou, J., Wan, H., Chen, Y., Zhang, J., & Wei, Z. (2024). Effect of CO2 Elevation on Tomato Gas Exchange, Root Morphology and Water Use Efficiency under Two N-Fertigation Levels. Plants, 13(17), 2373. https://doi.org/10.3390/plants13172373







