Maintenance of High Phytoplankton Diversity in the Danubian Floodplain Lake over the Past Half-Century
Abstract
:1. Introduction
2. Results
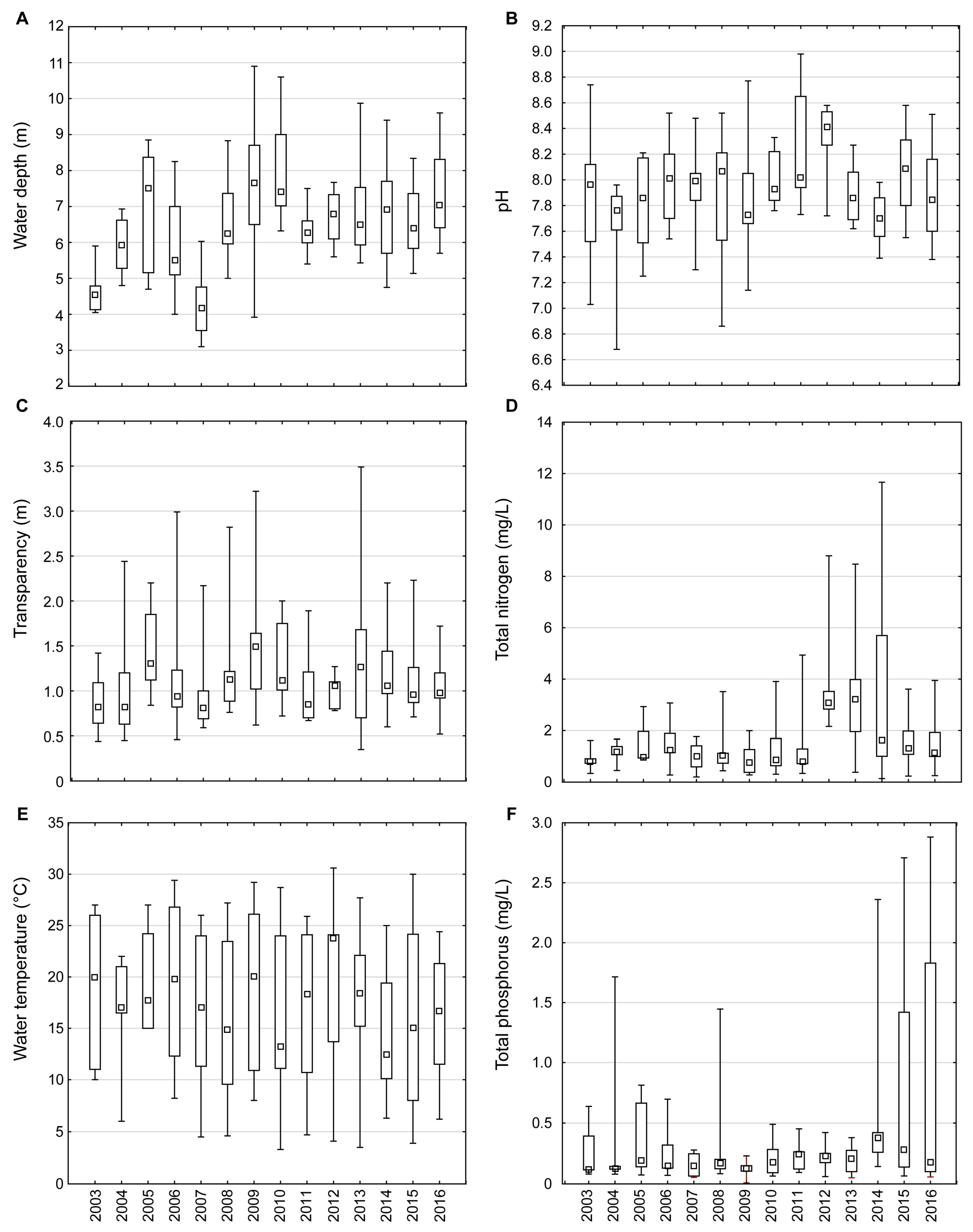
2.1. Environmental Characteristics
2.2. Phytoplankton Diversity and Dominant Taxa
2.3. Interannual Variation of Biomass and Diversity
2.4. Similarity Indices
2.5. Ordination
3. Discussion
3.1. Total Diversity
3.2. Diversity Drivers
3.3. Diversity Stressor—Anthropogenic Impact
3.4. Herbivore Pressure
3.5. Invasive Species
3.6. Global Change
4. Materials and Methods
4.1. Study Site
4.2. Sampling and Field Analyses
4.3. Laboratory Analyses
4.4. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thoms, M. Floodplain-river ecosystems: Lateral connections and the implications of human interference. Geomorphology 2003, 56, 335–349. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Agostinho, A.; Thomaz, S.; Minte-Vera, C.; Winemiller, K. Biodiversity in the high Paraná River floodplain. In Biodiversity in Wetlands: Assessment, Function and Conservation; Gopal, B., Junk, W.J., Davis, J.A., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2000; Volume 1, pp. 89–118. [Google Scholar]
- Ward, J.V.; Tockner, K.; Schiemer, F. Biodiversity of floodplain river ecosystems: Ecotones and connectivity. Regul. Rivers Res. Manag. 1999, 15, 125–139. [Google Scholar] [CrossRef]
- Dziock, F.; Henle, K.; Foeckler, F.; Follner, K.; Scholz, M. Biological indicator systems in floodplains—A review. Int. Rev. Hydrobiol. 2006, 91, 271–291. [Google Scholar] [CrossRef]
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van De Bund, W. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 205. [Google Scholar] [CrossRef]
- European Environment Agency. Available online: https://www.eea.europa.eu/publications/flood-risks-and-environmental-vulnerability (accessed on 15 June 2024).
- Cardinale, B.J.; Srivastava, D.S.; Emmett Duffy, J.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Wang, S.; Jia, J.; Ha, X.; Lu, Y. Floodplain lake response to climate-nutrient-hydrological pressure revealed through phytoplankton community succession over the past century. J. Hydrol. 2023, 623, 129838. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar5/wg2/ (accessed on 15 June 2024).
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Nõges, T.; Nõges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Stolz, R.; Prasch, M.; Weber, M.; Koch, F.; Weidinger, R.; Ebner, M.; Mauser, W. Climate change impacts on the water resources in the Danube River Basin and possibilities to adapt—The way to an adaptation strategy and its update. J. Environ. Geogr. 2018, 11, 13–24. [Google Scholar] [CrossRef]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Available online: https://www.unep.org/resources/report/convention-biological-diversity-june-1992 (accessed on 18 July 2024).
- Borics, G.; Görgényi, J.; Grigorszky, I.; László-Nagy, Z.; Tóthmérész, B.; Krasznai, E.; Várbíró, G. The role of phytoplankton diversity metrics in shallow lake and river quality assessment. Ecol. Indic. 2014, 45, 28–36. [Google Scholar] [CrossRef]
- Cardinale, B.; Matulich, K.; Hooper, D.; Byrnes, J.; Duffy, J.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.; Gonzalez, A. The Functional Role of Producer Diversity in Ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Li, L.; Lu, F.; Liu, Y.; Lu, X.; Fan, Y. Phytoplankton alpha diversity indices response the trophic state variation in hydrologically connected aquatic habitats in the Hrbin Section of the Songhua River. Sci. Rep. 2020, 10, 21337. [Google Scholar] [CrossRef]
- Bolgovics, Á.; Viktória, B.-B.; Varbiro, G.; Krasznai-K, E.Á.; Acs, E.; Keve Tihamér, K.; Borics, G. Groups of small lakes maintain larger microalgal diversity than large ones. Sci. Total Environ. 2019, 678, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: New York, NY, USA, 2006; p. 535. ISBN 9780511542145. [Google Scholar]
- European Union. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 18 July 2024).
- Görgényi, J.; Tóthmérész, B.; Várbíró, G.; Abonyi, A.; T-Krasznai, E.; B-Béres, V.; Borics, G. Contribution of phytoplankton functional groups to the diversity of a eutrophic oxbow lake. Hydrobiologia 2019, 830, 287–301. [Google Scholar] [CrossRef]
- Sommer, U. Trophic Cascades in Marine and Freshwater Plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Maileht, K.; Nõges, T.; Nõges, P.; Ott, I.; Mischke, U.; Carvalho, L.; Dudley, B. Water colour, phosphorus and alkalinity are the major determinants of the dominant phytoplankton species in European lakes. Hydrobiologia 2013, 704, 115–126. [Google Scholar] [CrossRef]
- Dembowska, E.A. Impacts of different hydrological conditions on phytoplankton communities in floodplain lakes of a regulated river (Lower Vistula, Poland). Hydrobiologia 2022, 849, 2549–2567. [Google Scholar] [CrossRef]
- Loverde-Oliveira, S.M.; Huszar, V.L.M. Phytoplankton functional groups driven by alternative states in a tropical floodplain lake (Pantanal, Brazil). Oecologia Aust. 2019, 23, 926–939. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Z.; Wang, W.; Zhou, Z.; Ye, X. Effects of flood on phytoplankton diversity and community structure in floodplain lakes connected to the Yangtze River. Diversity 2022, 14, 581. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F.; Horvatić, J.; Hackenberger Kutuzović, B. Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (Kopački Rit Nature Park, Croatia). Hydrobiologia 2009, 618, 77–88. [Google Scholar] [CrossRef]
- Schmid, M.; Haidvogl, G.; Friedrich, T.; Funk, A.; Schmalfuss, L.; Schmidt-Kloiber, A.; Hein, T. The Danube: On the environmental history, present, and future of a great european river. In River Culture—Life as a Dance to the Rhythm of the Waters; Wantzen, K.M., Ed.; UNESCO Publishing: Paris, France, 2023; pp. 637–671. ISBN 978-92-3-100540-4. [Google Scholar]
- Hein, T.; Schwarz, U.; Habersack, H.; Nichersu, I.; Preiner, S.; Willby, N.; Weigelhofer, G. Current status and restoration options for floodplains along the Danube River. Sci. Total Environ. 2016, 543, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Gucunski, D. A contribution to the knowledge of phytoplankton in the protected region of the swampy area of Kopachevo (Kopački rit). Acta Bot. Croat. 1973, 32, 205–216. [Google Scholar]
- Tadić, L.; Bonacci, O.; Dadić, T. Dynamics of the Kopački Rit (Croatia) wetland floodplain water regime. Environ. Earth Sci. 2014, 71, 3559–3570. [Google Scholar] [CrossRef]
- Tadić, L.; Tamás, E.A.; Mihaljević, M.; Janjić, J. Potential climate impacts of hydrological alterations and discharge variabilities of the Mura, Drava, and Danube rivers on the natural resources of the MDD UNESCO Biosphere Reserve. Climate 2022, 10, 139. [Google Scholar] [CrossRef]
- OECD. Eutrophication of Waters. Monitoring, Assessment and Control; Organisation for Economic Cooperation and Development: Paris, France, 1982; p. 154. [Google Scholar]
- Gucunski, D. Kvantitativna Istraživanja Fitoplanktona u Upravljanom Prirodnom Rezervatu Kopački Rit. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 1975. [Google Scholar]
- Gucunski, D.; Šomođi, I. The phytoplankton of Lake Sakadaš in relation to organic pollution. In Proceedings of the 2nd Congress of Ecologists of Yugoslavia, Zagreb, Croatia, 1–7 October 1979; pp. 515–525. [Google Scholar]
- Sørensen, T.J. A Method of establishing group of equal amplitude in plant sociobiology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Jaccard, P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull. Soc. Vaud. Des. Sci. Nat. 1901, 37, 547–579. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M. Development of the phytoplankton of the shallow Srebarna Lake (north-eastern Bulgaria) across the trophic gradient. Hydrobiologia 1998, 369, 259–267. [Google Scholar] [CrossRef]
- Török, L. The trend of phytoplankton development in Danube Delta’s lakes. Sci. Ann. Danub. Delta Inst. 2011, 17, 89–98. [Google Scholar]
- Beshkova, M.B.; Botev, I.S. Phytoplankton community structure of three temporary wetlands on Belene Island (Bulgarian sector of the Danube River). Phytol. Balc. 2004, 10, 11–19. [Google Scholar]
- Bortolini, J.C.; Bovo-Scomparin, V.M.; Paula, A.C.M.d.; Moresco, G.A.; Reis, L.M.; Jati, S.; Rodrigues, L.C. Composition and species richness phytoplankton in a subtropical floodplain lake: A long-term study. Acta Limnol. Bras. 2014, 26, 296–305. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Simões, N.R.; Bovo-Scomparin, V.M.; Jati, S.; Santana, N.F.; Roberto, M.C.; Train, S. Phytoplankton alpha diversity as an indicator of environmental changes in a neotropical floodplain. Ecol. Indic. 2015, 48, 334–341. [Google Scholar] [CrossRef]
- Bortolini, J.; Pineda, A.; Rodrigues, L.; Jati, S.; Velho, L. Environmental and spatial processes influencing phytoplankton biomass along a reservoirs-river-floodplain lakes gradient: A metacommunity approach. Freshw. Biol. 2017, 62, 1756–1767. [Google Scholar] [CrossRef]
- Salmaso, N.; Tolotti, M. Phytoplankton and anthropogenic changes in pelagic environments. Hydrobiologia 2021, 848, 251–284. [Google Scholar] [CrossRef]
- Borics, G.; Abonyi, A.; Salmaso, N.; Ptacnik, R. Freshwater phytoplankton diversity: Models, drivers and implications for ecosystem properties. Hydrobiologia 2021, 848, 53–75. [Google Scholar] [CrossRef]
- Ptacnik, R.; Moorthi, S.; Hillebrand, H. Hutchinson reversed, or why there need to be so many species. Adv. Ecol. Res. 2010, 43, 1–43. [Google Scholar] [CrossRef]
- Amoros, C.; Bornette, G. Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshw. Biol. 2002, 47, 761–776. [Google Scholar] [CrossRef]
- Weilhoefer, C.L.; Pan, Y.; Eppard, S. The effects of river floodwaters on floodplain wetland water quality and diatom assemblages. Wetlands 2008, 28, 473–486. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Stević, F.; Mihaljević, M.; Špoljarić Maronić, D. Changes of phytoplankton functional groups in a floodplain lake associated with hydrological perturbations. Hydrobiologia 2013, 709, 143–158. [Google Scholar] [CrossRef]
- Mihaljević, M.; Špoljarić, D.; Stević, F.; Žuna Pfeiffer, T. Assessment of flood-induced changes of phytoplankton along a river–floodplain system using the morpho-functional approach. Environ. Monit. Assess. 2013, 185, 8601–8619. [Google Scholar] [CrossRef] [PubMed]
- Stomp, M.; Huisman, J.; Mittelbach, G.G.; Litchman, E.; Klausmeier, C.A. Large-scale biodiversity patterns in freshwater phytoplankton. Ecology 2011, 92, 2096–2107. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F.; Špoljarić Maronić, D.; Pfeiffer, T. Spatial pattern of phytoplankton based on the morphology-based functional approach along a river-floodplain gradient. River Res. Appl. 2014, 31, 228–238. [Google Scholar] [CrossRef]
- Reynolds, C.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F. Cyanobacterial blooms in a temperate river-floodplain ecosystem: The importance of hydrological extremes. Aquat. Ecol. 2011, 45, 335–349. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Calheiros, D.F. Flood pulse influence on phytoplankton communities of the south Pantanal floodplain, Brazil. Hydrobiologia 2000, 427, 101–112. [Google Scholar] [CrossRef]
- Stendera, S.; Adrian, R.; Bonada, N.; Cañedo-Argüelles, M.; Hugueny, B.; Januschke, K.; Pletterbauer, F.; Hering, D. Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: A review. Hydrobiologia 2012, 696, 1–28. [Google Scholar] [CrossRef]
- Fu, H.; Chen, L.; Ge, Y.; Wu, A.; Liu, H.; Li, W.; Yuan, G.; Jeppesen, E. Linking human activities and global climatic oscillation to phytoplankton dynamics in a subtropical lake. Water Res. 2022, 208, 117866. [Google Scholar] [CrossRef]
- Borics, G.; Grigorszky, I.; Szabó, S.; Padisák, J. Phytoplankton Associations in a small hypertrophic fishpond in East Hungary during a change from bottom-up to top-down control. Hydrobiologia 2000, 424, 79–90. [Google Scholar] [CrossRef]
- Padisák, J.; Naselli-Flores, L. Phytoplankton in extreme environments: Importance and consequences of habitat permanency. Hydrobiologia 2021, 848, 157–176. [Google Scholar] [CrossRef]
- Yan, G.; Yin, X.; Huang, M.; Wang, X.; Huang, D.; Li, D. Dynamics of phytoplankton functional groups in river-connected lakes and the major influencing factors: A case study of Dongting Lake, China. Ecol. Indic. 2023, 149, 110177. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity Predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef]
- Gucunski, D. Sezonske oscilacije fitoplanktona u zaštićenom području Kopačkog rita. Acta Bot. Croat. 1974, 33, 163–173. [Google Scholar]
- Jacobsen, B.A. Hypertrophic phytoplankton: An overview. Freshwat. Forum 1992, 2, 184–199. [Google Scholar]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. The key role of zooplankton in ecosystem services: A perspective of interaction between zooplankton and fish recruitment. Ecol. Indic. 2021, 129, 107867. [Google Scholar] [CrossRef]
- Galir Balkić, A.; Špoljarić Maronić, D.; Žuna Pfeiffer, T.; Bek, N.; Stević, F.; Bogut, I.; Nikolašević, R.; Radočaj, D.; Kezerle, A. The effects of early spring stocking in an agricultural lake: A trophic cascade hypothesis. Hydrobiologia 2024, 851, 3061–3077. [Google Scholar] [CrossRef]
- Galir Balkić, A.; Ternjej, I.; Bogut, I. Impact of habitat heterogeneity on zooplankton assembly in a temperate river-floodplain system. Environ. Monit. Assess. 2018, 190, 143. [Google Scholar] [CrossRef] [PubMed]
- Galir Balkić, A.; Ternjej, I.; Katanić, N. Alteration in microcrustacean secondary production and herbivory effects between the river danube and its floodplain lake. Hydrobiologia 2019, 836, 185–196. [Google Scholar] [CrossRef]
- Galir Balkić, A.; Ternjej, I.; Špoljar, M. Hydrology Driven changes in the rotifer trophic structure and implications for food web interactions. Ecohydrology 2018, 11, e1917. [Google Scholar] [CrossRef]
- Sommer, U.; Sommer, F.; Santer, B.; Zöllner, E.; Jürgens, K.; Jamieson, C.; Boersma, M.; Gocke, K. Daphnia versus copepod impact on summer phytoplankton: Functional compensation at both trophic levels. Oecologia 2003, 135, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Leibold, M.A.; Hall, S.R.; Smith, V.H.; Lytle, D.A. Herbivory enhances the diversity of primary producers in pond ecosystems. Ecology 2017, 98, 48–56. [Google Scholar] [CrossRef]
- International Commission for the Protection of the Danube River. Available online: https://www.icpdr.org/ (accessed on 16 July 2024).
- Kaštovský, J.; Hauer, T.; Mareš, J.; Krautová, M.; Bešta, T.; Komárek, J.; Desortová, B.; Heteša, J.; Hindáková, A.; Houk, V.; et al. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biol. Invasions. 2010, 12, 3599–3625. [Google Scholar] [CrossRef]
- Antunes, J.; Leao, P.; Vasconcelos, V. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef]
- Padisák, J. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: Worldwide distribution and review of its ecology. Arch. Hydrobiol. 1997, 107, 563–593. [Google Scholar]
- Stoyneva, M.P. steady-state phytoplankton assemblages in shallow Bulgarian wetlands. Hydrobiologia 2003, 502, 169–176. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Mayer, J. Population dynamics and photosynthetic rates of a Cylindrospermopsis—Limnothrix Association in a highly eutrophic urban lake, Alte Donau, Vienna, Austria. Arch. Hydrobiol. Suppl. Algol. Stud. 1996, 83, 179–195. [Google Scholar] [CrossRef]
- Špoljarić Maronić, D.; Žuna Pfeiffer, T.; Bek, N.; Štolfa Čamagajevac, I.; Galir Balkić, A.; Stević, F.; Maksimović, I.; Mihaljević, M.; Lončarić, Z. distribution of selenium: A case study of the Drava, Danube and associated aquatic biotopes. Chemosphere 2024, 354, 141596. [Google Scholar] [CrossRef] [PubMed]
- McGregor, G.; Fabbro, L. Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in queensland tropical and subtropical reservoirs: Implications for monitoring and management. Lakes Reserv. Res. Manag. 2008, 5, 195–205. [Google Scholar] [CrossRef]
- Kiss, K.T.; Ács, É.; Kovács, A. Ecological observations on Skeletonema potamos (Weber) Hasle in the River Danube, near Budapest (1991–92, daily investigations). In Phytoplankton in Turbid. Environments: Rivers and Shallow Lakes, Proceedings of the 9th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP), Mont Rigi, Belgium, 10–18 July 1993; Descy, J.-P., Reynolds, C.S., Padisák, J., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 163–170. [Google Scholar]
- Duleba, M.; Ector, L.; Horváth, Z.; Kiss, K.T.; Molnár, L.F.; Pohner, Z.; Szilágyi, Z.; Tóth, B.; Vad, C.F.; Várbíró, G.; et al. Biogeography and phylogenetic position of a warm-stenotherm centric diatom, Skeletonema potamos (C.I. Weber) Hasle and its long-term dynamics in the River Danube. Protist 2014, 165, 715–729. [Google Scholar] [CrossRef]
- Döll, P.; Schmied, H.M. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ. Res. Lett. 2012, 7, 014037. [Google Scholar] [CrossRef]
- Padisák, J.; Borics, G.; Fehér, G.; Grigorszky, I.; Oldal, I.; Schmidt, A.; Zámbóné-Doma, Z. Dominant species, functional assemblages and frequency of equilibrium phases in late summer phytoplankton assemblages in Hungarian small shallow lakes. Hydrobiologia 2003, 502, 157–168. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Long-term adjustment of phytoplankton structure to environmental traits at timescales during lifetime development and over generations. Hydrobiologia 2024, 851, 823–847. [Google Scholar] [CrossRef]
- Krasznai, E.; Török, P.; Borics, G.; Lukács, Á.; Kókai, Z.; Lerf, V.; Görgényi, J.; Viktória, B.-B. Functional dynamics of phytoplankton assemblages in hypertrophic lakes: Functional- and species diversity is highly resistant to cyanobacterial blooms. Ecol. Indic. 2022, 145, 109583. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M.; Bozelli, R.L. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 2007, 579, 1–13. [Google Scholar] [CrossRef]
- Mihaljević, M.; Getz, D.; Tadić, Z.; Živanović, B.; Gucunski, D.; Topić, J.; Kalinović, I.; Mikuska, J. Kopački Rit—Research Survey and Bibliography; Croatian Academy of Arts and Sciences: Zagreb, Croatia, 1999; p. 188. ISBN 953-154-343-7. [Google Scholar]
- Gucunski, D. Die Entwicklung des Phytoplanktons und des Phytobenthos unter dem Einfluss der Abwässer. Schweiz. Z. Hydrol. 1982, 44, 216–229. [Google Scholar] [CrossRef]
- Croatian Waters. Available online: https://voda.hr/sites/default/files/dokumenti/PUVP3%20-%20KPV%20-%200042.pdf (accessed on 11 August 2024).
- Vereš, M.; Rožac, V.; Čerba, D.; Kučera, S.; Bolšec, B.; Jurčević Agić, I.; Bogdanović, T.; Koh, M.; Kresonja, M.; Šag, M.; et al. Ichthyofauna monitoring in Special Zoological Reserve Kopački rit. In Proceedings of the 8th Symposium with International Participation Kopački Rit: Past, Present, Future 2019, Kopačevo, Bilje, Croatia, 26–27 September 2019; Public Institution Nature Park Kopački rit: Kopačevo, Croatia, 2019; pp. 144–147. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd Edition. Bull.-Fish. Res. Board. Can. 1972, 167, 185–192. [Google Scholar] [CrossRef]
- SCOR-Unesco Determination of Photosynthetic Pigments in Sea-Water. In Monographs on Oceanographic Methodology; Unesco: Paris, France, 1966; Volume 1, pp. 11–17.
- APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- HR EN ISO 7150-1:1998; Water Quality—Determination of Ammonium—Part 1: Manual Spectrometric Method. ISO: Geneva, Switzerland, 1998.
- HRN ISO 7890-3:1998; Water Quality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. ISO: Geneva, Switzerland, 1998.
- HRN EN 26777:1998; Water Quality—Determination of Nitrite—Molecular Absorption Spectrometric Method. ISO: Geneva, Switzerland, 1998.
- HRN EN 25663:1993; Water Quality—Determination of Kjeldahl Nitrogen—Method after Mineralization with Selenium. ISO: Geneva, Switzerland, 1993.
- HRN ISO 5663:2001 +NO2-N+NO3-N; Water Quality—Determination of Total Nitrogen—Method after Mineralization with Selenium. ISO: Geneva, Switzerland, 2001.
- HRN ISO 6878:2008; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. ISO: Geneva, Switzerland, 2008.
- Hindák, F.; Cyrus, Z.; Marvan, P.; Javornicky, P.; Komarek, J.; Etll, H.; Rosa, K.; Sladečkova, A.; Popovsky, J.; Punčocharova, M.; et al. Slatkovodne Riasy; Hindák, F., Ed.; Slovenske Pedagogicke Nakladelstvo: Bratislava, Slovakia, 1978. [Google Scholar]
- Huber-Pestalozzi, G. Das Phytoplankton des Süsswassers: Systematik und Biologie; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1961–1983. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Süßwasserflora von Mitteleuropa, Bd. 19/2: Cyanoprokaryota; Bd. 2 Part 2: Oscillatoriales; Spektrum: Heidelberg, Germany, 2005. [Google Scholar]
- Komárek, J. Süßwasserflora von Mitteleuropa, Bd. 19/3: Cyanoprokaryota; 3. Teil 3rd part: Heterocytous Genera; Spektrum: Heidelberg, Germany, 2013. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd. 2/1: Bacillariophyceae, 1. Teil: Naviculaceae; Spektrum: Heidelberg, Germany, 1999. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd. 2/2: Bacillariophyceae, 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; Spektrum: Heidelberg, Germany, 1997. [Google Scholar]
- Krammer, K. Süßwasserflora von Mitteleuropa, Bd. 2/3: Bacillariophyceae. 3. Teil, Centrales, Fragilariaceae, Eunotiaceae; Spektrum: Heidelberg, Germany, 2008. [Google Scholar]
- AlgaeBase: Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 16 July 2024).
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Rott, E. Some results from phytoplankton counting intercalibrations. Schweiz. Z. Hydrol. 1981, 43, 34–62. [Google Scholar] [CrossRef]
- Javornický, P.; Komárková, J. The changes in several parameters of plankton primary productivity in Slapy Reservoir 1960-1967, their mutual correlations and correlations with the main ecological factors. Hydrobiol. Stud. 1973, 2, 155–211. [Google Scholar]
- Sakshaug, E. Phytoplankton Manual. In Monographs on Oceanographic Methodology, 6A; Sournia, A., Ed.; UNESCO: Paris, France, 1978; ISBN 93-3-101572-9. [Google Scholar]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; ISBN 978-0-521-89108-0. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: New York, NY, USA, 2002. [Google Scholar]





| Taxonomic Group | Species |
|---|---|
| Cyanobacteria | Aphanizomenon flos-aquae Ralfs ex Bornet and Flahault |
| Dolichospermum planctonicum (Brunnthaler) Wacklin, L.Hoffmann and Komárek | |
| Dolichospermum solitarium (Klebahn) P.Wacklin, L.Hoffmann and J.Komárek | |
| Limnothrix redekei (Goor) Meffert | |
| Planktolyngbya limnetica (Lemmermann) J.Komárková-Legnerová and G.Cronberg | |
| Planktothrix agardhii (Gomont) Anagnostidis and Komárek | |
| Pseudanabaena limnetica (Lemmermann) Komárek | |
| Raphidiopsis raciborskii (Wołoszyńska) Aguilera et al. | |
| Euglenophyta | Trachelomonas oblonga Lemmermann |
| Trachelomonas volvocina (Ehrenberg) Ehrenberg | |
| Dinophyta | Gymnodinium sp. |
| Peridinium aciculiferum Lemmermann | |
| Cryptophyta | Cryptomonas erosa Ehrenberg |
| Cryptomonas ovata Ehrenberg | |
| Cryptomonas sp. | |
| Plagioselmis nannoplanctica (Skuja) G.Novarino, I.A.N.Lucas and Morrall | |
| Chrysophyceae | Chrysococcus rufescens Klebs |
| Dinobryon divergens O.E.Imhof | |
| Synura uvella Ehrenberg | |
| Bacillariophyceae | Aulacoseira granulata (Ehrenberg) Simonsen |
| Aulacoseira sp. | |
| Lindavia comta (Kützing) T.Nakov et al. | |
| Melosira varians C.Agardh | |
| Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal and Kociolek | |
| Stephanodiscus hantzschii Grunow | |
| Ulnaria acus (Kützing) Aboal | |
| Ulnaria ulna (Nitzsch) Compère | |
| Chlorophyta | Pandorina morum (O.F.Müller) Bory |
| 1972/1973–1977/1978 | 1977/1978–2007/2008 | 1972/1973–2007/2008 | |
| Sørensen’s similarity coefficient (Ss; %) | 46.31 | 34.19 | 39.71 |
| Jaccard’s similarity coefficient (Sj; %) | 30.13 | 20.62 | 24.78 |
| 1972/1973 | 1977/1978 | 2007/2008 | |
| Hill’s diversity number one (N1) | 3.36 | 3.14 | 3.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaljević, M.; Špoljarić Maronić, D.; Stević, F.; Žuna Pfeiffer, T.; Zahirović, V. Maintenance of High Phytoplankton Diversity in the Danubian Floodplain Lake over the Past Half-Century. Plants 2024, 13, 2393. https://doi.org/10.3390/plants13172393
Mihaljević M, Špoljarić Maronić D, Stević F, Žuna Pfeiffer T, Zahirović V. Maintenance of High Phytoplankton Diversity in the Danubian Floodplain Lake over the Past Half-Century. Plants. 2024; 13(17):2393. https://doi.org/10.3390/plants13172393
Chicago/Turabian StyleMihaljević, Melita, Dubravka Špoljarić Maronić, Filip Stević, Tanja Žuna Pfeiffer, and Vanda Zahirović. 2024. "Maintenance of High Phytoplankton Diversity in the Danubian Floodplain Lake over the Past Half-Century" Plants 13, no. 17: 2393. https://doi.org/10.3390/plants13172393






