Intergrative Taxonomic Study of the Frullania parvistipula Complex with a Modern Circumscription of the Section Trachycolea (Frullaniaceae, Marchantiphyta)
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Study
2.2. Taxa Sampling
2.3. DNA Isolation, Amplification, and Sequencing
2.4. Phylogenetic Analysis
3. Results
3.1. Main Clades
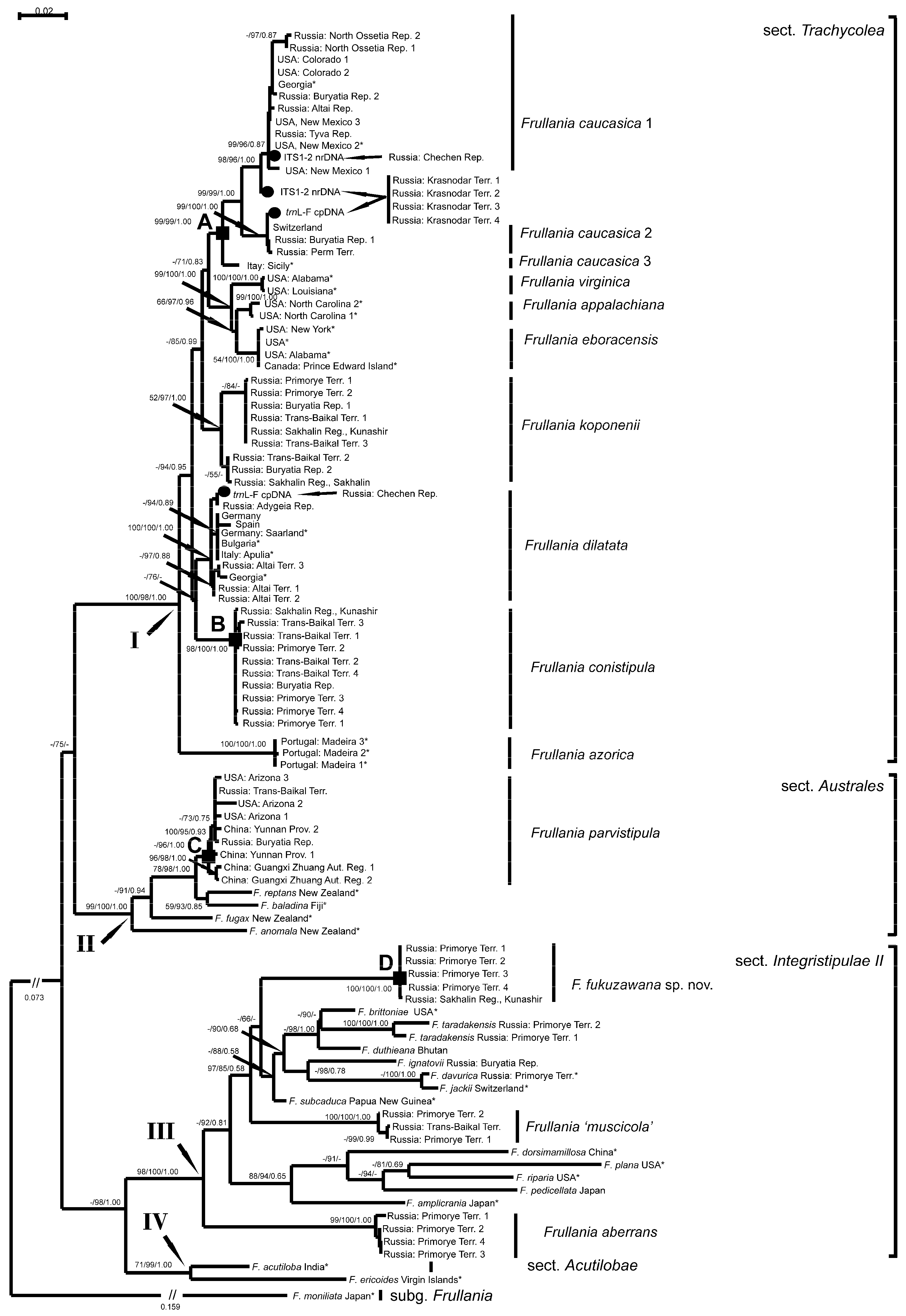
3.2. Subclades of Taxa of the Frullania parvistipula Complex
3.3. Other Groups
4. Discussion
4.1. Frullania parvistipula
4.2. Frullania caucasica
4.3. Frullania conistipula
4.4. Frullania fukuzawana
4.5. Frullania muscicola
4.6. Frullania aeolotis var. aberrans
4.7. Frullania dilatata subsp. asiatica and F. subdilatata
4.8. Frullania fuegiana, F. semivillosa, and F. tibetica
4.9. The Circumscription of Frullania sect. Trachycolea
4.10. Dichotomous Key to the Species of Frullania sect. Trachycolea and the F. dilalata and F. parvistipula Complexes
5. Taxonomy
5.1. Frullania parvistipula Steph., Sp. Hepat. 4: 397 (1910)
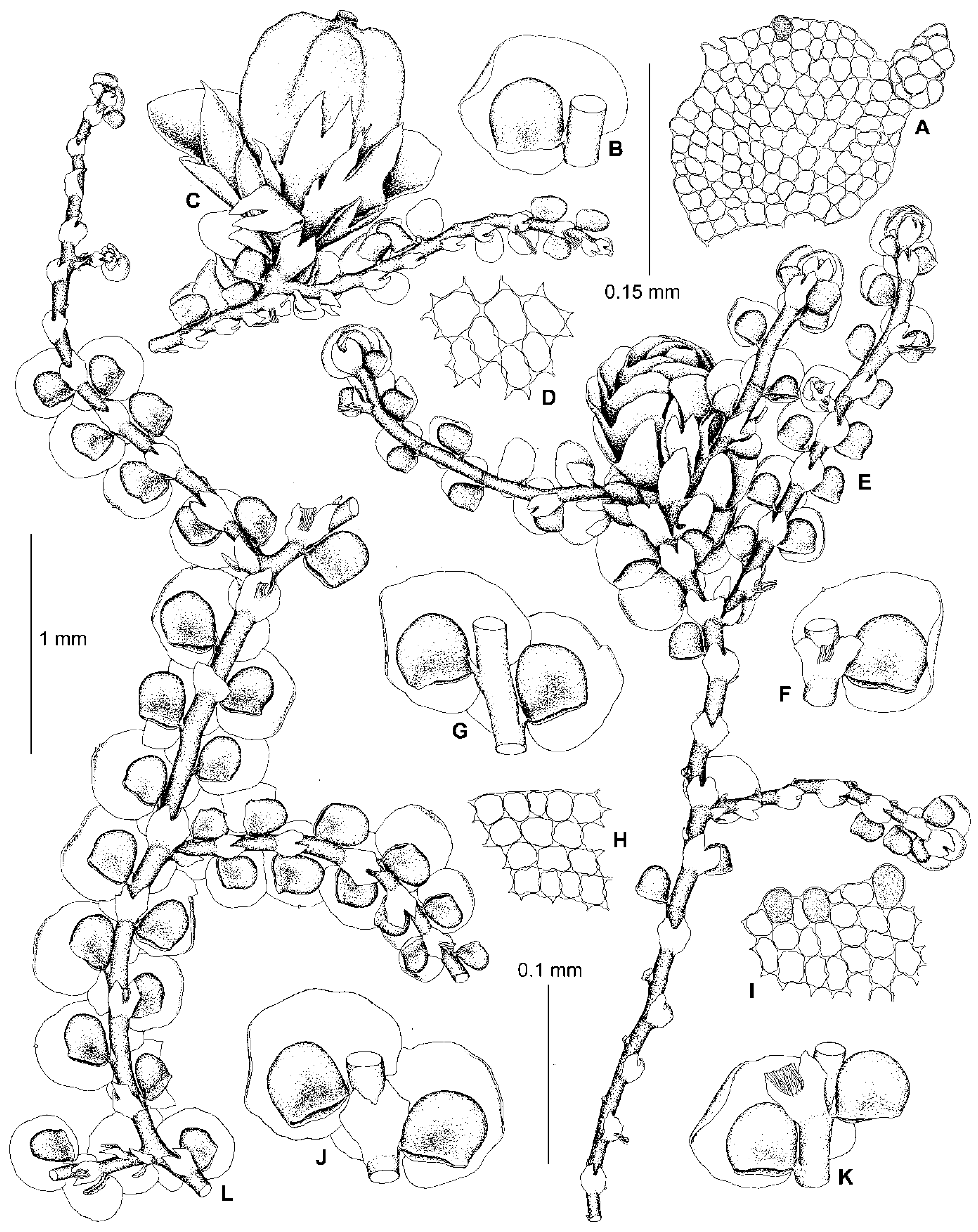
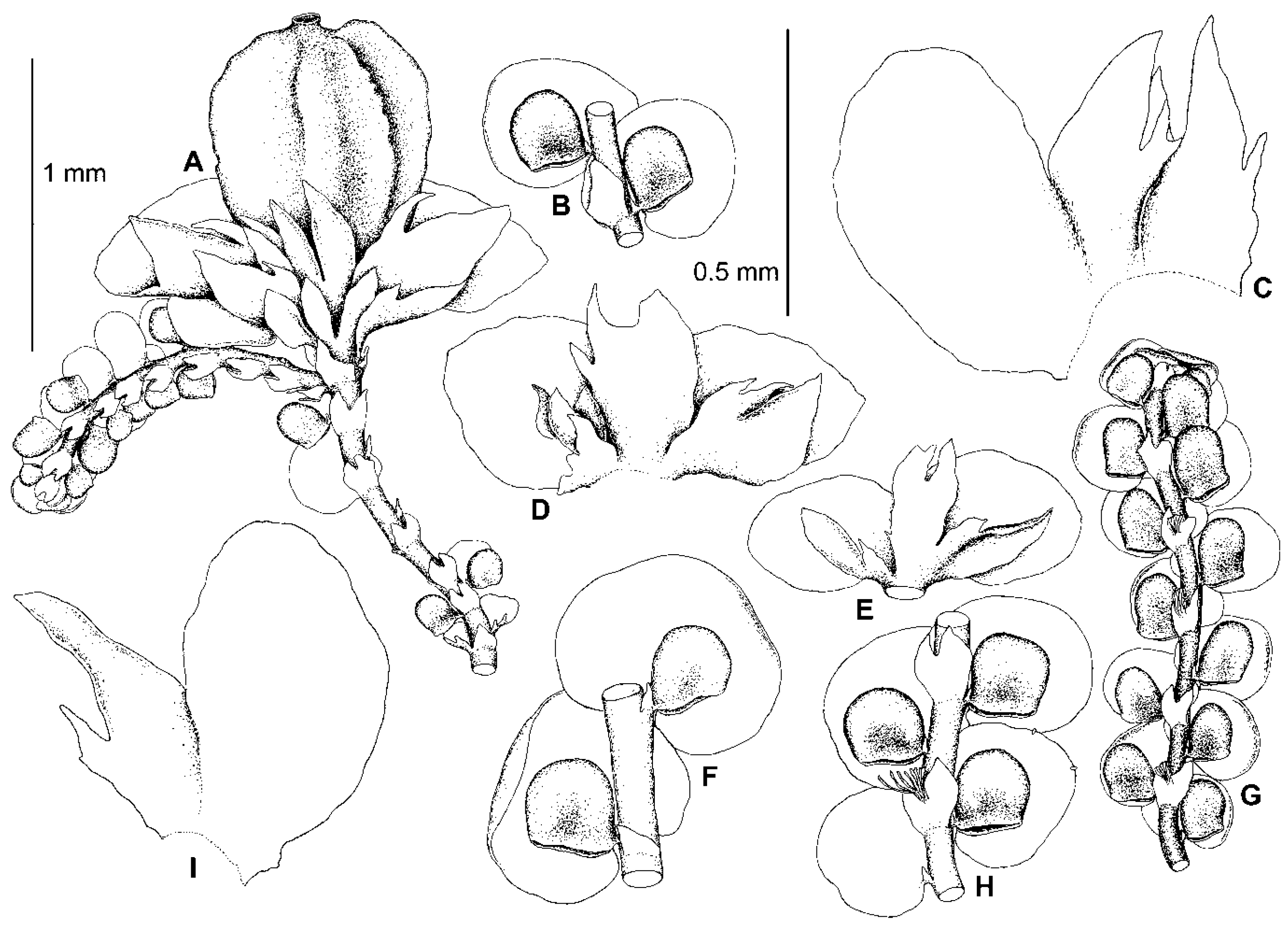
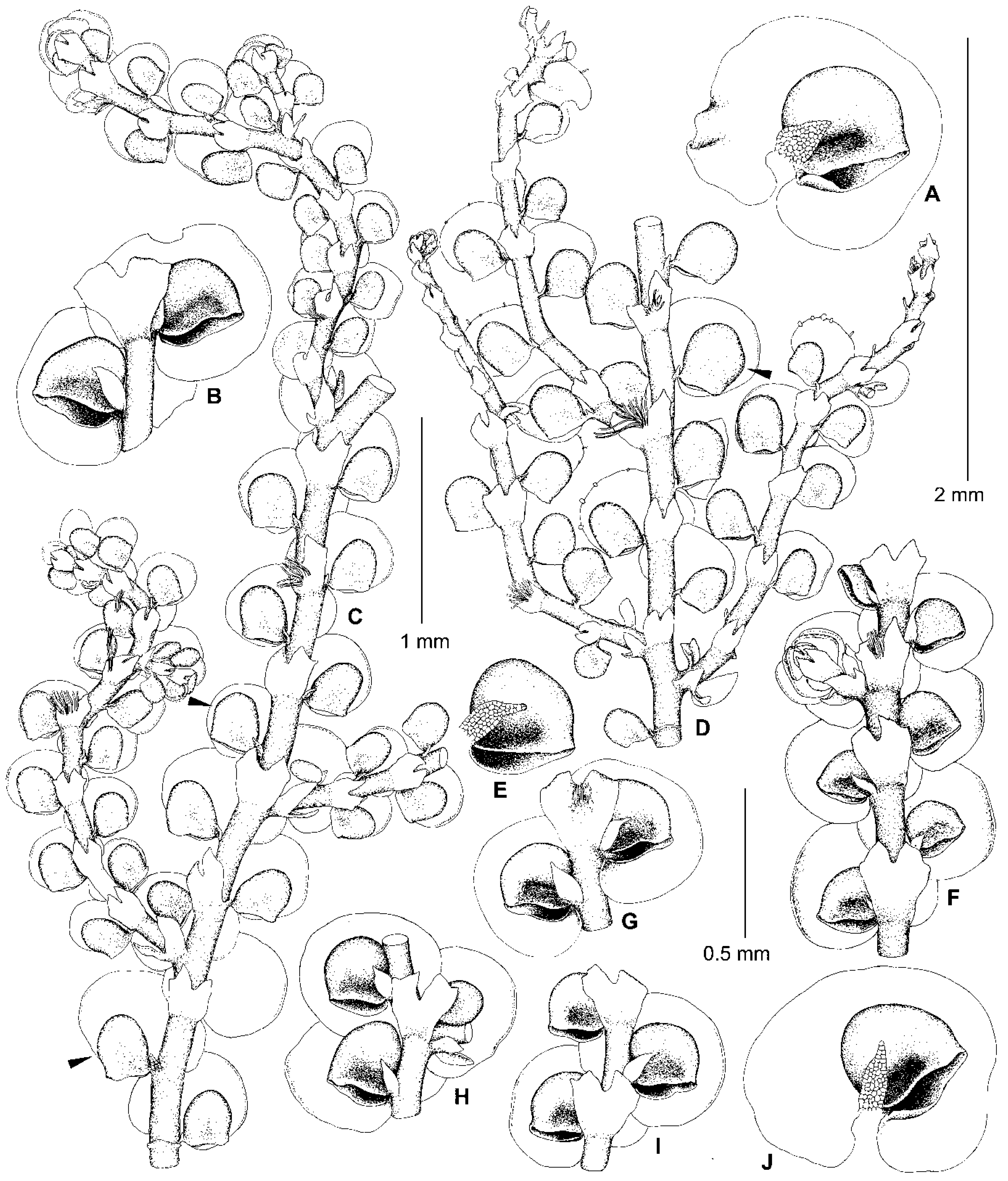
5.2. Frullania conistipula Steph., Sp. Hepat. 4: 399 (1910)
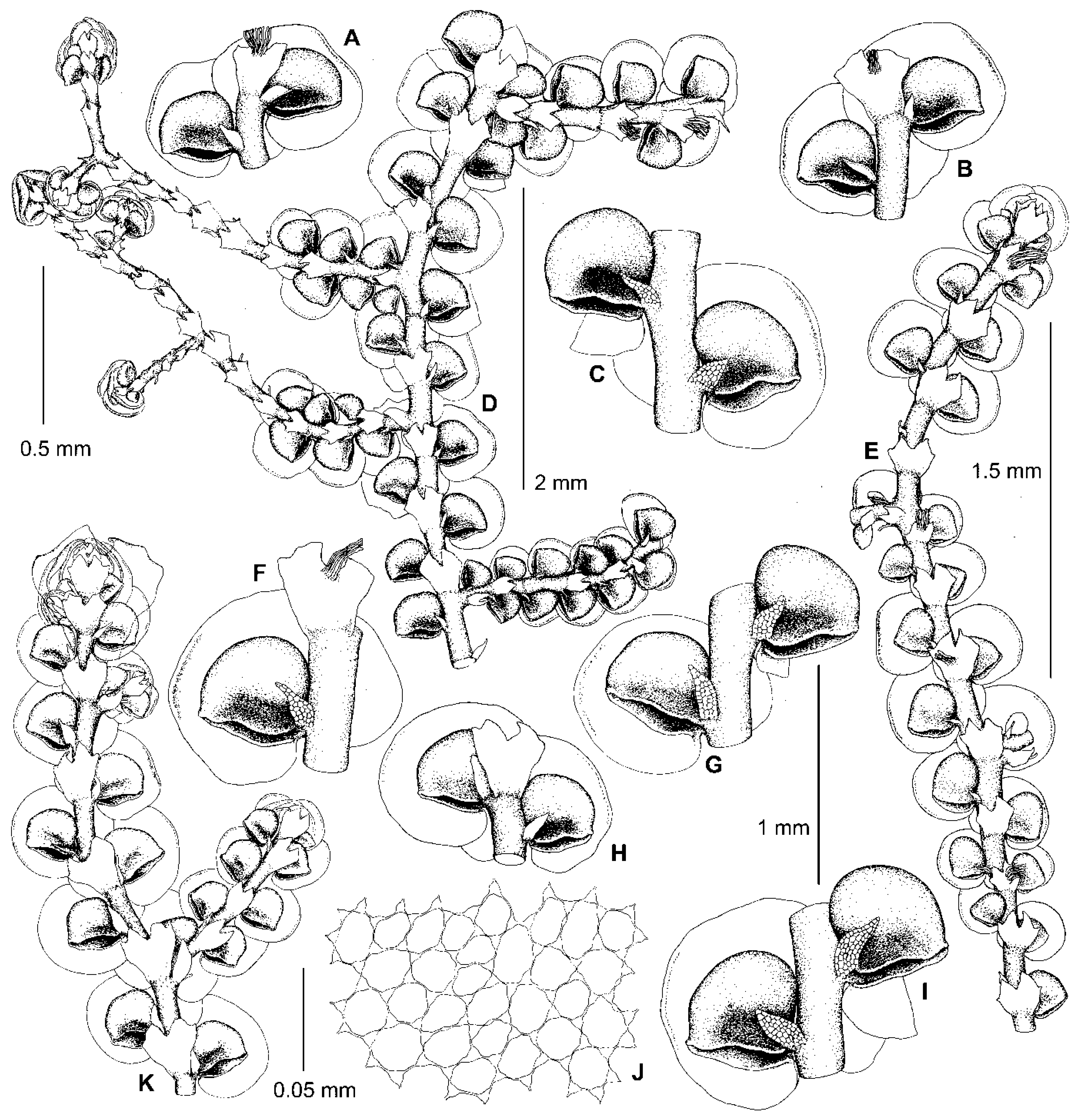
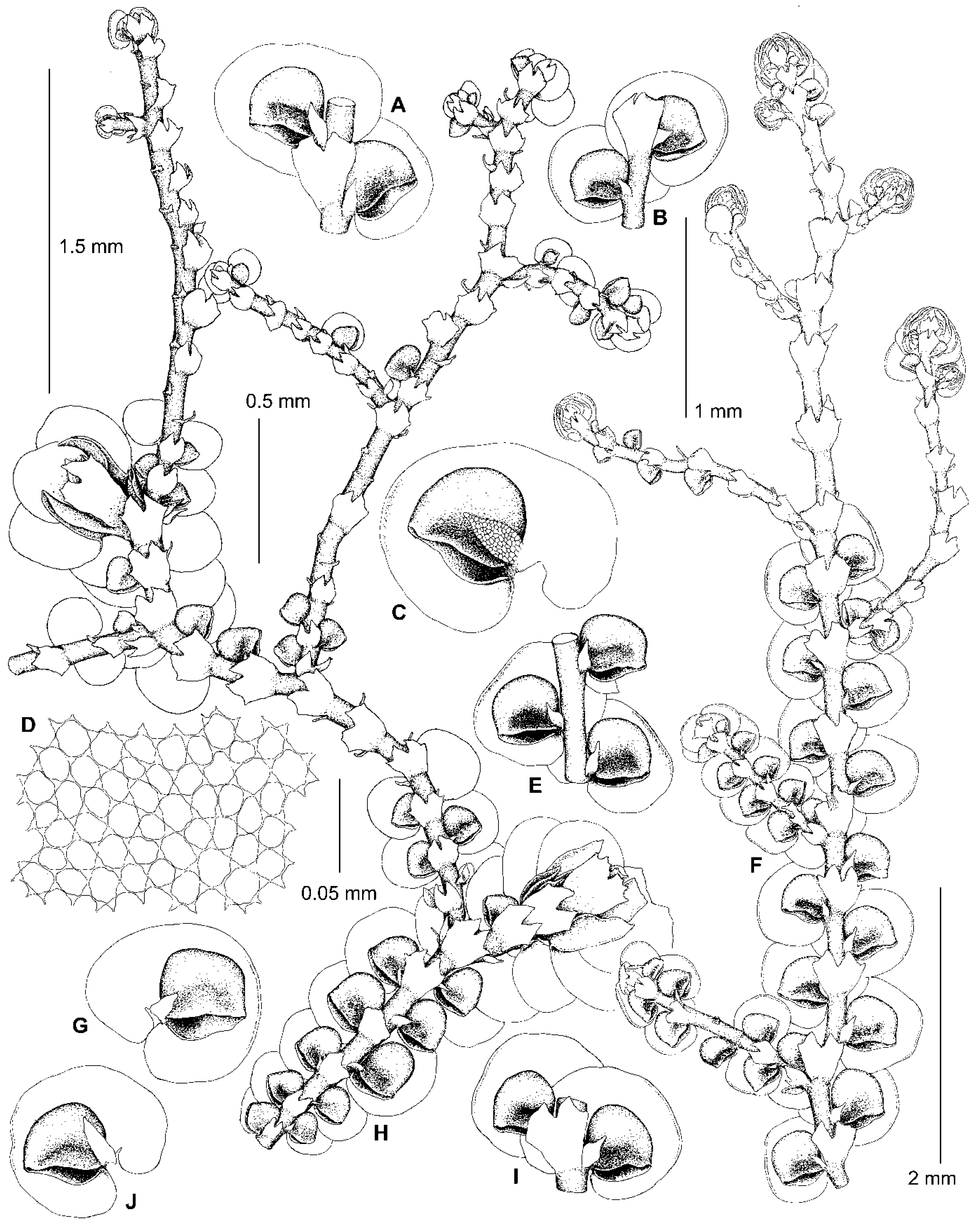
5.3. Frullania fukuzawana Steph. ex Mamontov, J.J.Atwood & Vilnet, sp. nov.

5.4. Frullania aberrans (C. Massal.) Mamontov, Vilnet & J. J. Atwood, comb. et stat. nov.
5.5. Frullania asiatica (S. Hatt.) Mamontov & J. J. Atwood, comb. et stat. nov.

6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heinrichs, J.; Klugmann, F.; Hentschel, J.; Schneider, H. DNA taxonomy, cryptic speciation and diversification of the Neotropical-African liverwort, Marchesinia brachiata (Lejeuneaceae, Porellales). Mol. Phylogenet. Evol. 2009, 53, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, J.; Hentschel, J.; Bombosch, A.; Fiebig, A.; Reise, J.; Edelmann, M.; Kreier, H.P.; Schäfer-Verwimp, A.; Caspari, S.; Schmidt, A.R.; et al. One species or at least eight? Delimitation and distribution of Frullania tamarisci (L.) Dumort. s.l. (Jungermanniopsida, Porellales) inferred from nuclear and chloroplast DNA markers. Mol. Phylogenet. Evol. 2010, 56, 1105–1114. [Google Scholar] [CrossRef]
- Bakalin, V.A.; Vilnet, A.A. Two new species of the liverwort genus Hygrobiella Spruce (Marchantiophyta) described from the North Pacific based on integrative taxonomy. Plant Syst. Evol. 2014, 300, 2277–2291. [Google Scholar] [CrossRef]
- Renner, M.A.M.; Heslewood, M.M.; Patzak, S.D.F.; Schäfer-Verwimp, A.; Heinrichs, J. By how much do we underestimate species diversity of liverworts using morphological evidence? An example from Australasian Plagiochila (Plagiochilaceae: Jungermanniopsida). Mol. Phylogenet. Evol. 2017, 107, 576–593. [Google Scholar] [CrossRef]
- Konstantinova, N.A.; Vilnet, A.A. A new species of the genus Jungermannia (Jungermanniales, Marchantiophyta) from the Caucasus with notes on taxa delimitation and taxonomy of Jungermannia s. str. Phytotaxa 2016, 255, 227–239. [Google Scholar] [CrossRef][Green Version]
- Bakalin, V.A.; Vilnet, A.A.; Choi, S.S.; Nguyen, V.S. Blepharostoma trichophyllum s.l. (Marchantiophyta): The complex of sibling species and hybrids. Plants 2020, 9, 1423. [Google Scholar] [CrossRef]
- Zhu, R.-L.; Bi, X.-F.; Shu, L. Mohamedia, a new genus of Lejeuneaceae (Marchantiophyta) from Oceania and tropical Asia. Bryologist 2019, 122, 84–97. [Google Scholar] [CrossRef]
- Bakalin, V.A.; Maltseva, Y.D.; Vilnet, A.A.; Choi, S.S. The transfer of Tritomaria koreana to Lophozia has led to recircumscription of the genus and shown convergence in Lophoziaceae (Hepaticae). Phytotaxa 2021, 512, 41–56. [Google Scholar] [CrossRef]
- Konstantinova, N.A.; Vilnet, A.A.; Mamontov, Y.S. The phylogenetic affinity, distribution and variability of Cryptocolea imbricata R.M.Schust. (Marchantiophyta). Arctoa 2023, 32, 101–106. [Google Scholar] [CrossRef]
- Hentschel, J.; von Konrat, M.J.; Pócs, T.; Schäfer-Verwimp, A.; Shaw, A.J.; Schneider, H.; Heinrichs, J. Molecular insights into the phylogeny and subgeneric classification of Frullania Raddii (Frullaniaceae, Porellales). Mol. Phylogenet. Evol. 2009, 52, 142–156. [Google Scholar] [CrossRef]
- Mamontov, Y.S.; Vilnet, A.A.; Atwood, J.J.; Konstantinova, N.A. Molecular phylogenetic study of Frullania subsect. Inflatae (Frullaniaceae, Marchantiophyta) in the Holarctic with description of a new subgenus and three new species. Nova Hedwig. Beih. 2020, 150, 201–242. [Google Scholar] [CrossRef]
- Atwood, J.J.; Vilnet, A.A.; Mamontov, Y.S. The taxonomic position and lectotypification of Frullania diversitexta Steph. (Frullaniaceae, Marchantiophyta) and its synonyms, with notes on the placement of F. ignatovii Sofronova, Mamontov & Potemkin. Cryptogam. Bryol. 2021, 42, 19–31. [Google Scholar] [CrossRef]
- Söderström, L.; Hagborg, A.; von Konrat, M.; Bartholomew-Began, S.; Bell, D.; Briscoe, L.; Brown, E.; Cargill, D.C.; Costa, D.P.; Crandall-Stotler, B.J.; et al. World checklist of hornworts and liverworts. PhytoKeys 2015, 59, 1–828. [Google Scholar] [CrossRef]
- Bombosch, A.; Wieneke, A.; Busch, A.; Jonas, R.; Hentschel, J.; Kreier, H.-P.; Shaw, B.; Shaw, A.J.; Heinrichs, J. Narrow species concepts in the Frullania dilatata-appalachiana-eboracensis complex: Evidence from nuclear and chloroplast-DNA markers. Plant Syst. Evol. 2010, 290, 151–158. [Google Scholar] [CrossRef][Green Version]
- Schuster, R.M. Frullania Raddi. In The Hepaticae and Anthocerotae of North America East of the Hundredth Meridian; Field Museum of Natural History: Chicago, IL, USA, 1992; Volume 5, ISBN 0-914-86820-9. [Google Scholar]
- Atwood, J.J.; Mamontov, Y.S. Notes on Frullania chilcootiensis (Frullaniaceae, Marchantiophyta) with a new synonym, lectotypification and an expanded distribution. Bot. Pacifica 2020, 9, 191–195. [Google Scholar] [CrossRef]
- Paton, J.A. The Liverwort Flora of the British Isles; Harley Books: Colchester, UK, 1999; ISBN 978-90-04-28537-8. [Google Scholar]
- Atwood, J.J.; Mamontov, Y.S. Frullania tibetica (Frullaniaceae), a new species from Tibet, China. Novon 2021, 29, 305–310. [Google Scholar] [CrossRef]
- Kamimura, M. A monograph of Japanese Frullaniaceae. J. Hattori Bot. Lab. 1961, 24, 1–109. [Google Scholar]
- Kozub, D.; Khmelik, V.; Shapoval, Y.; Chentsov, S.; Yatsenko, B.; Litovchenko, B.; Starykh, V. Helicon Focus Software. 2008. Available online: http://www.heliconsoft.com (accessed on 9 September 2023).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Snisky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322, ISBN-13 978-0123721815. [Google Scholar]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non–coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Goloboff, P.A.; Catalano, S.T.N.T. version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Keane, T.M.; Creevey, C.J.; Pentony, M.M.; Naughton, T.J.; McInerney, J.O. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumption for choice of matrix are not justified. BMC Evol. Biol. 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How many bootstrap replicates are necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum Likelihood-based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S. Notes on the Asiatic species of the genus Frullania, Hepaticae, XI. J. Hattori Bot. Lab. 1978, 44, 525–554. [Google Scholar]
- Hattori, S. Notes on the Asiatic species of the genus Frullania, Hepaticae, XIII. J. Hattori Bot. Lab. 1981, 49, 147–168. [Google Scholar]
- Koponen, T.; Järvinen, I.; Isoviita, P. Bryophytes from the Soviet Far East, mainly the Khabarovsk Territory. Ann. Bot. Fenn. 1978, 15, 107–121. [Google Scholar]
- Stephani, F. Species Hepaticarum; George & Cie: Geneva/Basel, Switzerland, 1910; Volume 4, pp. 97–448. [Google Scholar]
- Bonner, C.E.B. Index Hepaticarum. Pars V. Delavayella to Geothallus; J. Cramer: Weinheim, Germany, 1965. [Google Scholar]
- Hattori, S. Notes on the Asiatic species of the genus Frullania, Hepaticae, V. J. Hattori Bot. Lab. 1974, 38, 185–221. [Google Scholar]
- Hattori, S. Hepaticae Japonicae Exsiccatae, Ser. 3 (1950). J. Hattori Bot. Lab. 1951, 5, i_1. [Google Scholar] [CrossRef]
- Hattori, S. Asian taxa of the Frullania dilatata complex. J. Jpn. Bot. 1982, 57, 257–260. [Google Scholar]
- Hattori, S.; Lin, P.-J. A preliminary study of Chinese Frullania flora. J. Hattori Bot. Lab. 1985, 59, 123–169. [Google Scholar]
- Schumacker, R.; Váňa, J. Identification Keys to the Liverworts and Hornworts of Europe and Macaronesia (Distribution and Status), 2nd ed.; Sorus Publishing: Poznań, Poland, 2005; ISBN-10 8389949113/ISBN-13 9788389949110. [Google Scholar]
- Montagne, J.P.F.C. Histoire Physique, Politique et Naturelle de l’Île de Cuba, Botanique, Plantes Cellulaire; Arthus Bertrand: Paris, France, 1842. [Google Scholar]
- Yuzawa, Y.; Hattori, S. A new Frullania species from Fukushima, Japan. J. Jpn. Bot. 1983, 58, 43–45. [Google Scholar] [CrossRef]
- Hässel de Menéndez, G.G.; Rubies, M.F. Catalogue of Marchantiophyta and Anthocerotophyta of southern South America. Nova Hedwig. Beih. 2009, 134, 1–672. [Google Scholar]
- Lima, E. Frullania Raddi (Frullaniaceae, Marchantiophyta) no Brasil. Orientador: Anna Luiza Ilkiu Borges Benkendorff. Master’s Thesis, Universidade Federal Rural da Amazônia, Museu Paraense Emílio Goeldi, Belém, Pará, Brasil, 2019. Available online: http://repositorio.ufra.edu.br/jspui/handle/123456789/679 (accessed on 11 July 2024).
- Hattori, S.; Kamimura, M. Some new or little-known Asiatic species of Frullania (Hepaticae), I. J. Hattori Bot. Lab. 1973, 37, 519–533. [Google Scholar]
- Peralta, D.F.; Souza, A.M.; Ilkiu-Borges, A.L.; Carmo, D.M.; Lima, E.; Santos, E.L.; Valente, E.B.; Oliveira, H.C.; Lima, J.S.; Amelio, L.A.; et al. Frullaniaceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil2020.jbrj.gov.br/FB97312 (accessed on 11 July 2024).
- Raddi, G. Jungermanniographica Etrusca; Presso la Società Tipographica: Modena, Italy, 1818. [Google Scholar]
- Evans, A.W. Hepaticae. Liverworts. In Flora of Bermuda; Britton, N.L., Ed.; Charles Scribner’s Sons: New York, NY, USA, 1918; pp. 448–469. [Google Scholar]
- Arthur, J.C.; Barnhart, J.H.; Britton, N.L.; Clements, F.; Cood, O.F.; Coville, F.V.; Earle, F.S.; Evans, A.W.; Hazen, T.E.; Hoolick, A.; et al. American Code of Botanical Nomenclature. Bull. Torrey Bot. Club 1907, 34, 167–178. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018; pp. 1–254. [Google Scholar] [CrossRef]
- Hentschel, J.; von Konrat, M.J.; Söderström, L.; Hagborg, A.; Larraín, J.; Sukkharak, P.; Uribe, J.; Zhang, L. Notes on Early Land Plants Today. 72. Infrageneric classification and new combinations, new names, new synonyms in Frullania. Phytotaxa 2015, 220, 127–142. [Google Scholar] [CrossRef][Green Version]
- Lima, E.; Ilkiu-Borges, A.L.; Gradstein, S.R. A new species of Frullania subg. Frullania (Marchantiophyta) from the Brazilian Amazon. Phytotaxa 2020, 456, 119–124. [Google Scholar] [CrossRef]
- McNeill, J.; Barrie, F.R.; Gandhi, K.N.; Hollowell, V.C.; Redhead, S.A.; Söderström, L.; Zarucchi, J.L. Report of the Special Committee on publications using a largely mechanical method of selection of types (Art. 10.5(b)) (especially under the American Code). Taxon 2016, 65, 1443–1448. [Google Scholar] [CrossRef]
- Frye, T.C.; Clark, L. Hepaticae of North America. Part V. University of Washington Publications in Biology 6; University of Washington: Seattle, WA, USA, 1947; pp. 735–1022. [Google Scholar]
- Schuster, R.M. Bazzania S. F. Gray corr. Carr. In The Hepaticae and Anthocerotae of North America East of the Hundredth Meridian; Columbia University Press: New York, NY, USA, 1969; Volume 2, ISBN-10 0231089821/ISBN-13 9780231089821. [Google Scholar]
- Bakalin, V.A. A revision of Lepidoziaceae (Hepaticae) in the Russian Far East. I. Bazzania. Bot. Pacifica 2016, 5, 33–52. [Google Scholar] [CrossRef]
- Gradstein, S.R. A Taxonomic Monograph of the Genus Acrolejeunea (Hepaticae). Bryophyt. Bibl. 1975, 4, 1–162. [Google Scholar]
- Schuster, R.M. Acrolejeunea (Spr.) Schiffn. In The Hepaticae and Anthocerotae of North America East of the Hundredth Meridian; Columbia University Press: New York, NY, USA, 1980; Volume 4, ISBN 0-231-04608-1. [Google Scholar]
- Hattori, S. A revision of the Australasian species of the genus Frullania, Hepaticae. III. J. Hattori Bot. Lab. 1983, 54, 133–182. [Google Scholar]
- von Konrat, M.J.; Braggins, J.E. Notes on five Frullania species from Australia including typification, synonyms and new localities. J. Hattori Bot. Lab. 2001, 91, 229–263. [Google Scholar]
- Choi, S.S.; Bakalin, V.; Park, S.J. Integrating continental mainland and islands in temperate East Asia: Liverworts and hornworts of the Korean Peninsula. PhytoKeys 2021, 176, 131–226. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, N.A.; Mamontov, Y.S.; Savchenko, A.N. On the liverwort flora of Tunkinskiy National Park (Republic of Buryatia, Russia). Arctoa 2018, 27, 131–139. [Google Scholar] [CrossRef]
- Hattori, S.; Thaithong, O. Dr. Parihar’s collection of Indian Frullania (Hepaticae). J. Jpn. Bot. 1978, 53, 129–133. [Google Scholar] [CrossRef]
| Taxon | Specimen Voucher | GenBank Accession Number | |
|---|---|---|---|
| ITS1-2 nrDNA | trnL-F cpDNA | ||
| F. aberrans | Russia: Primorye Terr. 1, Mamontov 808-1-4726 (MHA) | MW358434 | MW351720 |
| F. aberrans | Russia: Primorye Terr. 2, Mamontov 689-1-4724 (MHA) | no data | MW351721 |
| F. aberrans | Russia: Primorye Terr, 3, Mamontov 697-1-2 (MHA) | MW358435/MW358436 | MW351722 |
| F. aberrans | Russia: Primorye Terr. 4, Mamontov 686-1-4723 (MHA) | MW358437 | MW351723 |
| F. acutiloba | India, Schaefer-Verwimp & Verwimp 28265 (GOET) | FJ380421 | FJ380257 |
| F. amplicrania | Japan, Ohnishi 5840 (HIRO) | FJ380447 | FJ380285 |
| F. anomala | New Zealand, Engel & von Konrat 27,319 (GOET) | FJ380457 | FJ380297 |
| F. appalachiana | USA: North Carolina 1, Davison 7888 (UNA) | HQ330382 | HQ330418 |
| F. appalachiana | USA: North Carolina 2, Davison & Smith 6389 (UNA) | HQ330383 | HQ330419 |
| F. azorica | Portugal: Madeira 1, Eckstein 487 (GOET) | HQ330386 | HQ330422 |
| F. azorica | Portugal: Madeira 2, Mues 3743 (SAAR) | HQ330387 | HQ330423 |
| F. azorica | Portugal: Madeira 3, Schaefer-Verwimp & Verwimp 25,896 (GOET) | FJ380436 | FJ380272 |
| F. baladina | Fiji: Pocs & Pocs 03259/N (GOET) | FJ380454 | FJ380293 |
| F. brittoniae | USA: Davison & Kauffman 5334 (GOET) | FJ380442 | FJ380278 |
| F. caucasica 1 | Georgia: Zündorf 21852 (JE), as F.parvistipula in Bombosch et al. [14] | HQ330415 | no data |
| F. caucasica 1 | Russia: Altai Rep., Mamontov 333-1-39 (MHA) | MW358439 | MW351725 |
| F. caucasica 1 | Russia: Buryatia Rep. 2, Mamontov 639-1-1 (MHA) | MW358441 | MW351727 |
| F. caucasica 1 | Russia: Krasnodar Terr. 1, Konstantinova K235b-18 (KPABG) | MW358442 | MW351728 |
| F. caucasica 1 | Russia: Krasnodar Terr. 2, Konstantinova K270-18 (KPABG) | MW358443 | MW351729 |
| F. caucasica 1 | Russia: Krasnodar Terr. 3, Konstantinova K284-1-18 (KPABG) | MW358444 | MW351730 |
| F. caucasica 1 | Russia: Krasnodar Terr. 4, Konstantinova K287-18 (KPABG) | MW358445 | MW351731 |
| F. caucasica 1 | Russia: North Ossetia Rep. 1, Rumyanzeva AR-3-2-11 (KPABG) | MW358446 | MW351732 |
| F. caucasica 1 | Russia: North Ossetia Rep. 2, Rumyanzeva AR-2-11 (KPABG) | no data | MW351733 |
| F. caucasica 1 | Russia: Tyva Rep., Otnyukova (KPABG-101204) | MW358448 | MW351735 |
| F. caucasica 1 | USA: Colorado 1, Weber et al. B-112022 (MO) | MW358450 | MW351737 |
| F. caucasica 1 | USA: Colorado 2, Wittmann et al. B-112078 (MO) | MW358451 | MW351738 |
| F. caucasica 1 | USA: New Mexico 1, Worthington 31081 (MO, GOET), as F. parvistipula in Bombosch et al. [14] |
MW358452/MW358453
HQ330379/FJ380435 |
MW351739
FJ380271 |
| F. caucasica 1 | USA: New Mexico 2, Worthington 32,814 (GOET), as F. parvistipula in Bombosch et al. [14] | FJ380433 | FJ380269 |
| F. caucasica 1 | USA: New Mexico 3, Schofield 96,656 (MHA) | PP239508 | PP261943 |
| F. caucasica 2 | Russia: Perm Terr., Bezgodov AB204a-18 (KPABG) | MW358447 | MW351734 |
| F. caucasica 2 | Switzerland: Rueegsegger 1303-III (KPABG) | MW358449 | MW351736 |
| F. caucasica 2 | Russia: Buryatia Rep. 1, Mamontov 560-6-1, 4610 (MHA) | MW358440 | MW351726 |
| F. caucasica 3 | Italy: Sicily, Eckstein 4684 (GOET), as F. parvistipula in Bombosch et al. [14] | FJ380438 | FJ380274 |
| F. conistipula | Russia: Buryatia Rep., Mamontov 450-1-5592 (MHA) | MW358454 | MW351740 |
| F. conistipula | Russia: Primorye Terr. 1, Mamontov 697-1-4584 (MHA) | MW358455 | MW351741 |
| F. conistipula | Russia: Primorye Terr. 2, Mamontov 807-1-4522 (MHA) | MW358456/MW358457 | MW351742 |
| F. conistipula | Russia: Primorye Terr. 3, Mamontov 807-1-4 (MHA) | MW358458 | MW351743 |
| F. conistipula | Russia: Primorye Terr. 4, Mamontov 801-1-9 (MHA) | MW358459 | MW351744 |
| F. conistipula | Russia: Sakhalin Reg., Kunashir, Bakalin K-37-5-06 (KPABG-110245) | MW358460 | MW351745 |
| F. conistipula | Russia: Trans-Baikal Terr. 1, Afonina A5510-1 (KPABG-116339) | MW358461 | MW351746 |
| F. conistipula | Russia: Trans-Baikal Terr. 2, Afonina A5510-8 (KPABG-118647) | MW358462 | MW351747 |
| F. conistipula | Russia: Trans-Baikal Terr. 3, Afonina A5710-1 (KPABG-116341) | MW358463 | MW351748 |
| F. conistipula | Russia: Trans-Baikal Terr. 4, Mamontov 544-1-6073 (MHA) | PQ213807 | PQ213858 |
| F. davurica | Russia: Primorye Terr., Gambaryan VLA-h-764 (GOET) | FJ380444 | FJ380280 |
| F. dilatata | Bulgaria: Hentschel 0758 (GOET) | FJ380434 | FJ380270 |
| F. dilatata | Georgia: Bordshomi, Zündorf 21940 (JE) | HQ330390 | HQ330425 |
| F. dilatata | Germany: Saarland, Sesterhenn 5474 (SAAR) | HQ330396 | HQ330430 |
| F. dilatata | Germany: Nordrhein-Westfalen, Mamontov 244-12-1 (MHA) | MW358465 | MW351750 |
| F. dilatata | Italy: Apulia, Sauer 3090 (GOET) | HQ330401 | HQ330435 |
| F. dilatata | Russia: Adygeia Rep., Konstantinova K472-1-07 (KPABG-111725) | PP239515 | PP261950 |
| F. dilatata | Russia: Altai Terr. 1, Mamontov 219-15 (KPABG) | MW358466 | MW351751 |
| F. dilatata | Russia: Altai Terr. 2, Mamontov 219-18 (KPABG) | MW358467 | MW351752 |
| F. dilatata | Russia: Altai Terr. 3, Mamontov 219-32-8 (KPABG, MHA) | MW358468 | MW351753 |
| F. dilatata | Spain: Konstantinova K12-9a-19 (KPABG) | MW358470 | MW351755 |
| F. dorsimamillosa | China: Koponen 45098 (JE), as F. fuscovirens var. gemmipara in Hentschel et al. [10] | FJ380440 | FJ380276 |
| F. duthieana | Bhutan: Miehe & Miehe 00-132-21 (GOET), as F. duthiana in Hentschel et al. [10] | FJ380443 | FJ380279 |
| F. eboracensis | Canada: Prince Edward Island, Belland | HQ330409 | HQ330442 |
| F. eboracensis | USA: Alabama, Davison 6875 (UNA) | HQ330410 | HQ330443 |
| F. eboracensis | USA: New York, Smith 50725 (UBC) | HQ330414 | HQ330446 |
| F. eboracensis | USA: Davison 5193 (GOET) | FJ380437 | FJ380273 |
| F. ericoides | Virgin Islands, Gradstein 6421 (GOET) | FJ380405 | FJ380240 |
| F. fugax | New Zealand: Engel & von Konrat 24698 (GOET) | FJ380455 | FJ380295 |
| F. fukuzawana | Russia: Primorye Terr. 1, Mamontov 851-1-6016 (MHA, KPABG-126113) | PP239511 | PP261946 |
| F. fukuzawana | Russia: Primorye Terr. 2, Mamontov 850-1-6021 (MHA, KPABG-126089) | PP239512 | PP261947 |
| F. fukuzawana | Russia: Primorye Terr. 3, Mamontov 850-1-6301 (MHA, KPABG-126112) | PP239513 | PP261948 |
| F. fukuzawana | Russia: Primorye Terr. 4, Mamontov 855-1-6303 (MHA, KPABG-126088) | PP239514 | PP261949 |
| F. fukuzawana | Russia: Sakhalin Reg., Kunashir, Mamontov 815-1-5628 (MHA, KPABG-126092) | PP239510 | PP261945 |
| F. ignatovii | Russia: Buryatia Rep., Mamontov 384-8 (KPABG-120318, isotype) | MT408599 | no data |
| F. jackii | Switzerland: Urmi & Schaefer-Verwimp 16,328 (GOET) | FJ380445 | FJ380281 |
| F. koponenii | Russia: Buryatia Rep. 1, Mamontov 565-3-4730 (MHA) | MW358471 | MW351756 |
| F. koponenii | Russia: Buryatia Rep. 2, Mamontov 658-1-1 (MHA) | MW358472 | MW351757 |
| F. koponenii | Russia: Primorye Terr. 1, Mamontov 692-1-1 (MHA) | MW358473 | MW351758 |
| F. koponenii | Russia: Primorye Terr. 2, Mamontov 805-1-1 (MHA) | MW358474 | MW351759 |
| F. koponenii | Russia: Sakhalin, Bakalin S-24-18-06 (KPABG) | MW358475 | MW351760 |
| F. koponenii | Russia: Sakhalin Reg., Kunashir, Mamontov 837-1-6005 (MHA, KPABG-126091) | PP239509 | PP261944 |
| F. koponenii | Russia: Trans-Baikal Terr. 1, Mamontov 61-1 (LE) | MW358476 | no data |
| F. koponenii | Russia: Trans-Baikal Terr. 2, Mamontov 511-1-2 (MHA) | MW358477 | MW351761 |
| F. koponenii | Russia: Trans-Baikal Terr. 3, Afonina A07208 (KPABG-113885) | no data | MW351762 |
| F. moniliata | Japan: Ohnishi, 5346 (HIRO) | FJ380500 | FJ380346 |
| F. ‘muscicola’ | Russia: Primorye Terr. 1, Mamontov 739-3-1 (MHA) | MW358478 | MW351763 |
| F. ‘muscicola’ | Russia: Primorye Terr. 2, Mamontov 805-1-4 (MHA) | MW358479 | MW351764 |
| F. ‘muscicola’ | Russia: Trans-Baikal Terr., Mamontov 264-6 (MHA) | MW358480 | no data |
| F. parvistipula | China: Yunnan Prov., 1, Mamontov 722-1-4 (MHA) | MW358485 | MW351768 |
| F. parvistipula | China: Yunnan Prov., 2, Mamontov 737-1-2 (MHA) | MW358486 | MW351769 |
| F. parvistipula | Russia: Buryatia Rep., Mamontov 376-1-1 (KPABG) | MW358487 | MW351770 |
| F. parvistipula | Russia: Trans-Baikal Terr., Mamontov 182-6-1 (KPABG-118618) | MW358488 | MW351771 |
| F. parvistipula | USA: Arizona 1, Brinda 9978 (MO) | MW358489 | MW351772 |
| F. parvistipula | USA: Arizona 2, Brinda 1398 (MO) | MW358490 | MW351773 |
| F. parvistipula | USA: Arizona 3, Brinda 1396b (MO) | MW358491 | MW351774 |
| F. parvistipula | China: Guangxi 1, Mamontov 716-1-1 (MHA) | MW358492 | MW351775 |
| F. parvistipula | China: Guangxi 2, Mamontov 719-1-1 (MHA) | MW358493 | MW351776 |
| F. pedicellata | Japan: Inoue s.n., as ‘No. 705. Frullania muscicola Steph.’ of Bryophyta Selecta Exsiccata (KPABG) | MW358481/MW358482 | MW351765 |
| F. plana | USA: Davison 4325 (GOET) | FJ380431 | FJ380267 |
| F. reptans | New Zealand: Engel & von Konrat 24,974 (GOET) | FJ380450 | FJ380288 |
| F. riparia | USA: Worthington 29,965 (GOET) | FJ380446 | FJ380284 |
| F. subcaduca | Papua New Guinea, Norris 62427 (JE) | no data | FJ380283 |
| F. taradakensis | Russia: Primorye Terr. 1, Mamontov 801-1-5 (MHA) | MW358483 | MW351766 |
| F. taradakensis | Russia: PrimoryeTerr. 2, Bakalin P-69-10-06 (KPABG-110319) | MW358484 | MW351767 |
| F. virginica | USA: Louisiana, Hyatt s.n. (GOET) | HQ330416 | HQ330447 |
| F. virginica | USA: Alabama, Davison 3550 (GOET) | HQ330417 | HQ330448 |
| F. dilatata × caucasica | Russia: Chechen Rep., Doroshina 467-2-18 (KPABG) | MW358469 | MW351754 |
| Taxon | Infraspecific p-Distances, ITS2/trnL-F (%) | Infrageneric p-Distances, ITS2/trnL-F (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |||
| 1 | caucasica 1 | 0.4/0.2 | |||||||||||||||||
| 2 | caucasica 2 | 0.1/0.1 | 2.1/0.8 | ||||||||||||||||
| 3 | caucasica 3 | n/c/n/c | 1.9/1.2 | 1.2/1.4 | |||||||||||||||
| 4 | virginica | 0.2/0.0 | 4.0/1.5 | 4.1/1.8 | 3.0/1.5 | ||||||||||||||
| 5 | appalachiana | 0.4/0.2 | 3.7/1.3 | 3.9/1.5 | 2.1/1.5 | 2.5/0.7 | |||||||||||||
| 6 | eboracensis | 0.2/0.0 | 3.6/0.9 | 3.9/1.0 | 2.9/1.3 | 2.3/0.6 | 1.8/0.5 | ||||||||||||
| 7 | koponenii | 1.2/0.0 | 3.7/1.1 | 3.6/1.5 | 2.5/1.2 | 3.6/1.6 | 3.2/1.5 | 3.5/1.4 | |||||||||||
| 8 | conistipula | 0.3/0.0 | 4.4/0.9 | 4.7/1.3 | 2.5/1.0 | 4.9/1.4 | 4.2/1.3 | 4.5/1.2 | 3.4/1.1 | ||||||||||
| 9 | dilatata | 0.4/0.3 | 4.0/0.6 | 3.9/0.9 | 3.1/0.8 | 4.1/1.1 | 3.4/1.0 | 3.9/0.9 | 3.0/0.7 | 3.0/0.5 | |||||||||
| 10 | azorica | 0.2/0.0 | 7.0/1.4 | 6.2/1.6 | 6.2/1.5 | 6.8/1.9 | 6.3/1.9 | 6.8/1.7 | 6.2/1.7 | 6.6/1.5 | 5.4/1.1 | ||||||||
| 11 | parvistipula | 0.8/0.3 | 11.2/2.5 | 10.8/2.5 | 9.6/2.4 | 10.6/3.1 | 10.6/3.2 | 10.3/2.7 | 10.6/2.7 | 11.7/2.6 | 10.0/2.2 | 11.5/3.0 | |||||||
| 12 | baladina | n/c/n/c | 10.0/2.9 | 7.5/3.2 | 9.9/2.6 | 9.8/2.9 | 9.9/3.3 | 9.9/3.1 | 10.0/3.0 | 10.4/2.8 | 9.8/2.5 | 11.8/3.2 | 3.4/0.9 | ||||||
| 13 | reptans | n/c/n/c | 10.7/2.7 | 8.0/3.0 | 10.6/2.4 | 10.4/3.1 | 10.6/3.1 | 10.6/2.9 | 10.8/2.8 | 11.2/2.6 | 10.1/2.3 | 12.6/3.1 | 3.4/0.7 | 3.8/0.9 | |||||
| 14 | fugax | n/c/n/c | 8.9/2.7 | 7.1/3.0 | 8.8/2.1 | 8.7/3.1 | 8.8/3.1 | 8.8/2.9 | 8.9/2.8 | 9.2/2.7 | 8.3/2.3 | 9.9/3.1 | 5.1/1.2 | 6.7/1.5 | 6.0/1.3 | ||||
| 15 | anomala | n/c/n/c | 10.0/3.0 | 7.9/3.4 | 10.3/2.8 | 10.0/3.5 | 9.2/3.5 | 9.7/3.2 | 10.4/3.2 | 10.6/3.0 | 9.8/2.7 | 9.9/3.1 | 7.8/1.4 | 8.2/1.7 | 8.6/1.5 | 7.3/1.7 | |||
| 16 | fukuzawana | 0.1/0.0 | 16.4/3.1 | 15.3/3.4 | 13.1/3.1 | 16.4/3.7 | 16.3/3.7 | 15.4/3.5 | 15.6/3.3 | 16.9/3.1 | 15.8/2.7 | 16.3/3.5 | 16.5/2.9 | 13.8/2.9 | 14.0/3.1 | 12.9/2.7 | 15.0/3.5 | ||
| 17 | ‘muscicola’ | 0.4/0.0 | 15.0/3.1 | 13.9/3.6 | 12.2/2.7 | 15.0/3.6 | 15.2/3.7 | 14.7/3.4 | 14.4/3.4 | 15.8/3.2 | 14.5/2.9 | 14.4/3.8 | 14.2/3.1 | 13.0/3.1 | 12.5/3.3 | 12.1/2.9 | 14.3/3.3 | 9.1/2.7 | |
| 18 | aberrans | 0.5/0.0 | 17.4/2.4 | 15.0/2.8 | 16.1/2.0 | 16.9/2.9 | 17.5/2.9 | 17.4/2.7 | 17.0/2.6 | 17.9/2.5 | 17.5/2.1 | 18.5/3.1 | 15.9/2.2 | 15.8/2.2 | 16.3/2.4 | 15.3/2.4 | 16.8/2.8 | 13.6/1.9 | 12.2/2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamontov, Y.S.; Vilnet, A.A.; Atwood, J.J.; Konstantinova, N.A. Intergrative Taxonomic Study of the Frullania parvistipula Complex with a Modern Circumscription of the Section Trachycolea (Frullaniaceae, Marchantiphyta). Plants 2024, 13, 2397. https://doi.org/10.3390/plants13172397
Mamontov YS, Vilnet AA, Atwood JJ, Konstantinova NA. Intergrative Taxonomic Study of the Frullania parvistipula Complex with a Modern Circumscription of the Section Trachycolea (Frullaniaceae, Marchantiphyta). Plants. 2024; 13(17):2397. https://doi.org/10.3390/plants13172397
Chicago/Turabian StyleMamontov, Yuriy S., Anna A. Vilnet, John J. Atwood, and Nadezhda A. Konstantinova. 2024. "Intergrative Taxonomic Study of the Frullania parvistipula Complex with a Modern Circumscription of the Section Trachycolea (Frullaniaceae, Marchantiphyta)" Plants 13, no. 17: 2397. https://doi.org/10.3390/plants13172397
APA StyleMamontov, Y. S., Vilnet, A. A., Atwood, J. J., & Konstantinova, N. A. (2024). Intergrative Taxonomic Study of the Frullania parvistipula Complex with a Modern Circumscription of the Section Trachycolea (Frullaniaceae, Marchantiphyta). Plants, 13(17), 2397. https://doi.org/10.3390/plants13172397






