Abstract
The success of using active restoration in Mediterranean-type climate zones mostly depends on an appropriate matching of plant species and specific management prescriptions upon establishment. In this study, we assessed the early growth and short-term physiological acclimation of seven common species found in the sclerophyllous forests in central Chile to water restriction and shading. We established a nursery experiment that included three treatments (T0: sun-exposed and water-restricted, T1: sun-exposed and fully irrigated, and T2: shaded and fully irrigated) and seven tree species differing in their shade and drought tolerance (Quillaja saponaria Molina, Aristotelia chilensis (Mol.) Stuntz, Peumus boldus Molina, Lithraea caustica (Mol.) Hook. and Arn, Luma apiculata (DC.) Burret, Colliguaja odorifera Molina, and Escallonia pulverulenta (Ruiz and Prav.) Pers). We measured the increment in seedling height and different leaf morpho-physiological traits during two months in the dry season. Based on the measured traits, none of the species took advantage of the higher water availability in T1 relative to T0, but most of the species responded to the shade in T2, regardless of their shade or drought tolerance. Height increments due to shade varied from 0% in P. boldus to 203% in L. apiculata. Overall, all the species responded similarly to the treatments in specific leaf area, chlorophyll content index, photosynthetic rate, stomatal conductance, and intrinsic water use efficiency. This suggests that the species exhibited similar acclimation patterns of these parameters to shade and drought, even regarding the variation in midday xylem water potential found in the water-restricted treatment T0 (from −1.5 MPa in P. boldus to −3.1 MPa in E. pulverulenta). In this study, shading had a higher positive effect on the seedling performance of sclerophyllous species than watering, which at operational level highlights the need for investing in tree shelters when using these species in restoration programs.
1. Introduction
The more intense and prolonged droughts due to climate change are threatening the recruitment of young seedlings in Mediterranean ecosystems [1]. Assisted reforestation (i.e., active restoration) is seen as the main strategy to speed up the recovery of forest functions and the structure of degraded zones in this ecosystem type. However, there is still a lack of knowledge on specific management prescriptions for successful seedling establishment and survival in restoration projects, which emphasizes the importance of screening in controlled experiments (i.e., at the nursery level) before scaling projects at the landscape level. Overall, seedlings are more susceptible to drought than adult trees. Thus, information at the seedling stage might give indications on the capacity of a species to grow and survive under environmental stresses and provide guidelines for specific management prescriptions at establishment.
Central Chile possesses one of the five Mediterranean-type climate zones in the world, corresponding to the transition between the Atacama Desert in the north and the mixed deciduous–evergreen temperate forests in the southern part of the country [2]. This zone is considered a hotspot of biodiversity, characterized by its high richness and endemism of plant species [3] but is also considered one of the most degraded ecosystems in the country by anthropogenic disturbances [4]. Overall, the semi-arid region is extensively dominated by sclerophyllous forest species (i.e., matorral shrublands) [5,6]. In this ecosystem, sclerophyllous plant species are adapted to the cool wet winters and warm dry summers historically found in this zone, with many species presenting leathered and small leaves, shrubby habits, and evergreenness [7]. However, despite these adaptation attributes, in recent years, a large-scale browning of this ecosystem type due to the desiccation of vegetation has been observed [8], which has been associated with the mega-drought reported since 2010 [9]. This phenomenon is reducing the canopy cover and creating new microenvironments at the understory level, which threatens the recruitment and regeneration of sclerophyllous species, mainly because young seedlings are unable to establish and survive under the new conditions of high radiation and water scarcity [10,11,12]. In addition, the future intensification of droughts, combined with higher temperatures, also jeopardizes the success of restoration efforts because severe drought and high levels of irradiance during summer severely constrain seedling establishment [11]. Therefore, understanding the responses to drought and high irradiance in sclerophyllous species at the early stages of development is urgently needed as it may provide knowledge to guide the design of species-specific establishing prescriptions.
Most of the success in active restoration relies on seedling survival during the first years after establishment [13,14], where small seedlings struggle with the transplant shock. This is especially the case of Mediterranean-type clime zones, such as the one in central Chile, where post-planting seedling survival depends mainly on the soil water availability, the susceptibility of different plant species to drought, and the effectiveness of the establishment prescriptions addressed to ameliorate that stress. In this context, irrigation of seedlings during the first growing seasons in this climate type has been found to be crucial for improving survival and growth [11,12,15]. Depending on the duration and intensity of drought, plant mortality is caused due to a dysfunction of the xylem hydraulic conductivity (i.e., hydraulic failure) and through the stomatal closure, which negatively affects the carbon balance (i.e., carbon starvation) [16,17]. In this regard, plant species have different mechanisms to cope with drought (i.e., escaping, avoidance, and tolerance) [18,19], but their response to irrigation is still poorly understood in sclerophyllous species. The few studies addressing the responses of sclerophyllous species of central Chile to drought have been mostly carried out on few species under different experimental conditions [20,21,22,23,24], which complicates the comparison between species and studies. Thus, there is a need to expand this type of research to a higher number of species [25] since the restoration of this type of ecosystem must be addressed toward the development of mixed forests.
Excess irradiation and temperature may also exacerbate water stress [10,13], leading plants to their physiological limits determining growth and survival [26]. Plant species have different adaptations to cope with the light spectrum in their lifetime. Depending on their capacity to develop and grow in environments with low light, they are classified as shade-tolerant or intolerant species [27]. Regardless of the shade tolerance of sclerophyllous species, shading by using any type of tree shelter has become a more common practice in restoration projects in Mediterranean-type climates, improving seedlings’ performance [13]. Operationally, and depending on the shelter type, shading improves the microclimate conditions that lead to lower evaporative demand [28], such as lower air temperatures and higher humidity [13]. The acclimation of plants to shade includes a change in stem slenderness (higher height and lower diameter growth) [29]; a decrease in root growth in shade-intolerant species but not in shade-tolerant species [30]; a decrease in photosynthetic rate, stomatal conductance, and photochemical efficiency [31,32,33]; and an increase in specific leaf area (SLA) and chlorophyll content [27,34]. However, under water deficit, plant short-term responses to shading are species-specific [31,35,36,37] since the acclimation responses to light and water restriction seem to be uncoupled for some species (i.e., no trade-off) [38,39].
In general, restoration programs in Mediterranean-type climates are not driven economically because most of the species are not of commercial interest. Moreover, management prescriptions such as irrigation and the use of shelters may be very expensive, both operationally and experimentally, which delays the effort in the ecosystem restoration. Thus, the screening of species in controlled conditions at the nursery level is encouraged. In this study, we established a nursery experiment with seven sclerophyllous forest species commonly found in the Mediterranean zone of central Chile, which differ in their shade tolerance. We hypothesized that the species differed in their response to shade and water restriction and that the combined effect of watering and shade will favor the shade-tolerant more than intolerant–intolerant species. In this context, the objective of this study was to assess the growth and short-term physiological acclimation of the species to water restriction and shading. This type of study may help to improve the prescriptions for seedling establishment in restoration programs in Mediterranean zones.
2. Results
The water restriction was successfully imposed for two months during the experiment, with the substrate moisture content in a range of 0.10–0.26 m3 m−3 and 0.27–0.39 m3 m−3 for T0 and T2, respectively (Figure 1A). Additionally, conditions in treatment T2 (shading and irrigation) led to a considerably decrease in the substrate temperature during the experiment (10 °C less, approximately) when compared to T0 (Figure 1B).
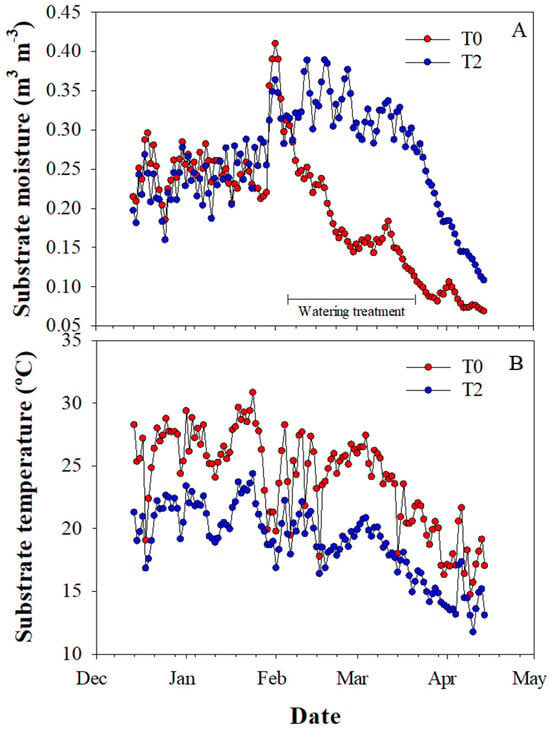
Figure 1.
Daily mean moisture (A) and temperature (B) of the substrate for the full (T2) and restricted (T0) water treatments during the experimental period.
Overall, seedling survival by species in the experiment was 100% for L. apiculata, and Q. saponaria, 94% for P. boldus and C. odorifera, 83% for L. caustica and A. chilensis, and 74% for E. pulverulenta. At the treatment level, survival was 98% for T2, 97% for T1, and 80% for T0. The analysis of variance showed a significant interaction between treatments and species for the height increments (Table 1), implying a differential response of the species to the treatments (Figure 2). In the experimental conditions, none of the species improved their height growth by the extra amount of water added, as expected (treatment T0 versus T1). Nonetheless, all the species had significant increments in height in T2, except P. boldus (Figure 2). The height increment responses of the species in T2 relative to the average of the other treatments were, from high to low, L. apiculata (203%), C. odorifera (158%), Q. saponaria (130%), L. caustica (125%), A. chilensis (104%), E. pulvurulenta (97%), and P. boldus (0%) (Figure 2).

Table 1.
p-values on the analysis of variance for increment in height (IncH), specific leaf area (SLA), chlorophyll content index (CCI), light-saturated photosynthetic rate (Asat), stomatal conductance (gs), intrinsic water use efficiency (WUEint), and xylematic hydric potential (Ψpot). Significant effects (p-values < 0.05) are shown in bold font.
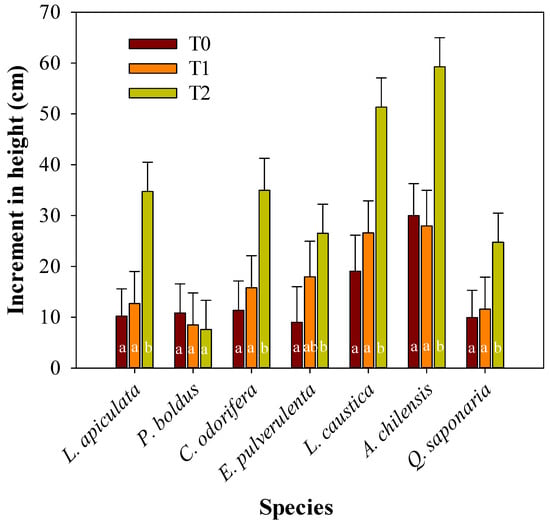
Figure 2.
Mean height increment during the experimental period per plant species and treatment (T0, T1, and T2). Different letters denote significant differences among treatments for a specific plant species according to Tukey’s test, with a significance level of 0.05.
At the leaf level, significant differences in SLA were found among species (Table 1 and Figure 3A). A. chilensis had the highest SLA, whereas C. odorifera, L. caustica, and Q. saponaria had the lowest values, with L. apiculata, P. boldus, and E. Pulverulenta having intermediate SLA values (Figure 3A). There was also a main effect of the treatments on SLA (Table 1), with no differences between T0 (56 cm2 g−1) and T1 (61.6 cm2 g−1) and with a significant increase in SLA in treatment T2 (101.7 cm2 g−1). Similarly, species significantly differed in CCI, with L. apiculata having over 3-fold more chlorophyll than other species such as E. pulverulenta and A. chilensis, whereas other species had intermediate values (Table 1 and Figure 3B). We did not observe any effect of the treatments on CCI (Table 1, p = 0.0907).
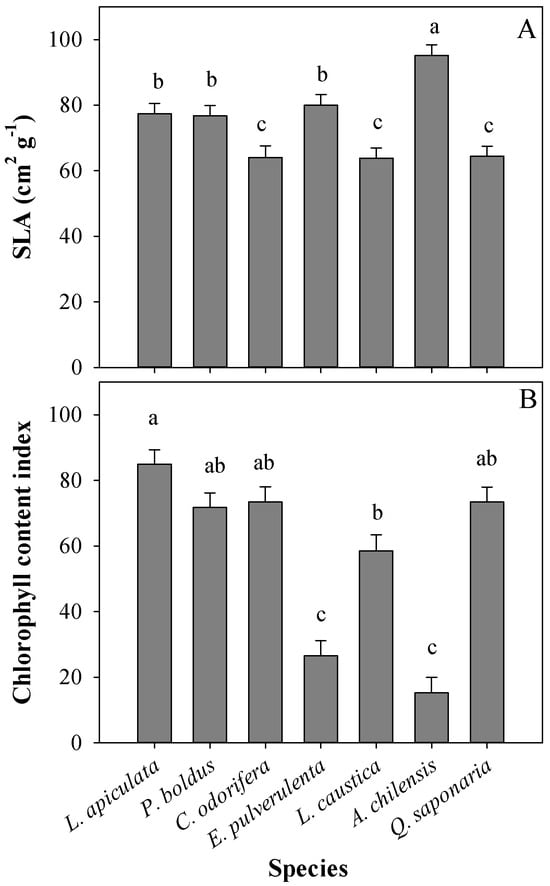
Figure 3.
Mean specific leaf area (SLA) (A) and chlorophyll content index (B) per plant species. Different letters denote significant differences among plant species according to Tukey’s test, with a significance level of 0.05.
Species did not differ in the responses in gas-exchange parameters (Asat, gs, and WUEint) to the treatments (no treatment by species interaction) (Table 1). However, the differences found both among species and among treatments varied over time (Table 1 and Figure 4). C. odorifera and L. caustica tended to have the highest Asat over the period, which almost doubled the species with the lowest values, L. apiculata and A. chilensis (Figure 4A). For gs, C. odorifera and L. apiculata tended to have the highest and lowest values during the period, respectively (Figure 4B). Species such as Q. saponaria and A. chilensis had intermediate values of gs during February, but they had an important decline in this parameter towards the middle of March. Overall, peaks of Asat and gs occurred in early March for L. apiculata, P. boldus, and E. pulverulenta relative to those of the other species.
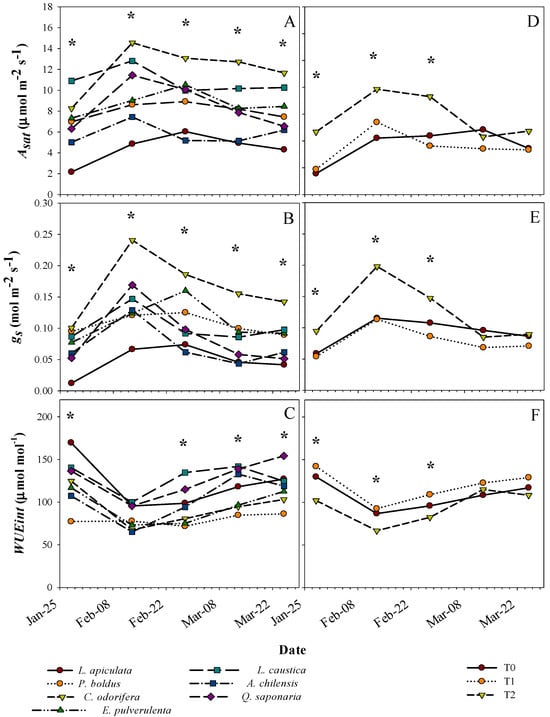
Figure 4.
Means for net saturated photosynthetic rate (Asat), stomatal conductance (gs), and intrinsic water use efficiency (WUEint) over time per plant species (left panels) and treatment (T0, T1, and T2) (right panels). * indicates significant differences between species and treatments at a specific date, respectively, with a significance level of 0.05.
On average, P. boldus had the lowest WUEint, where the highest values were observed in L. caustica, Q. saponaria, and L. apiculata (Figure 4C). C. odorifera and E. pulverulenta had almost the same WUEint values across the period, being among the lowest along with P. boldus. Otherwise, general differences in gas-exchange parameters between treatments were expressed only on measurements during February (Figure 4D,E). Similar to other traits, treatments T0 and T1 had similar gas-exchange parameters, but they significantly differed from T2 at some dates (Figure 4D,E). Asat and gs were higher in T2 than in the other treatments (Figure 4D,E), whereas the opposite was observed for WUEint (Figure 4F). Asat and gs were higher in T2 in February and decreased toward March, while in the other treatments, these parameters tended to be more stable across the period. Additionally, we found a differential response of the species in xylem water potential to the treatments across dates (Table 1 and Figure 5). Treatments differed in Ψx only on some dates, which vary with the species. The lowest and highest Ψx across the period were for treatments T0 and T2, respectively, whereas T1 tended to have intermediate values (Figure 5). The average Ψx for the last two dates (lowest values) for the control treatment T0 by species were, from low to high, E. pulvurulenta (−3.1 MPa), Q. saponaria (−2.5 MPa), L. apiculata (−2.3 MPa), A. chilensis (−2.1 MPa), L. caustica (−1.7 MPa), C. odorifera (−1.7 MPa), and P. boldus (−1.5) (Figure 5).
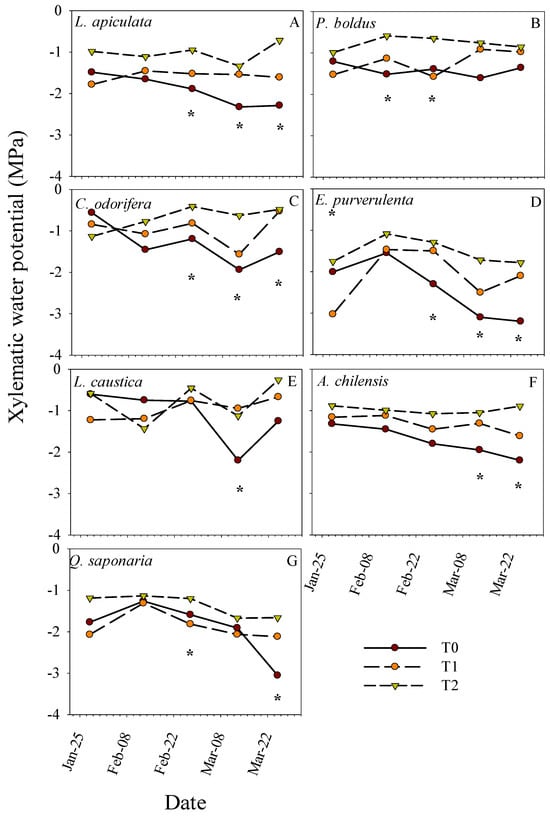
Figure 5.
Means for midday xylematic hydric potential per treatment (T0, T1, and T2) over time and separated by species. * indicates significant differences between treatments at a specific date, respectively, with a significance level of 0.05.
3. Discussion
The species assessed in this study are some of the most common in the sclerophyllous forests of the Mediterranean-type climate zone of central Chile, with some of them growing in close association depending on the geographic location [40]. Although this study was carried out at the nursery level, the experimental conditions mimic to some extent the establishment scenarios typically used in the restoration of this ecosystem type, which is the seedling establishment with some irrigation level and the incorporation of shading by using tree shelters. Overall, the evergreen species tested in this study exhibited great differences in growth and leaf morpho-physiological traits, but the way in which they responded to the treatments was similar, regardless of their shade or drought tolerance. Although a gradient was observed in the water stress imposed in the experiment, the acclimation response of the species was higher for shading than for the water restriction.
Because the shading dome in T2 was built seven months before imposing the water restriction treatments, we assumed the plants were already acclimated to shade during this period. Then, during summer, the mesh ventilation in T2 cooled the air inside, especially at midday as has been reported by [28], creating a microclimate that decreased water and heat stress and favored better growth and physiological performance for both shade- tolerant and intolerant species. This is also supported by the lowest substrate temperature being recorded in T2 relative to T0 across the period (Figure 1), likely favoring root growth and functions. In Vaccinuum spp., Spiers [41] found that root and shoot growth was higher at substrate temperatures around 16 °C than at 27 °C and 38 °C, values that are in the range for the monitored treatments in our study. On the contrary, the black color of the pots exacerbated the highest substrate temperatures in the sun-exposed treatments (T0 and T1). Black pots significantly increase the substrate temperature, which consequently may decrease the root density in different species [42,43]. Therefore, although root growth was not measured, the lack of differences in the assessed traits among treatments T0 and T1 might be due to poor root development as a consequence of the high temperatures, which prevented seedlings from taking advantage of the extra water added in T1 relative to T0.
Although the period of water restriction in this study was short, it was applied during midsummer, when the higher air temperatures predominate. We are aware that pot studies do not represent the real conditions in the field, and in dry years, the water stress may start earlier in spring, extending the duration of the drought. However, because our interest was to characterize the short-term acclimation responses of the species to drought as a strategy to cope with the stress, we tried to exacerbate the intensity more than the duration of the stress. Several studies showed acclimation to drought in these types of sclerophyllous species in similar periods [20,21,22,24,44]. Therefore, the water restriction at T0 should have been enough to provoke the water stress on seedlings, especially since they were not previously acclimated to this driest condition. This is supported by the xylem water potential observed at the species level, where the most stressed seedlings were in T0 (i.e., lower Ψx), followed by an ascendent gradient by T1 and T2. The fact that none of the species were able to efficiently use the extra amount of water in T1 relative to T0 may suggest that: (1) the degree of water restriction applied in T0 was just likely moderated and not enough to cause differences with T1; (2) the seedling stress by the irradiance and temperature in T1 (typically of potted studies as explained before) was high that was not alleviated by watering, which is supported by the great responses of the species to T2; or (3) seedlings used in the study were c.a., 70 cm in height with root collar diameter of c.a., 5 mm, which could have positively influenced the tolerance of T0 and T1 to water restrictions. Hence, all the species could hold their growth and physiological performance unaltered with water restrictions such as the one imposed by T0, with Ψx varying from −1.5 MPa in P. boldus to −3.1 MPa in E. pulverulenta. It is possible that the lower survival recorded in T0 relative to the other treatments was due to other ecophysiological processes not considered in this study, such as an increase in respiration rates by the highest temperature that led to a negative carbon budget, which needs further research. Although other studies in nursery and field trials have reported low pre-dawn potential for C. odorifera (−4.5 MPa, Montenegro and Riveros de la Puente [45]), Q. saponaria (−3.9 MPa, Donoso et al. [20]), P. boldus (−3.9 MPa, Peña-Rojas et al. [21]), L. caustica (−4.3 MPa, Peña-Rojas et al. [46]), and A. chilensis (−3.6 MPa, González-Villagra et al. [44]), it is not clear how species compare between them or how to use this information for management purposes, which was attempted in our study. A possible explanation for the low water potential reported by the latter authors could be the age and quality of seedlings at the beginning of their experiment in terms of height and diameter, which should be addressed in further studies.
Height increments in the shaded treatment were 94% to 203% higher than in the other treatments, except in P. boldus, which showed no response (0%). This null response of P. boldus may be explained by the slow growth of the species and the need for a longer acclimation period, which likely goes beyond the experimental period considered in this study. Moreover, the high xylem water potential reached by P. boldus in T0 suggests that this species was less susceptible to water restriction in our study. Similarly, Peña-Rojas et al. [21] found no differences in height increments in the species in well-watered and water-restricted treatments (−0.56 MPa vs. −3.9 MPa, respectively). Moreover, Vogel et al. [47] found that growth and leaf production in the species were not altered by shading or irrigation after four seasons, which agrees with our findings.
Evergreenness is a strategy that allows plants to grow the entire year [6], and in sclerophyllous species, it is an adaptation that also serves to cope with drought during summer [25]. Other adaptations of sclerophyllous species are reduced leaf area [48], stomatal control over transpiration [49], and the ease to extend roots towards new volumes of soil [50]. In our study, L. caustica and Q. saponaria are characterized by a deep root system as an important adaptation to survive the long summer periods [51,52,53]. However, both species exhibit no differences in height increment in the treatment T0, which corroborates that T0 imposed a moderate stress on the species under study. In addition, the fact that no species was found by treatment interaction for most leaf morpho-physiological traits (term ‘Trt × Spe’ in Table 2) suggests that the species under study have similar leaf acclimation to the stressor factors assessed, which is explained because all of them coexist under the same environmental conditions in Mediterranean-type climates. Among the most common traits reported as shade acclimation are an increase in SLA, an increase in chlorophyll content, and lower maximum net photosynthetic rates [27,33,34,54]. However, the magnitude of acclimation of different attributes and their phenotypic plasticity depend on the shade tolerance of the species [27], which did not coincide with our results since the leaf morphology of all the species was similarly affected by the treatments. In our study, the SLA in T2 almost doubled the values of the other treatments, whereas Asat was increased, and CCI showed no change with shading. The shading treatment intercepted approximately 80% of the photosynthetic active radiation (PAR), an interception value that is extremely high regarding the natural dispersion of sclerophyllous forests in central Chile and corroborates the need for sun protection at the early stages of development in the new environments created by the large-scale browning phenomenon on this ecosystem. This reinforces the phenotypic plasticity of all the species to shade at the seedling stage and that the lower Asat and gs in the sun-exposed treatments were likely limited due to other factors rather than water restriction, such as the excess of heat, irradiance, and substrate temperature in the pots. Moreover, we found a great variation in CCI among species (from c.a., 20 for species such as E. pulverulenta and A. chilensis to 85 for L. apiculata), which coincides with other studies showing high variation in chlorophyll content even in coexisting species [55]. Chen et al. [56] found a quick increase in chlorophyll content in response to shade (7 days) in tea leaves, whereas in our study, CCI was not increased by shading in any of the species. We argue that leaf chlorophyll content in evergreen species is not so sensitive to light compared to that in other species since leaves continue functioning over winter with low temperatures and light.

Table 2.
Life-form and functional group of the species under study.
Overall, drought combined with excess light induces photo oxidation and decreases water potential, stomatal conductance, and chlorophyll content, with further consequences in CO2 assimilation [57,58]. On the other hand, Marchin et al. [59] mentioned that some broadleaf evergreen species increase their stomatal conductance under extreme heat conditions to prevent damage by leaf overheating, but this accelerates dehydration and turgor loss. Nevertheless, neither of the above-mentioned claims precisely represent the results found in our study. First, Asat, gs, and WUEint did not vary between the water-restricted (T0) and well-watered (T1) treatments, likely for the above-mentioned explanations. Second, these parameters were relatively stable across the period in the sun-exposed treatments (T0 and T1), with higher fluctuations only in the shaded treatment, which reinforces that higher temperatures and excess of light are important factors limiting the physiological performance of these sclerophyllous evergreen species, even if water restriction is ameliorated. The values of gas-exchange parameters found in this study are in the range of other studies for some of the species, but especially for well-watered seedlings, with a considerable decrease in the parameters in more severe drought treatments [20,22,60]. Otherwise, it has been reported that sclerophyllous species with thicker and more-leathery leaves tend to have lower photosynthetic rates [6]. This was not the case for our findings, since, based on the SLA values, species such as A. chilensis would have thinner leaves (i.e., higher SLA) and lower Asat, whereas C. odorifera had the thicker leaves and the highest Asat among the species.
The effect of shading and watering on seedlings’ growth in Mediterranean climates depends mostly on what practice may better alleviate water stress. Excessive light and temperature may exacerbate the water stress on seedlings and, consequently, survival [13], which is ameliorated by using shelters in restoration programs. Regarding the current climate scenario, shading seems to be critical at the establishment phase to promote stem growth for both shade-tolerant and -intolerant species in this type of semi-arid region, which agrees with other studies in nursery [54,61] and field conditions [36,62,63]. Studies on other Mediterranean zones show that the use of shading may be effective only for drought-tolerant species, even when seedlings receive some pulses of watering during summer [13,37]. Nonetheless, Quiroz et al. [62] found that the shade-intolerant temperate tree Nothofagus alessandrii Espinosa in central Chile had better growth and survival responses to shading (via using shelters) than to irrigation during the first 5 years after outplanting, supporting our findings that at early stages, there is a need for sun protection. Based on some limitations of our study, future research must consider a longer time of experimentation, more precise monitoring of water restriction, incorporation of more integrated measures of water stress such as carbon isotope discrimination and non-structural carbon analysis, and carbon partitioning, especially to assess the effect of temperature on the root formation of the different species.
Our results suggest that in environments with restricted water availability, shading positively influences the early growth and physiological acclimation of sclerophyllous species of the Chilean forests. From the restoration point of view, a specific management prescription at establishment could be the use of tree shelters. The positive effects exerted by tree shelters in the initial establishment of small seedlings in sites with water restriction and high radiances are largely known. In the case of watering, despite there being several options from deep pipes to dew harvesters [64], this technique is still expensive at an operational scale, especially if the proposed species are not of commercial interest. There are also uncertainties with the constant and sufficient water supply during the dry season. Instead of watering seedlings in the field, it would be more efficient to apply polymer gels [65] to support and secure the establishment of seedlings the first year after outplanting.
4. Materials and Methods
4.1. Plant Material and Study Design
The study was conducted at the nursery facilities at the Universidad de Talca, Chile (35° 24′ S, 71° 38′ W, 112 m. asl). In the fall of 2020, seedlings of seven tree sclerophyllous species (grown in 1 L plastic bags) were obtained from local commercial nurseries. The species corresponded to Quillaja saponaria Molina (Quillay), Aristotelia chilensis (Mol.) Stuntz (Maqui), Peumus boldus Molina (Boldo), Lithraea caustica (Mol.) Hook. and Arn (Litre), Luma apiculata (DC.) Burret (Arrayán), Colliguaja odorifera Molina (Colliguay), and Escallonia pulverulenta (Ruiz and Prav.) Pers (Corontillo). We checked that all seedlings were in good health conditions. Species life forms and functional groups (i.e., shade tolerance) are presented in Table 2. In these new nursery conditions, seedlings were acclimated for 1 month. Afterward, they were transplanted into 11 L black pots. At this stage, all seedlings were 3 years old and produced from seeds, except A. chilensis, whose plants were 2 years old and propagated vegetatively from 5 clonal genotypes. In the nursery, the experiment was established under outdoor conditions (i.e., environmental conditions of the site). The substrate corresponded to a mixture of decomposed pine bark (90%) and perlite (particle size 0.5–3 mm, 10%). We added 15 g per seedling of slow-released fertilizer (Basacote Plus 9M, Compo Expert S.A. Jalisco, Mexico), which provided macro- and micronutrients.
The experimental design was a split-plot design, arranged in a complete randomized design (CRD), with six replicates (single tree plot). Three treatments representing different scenarios at establishment in restoration programs were the whole plot, whereas the plant species were the split-plot (i.e., 3 treatments × 7 plant species × 6 replicates = 126 seedlings in total). Seedlings were separated enough to avoid potential shading between plants. The treatments were: T0: sun-exposed and water-restricted, T1: sun-exposed and fully irrigated, and T2: shaded and fully irrigated. The treatments were designed to assess the separated additive effect of watering and shading. All seedlings were equally watered to maintain the substrate at field capacity until the end of January 2021 (mid-summer), when the water-restricted treatment (T0) started. Afterward, T0 was watered at a dose of 250 mL of water every other day, whereas seedlings in the fully irrigated treatments (T1 and T2) were watered daily to substrate field capacity (700 mL per pot, approximately). This watering regime remained until mid-March and then was cut off. During the experiment, the substrate moisture and temperature were monitored on an hourly basis with a Thetaprobe soil moisture sensor (Delta-T Devices Ltd., Cambridge, UK) in the most extreme treatments, T0 and T2 (Figure 1). For the shading treatment T2, in winter 2020, seedlings were covered by a 14 m3 dome built with 80% black polyethylene mesh (Raschel®, Santiago, Chile) and secured with 1.3 cm diameter polyvinyl chloride (PVC). This gave six months of shade acclimation before the other treatments were imposed. We chose this type of net as it represents one of the cheaper options for building shelters in this ecosystem.
4.2. Growth and Physiological Measurements
Seedlings’ height was measured with a metric tape at the beginning and the end of the watering treatment application to obtain the increments in height (IncH). Since the application of the water restriction, leaf-level physiological measurements were obtained in five instances (from 28 January to 25 March 2021) on 4 plants per treatment and species combination. During this period, the average temperature and relative humidity were 18.5 °C and 71.8%, respectively. Net saturated photosynthesis rate (Asat, μmol m−2 s−1), stomatal conductance (gs, mmol m−2 s−1), and intrinsic water use efficiency (WUEint, μmol mmol−1) were measured for one fully expanded leaf in the upper third of the plant, using a portable gas-exchange system LI 6800 (LICOR Inc., Lincoln, NE, USA). Initial chamber conditions were set up at ambient conditions during measurements, with a temperature of 20 °C, CO2 concentration of 400 ppm, relative humidity of 50%, and PAR of 1800 µmol m−2 s−1. Measurements were performed between 09.00 and 12.00 h at local time. On the same dates, midday xylem water potential (Ψx) was measured on one leaf using a Scholander pressure chamber (PMS Instrument Co., Albany, OR, USA). Before the measurements, the attached leaves were covered with a plastic sheet and wrapped in aluminum paper to equalize the water potential between the leaf and stem. Additionally, at each date, we measured the chlorophyll content index (CCI) using the chlorophyll meter MC-100 (Apogee Instruments, Inc., Logan, UT, USA). At the end of the growing period, we collected foliage from the different seedlings, which were scanned to determine leaf area using the software Image J 1.46r (developed by the National Institute of Health). Then, the foliage was oven-dried at 65 °C to a constant weight to determine the specific leaf area (SLA, leaf area to dry weight ratio).
4.3. Statistical Analysis
Data analyses were made with the PROC MIXED procedure of the software SAS 9.4 (SAS Institute Inc., Cary, NC, USA). We used a split-plot design analysis for more integrated measurements such as IncH and SLA, with factor treatment, species, and their interaction being considered fixed effects. For measurements monitored through time (leaf physiological traits and CCI), we expanded the model to a repeated measures analysis, with the factors treatment, species, date, and the interaction considered fixed effects. To improve the inference over the repeated measures analysis, preliminarily for each trait we modeled different variance–covariance structures (compound symmetry, unstructured, and first-order autoregressive) for the residual effects and selected the best model according to the Akaike information criteria. When needed, we used Tukey’s test for mean multiple comparisons. Significant differences were considered at a probability level of 0.05.
5. Conclusions
The evergreen sclerophyllous species tested in this study exhibited great differences in growth and leaf-morpho-physiological traits. However, the way in which they responded to the treatments was similar in six of the seven species, regardless of their shade or drought tolerance. P. boldus was the exception, and its null response in growth to the treatment was likely due to its slow growth and the need for a longer period of acclimation to the stressors factors. Moreover, species acclimation was higher for the shading than for the watering treatments. Given the excess of temperature and light in the Mediterranean zone of central Chile, the use of shading (i.e., via shelters) should be mandatory for these species; otherwise, irrigation might be insufficient to secure seedling establishment. Regarding the high costs involved in ecosystem restoration, species-specific responses to establishment treatments must be identified to increase the success of seedling establishment after outplanting, first with screening trials in nursery experiments and then validation in field conditions.
Author Contributions
Conceptualization, M.A.Y. and S.E.E.; methodology M.A.Y. and E.M.-H.; formal analysis, M.A.Y.; writing—original draft preparation, M.A.Y. and E.M.-H.; writing—review and editing, M.A.Y., S.E.E., C.R.M., and E.M.-H.; funding acquisition, M.A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chilean government through the Agencia Nacional de Investigacion y Desarrollo (ANID), throughout the “Programa FONDECYT Iniciacion en la Investigacion, año 2018”, Grant no. 11180259.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank all the people who contributed in some way to this research, among them Javier Urzua, and Vicente Silva. We acknowledge the Pantanillos Experimental Station from the Universidad de Chile for providing us with seedlings of some of the species under study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- David, T.; Pinto, C.; Nadezhdina, N.; David, J. Water and Forests in the Mediterranean Hot Climate Zone: A Review Based on a Hydraulic Interpretation of Tree Functioning. For. Syst. 2016, 25, eR02. [Google Scholar] [CrossRef]
- Armesto, J.; Arroyo, M.T.K.; Hinojosa, L. The Mediterranean Environment of Central Chile. In The Physical Geography os South America; Oxford University Press: New York, NY, USA, 2007; pp. 184–199. [Google Scholar]
- Bannister, J.R.; Vidal, O.J.; Teneb, E.; Sandoval, V. Latitudinal Patterns and Regionalization of Plant Diversity along a 4270-Km Gradient in Continental Chile. Austral Ecol. 2012, 37, 500–509. [Google Scholar] [CrossRef]
- Cartes, E.; Alvarez, C.; Acevedo, M.; Gonzalez, M.; Urbina-Parra, A.; León-Lobos, P. Pre-Germination Treatments at Operational Scale for Six Tree Species from the Sclerophyll Forest of Central Chile. Plants 2022, 11, 608. [Google Scholar] [CrossRef]
- Rundel, P.; Arroyo, M.; Cowling, R. Mediterranean Biomes: Evolution of Their Vegetation, Floras, and Climate. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- Rundel, P.W.; Sharifi, M.R.; Vu, M.K.; Montenegro, G.; Mooney, H.A. Seasonal Patterns of Growth Phenology and Nutrient Dynamics in Four Matorral Shrubs in Central Chile. Gayana Botánica 2019, 76, 208–219. [Google Scholar] [CrossRef]
- Miller, P. Resource Use by Chaparral and Matorral: A Comparison of Vegetation Function in Two Mediterranean Type Ecosystems; Springer: New York, NY, USA, 1981. [Google Scholar]
- Miranda, A.; Lara, A.; Altamirano, A.; Di Bella, C.; González, M.E.; Julio Camarero, J. Forest Browning Trends in Response to Drought in a Highly Threatened Mediterranean Landscape of South America. Ecol. Indic. 2020, 115, 106401. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Alvarez-Garreton, C.; Barichivich, J.; Boisier, J.P.; Christie, D.; Galleguillos, M.; LeQuesne, C.; McPhee, J.; Zambrano-Bigiarini, M. The 2010–2015 Megadrought in Central Chile: Impacts on Regional Hydroclimate and Vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef]
- Benayas, J.; López-Pintor, A.; García, C.; Cámara, N.; Strasser, R.; Sal, A. Early Establishment of Planted Retama sphaerocarpa Seedlings under Different Levels of Light, Water and Weed Competition. Plant Ecol. 2002, 159, 201–209. [Google Scholar] [CrossRef]
- Siles, G.; Rey, P.J.; Alcántara, J.M.; Bastida, J.M.; Herreros, J.L. Effects of Soil Enrichment, Watering and Seedling Age on Establishment of Mediterranean Woody Species. Acta Oecologica 2010, 36, 357–364. [Google Scholar] [CrossRef]
- Becerra, P.; Cruz, G.; Ríos, S.; Castelli, G. Importance of Irrigation and Plant Size in the Establishment Success of Different Native Species in a Degraded Ecosystem of Central Chile. Bosque 2013, 34, 103–111. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Dios Miranda, J.; Ortega, R.; Hervás, M.; Sánchez, J.; Pugnaire, F.I. Does Shelter Enhance Early Seedling Survival in Dry Environments? A Test with Eight Mediterranean Species. Appl. Veg. Sci. 2011, 14, 31–39. [Google Scholar] [CrossRef]
- Grossnickle, S. Seedling Establishment on a Forest Restoration Site—An Ecophysiological Perspective. Reforesta 2018, 6, 110–139. [Google Scholar] [CrossRef]
- Bown, H.; Fuentes, J.; Martínez, A. Assessing Water Use and Soil Water Balance of Planted Native Tree Species under Strong Water Limitations in Northern Chile. New For. 2018, 49, 871–892. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.; Allen, C.; Breshears, D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; William, D.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Time, A.; Garrido, M.; Acevedo, E.; Time, A.; Garrido, M.; Acevedo, E. Water Relations and Growth Response to Drought Stress of Prosopis Tamarugo Phil. A Review. J. Soil Sci. Plant Nutr. 2018, 18, 329–343. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Salleo, S. Different Strategies of Drought Resistance in Three Mediterranean Sclerophyllous Trees Growing in the Same Environmental Conditions. New Phytol. 1988, 108, 267–276. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef]
- Donoso, S.; Peña, K.; Pacheco, C.; Luna, G.; Aguirre, A. Respuesta Fisiológica y de Crecimiento En Plantas de Quillaja Saponaria y Cryptocarya Alba Sometidas a Restricción Hídrica. Bosque 2011, 32, 187–195. [Google Scholar] [CrossRef]
- Peña-Rojas, K.; Donoso, S.; Gangas, R.; Durán, S.; Ilabaca, D. Efectos de La Sequia En Las Relaciones Hidricas, Crecimiento y Distribucion de Biomasa de Peumus Boldus Molina (Monimiaceae) Cultivadas En Vivero. Interciencia 2018, 43, 36–42. [Google Scholar]
- Magni, C.; Espinoza, S.; Poch, P.; Abarca, B.; Grez, I.; Martínez, E.; Yáñez, M.; Santelices, R.; Cabrera, A. Growth and Biomass Partitioning of Nine Provenances of Quillaja Saponaria Seedlings to Water Stress. South. For. J. For. Sci. 2019, 81, 103–109. [Google Scholar] [CrossRef]
- Alvarez-Maldini, C.; Acevedo, M.; Dumroese, R.K.; González, M.; Cartes, E. Intraspecific Variation in Drought Response of Three Populations of Cryptocarya alba and Persea Lingue, Two Native Species From Mediterranean Central Chile. Front. Plant Sci. 2020, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.; Arce, M.A.Y.; Díaz, C.R.M.; Herrera, E.E.M.; Ortega, J.F.O.; Miranda, S.A.V. Growth of Provenances of Cryptocarya alba during Water Stress and after Re–Watering in the Nursery. Sci. Agric. 2021, 78, e20200292. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Opportunities and Threats of Mediterranean Evergreen Sclerophyllous Woody Species Subjected to Extreme Drought Events. Appl. Sci. 2020, 10, 8458. [Google Scholar] [CrossRef]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for Drought Assessment in the Mediterranean Region under Future Climate Scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Devine, W.; Harrington, C. Influence of Four Tree Shelter Types on Microclimate and Seedling Performance of Oregon White Oak and Western Redcedar; USDA Forest Service: Washington, DC, USA, 2008; p. 42. [Google Scholar]
- Oliet, J.A.; Planelles, R.; Artero, F.; Jacobs, D.F. Mesh-Shelters Provide More Effective Long-Term Protection than Tube-Shelters or Mulching for Restoration of Pinus Halepensis in a Mediterranean Arid Ecosystem. Front. For. Glob. Change 2023, 5, 1092703. [Google Scholar] [CrossRef]
- Puértolas, J.; Oliet, J.A.; Jacobs, D.F.; Benito, L.F.; Peñuelas, J.L. Is Light the Key Factor for Success of Tube Shelters in Forest Restoration Plantings under Mediterranean Climates? For. Ecol. Manag. 2010, 260, 610–617. [Google Scholar] [CrossRef]
- Valladares, F.; Zaragoza-Castells, J.; Sánchez-Gómez, D.; Matesanz, S.; Alonso, B.; Portsmuth, A.; Delgado, A.; Atkin, O.K. Is Shade Beneficial for Mediterranean Shrubs Experiencing Periods of Extreme Drought and Late-Winter Frosts? Ann. Bot. 2008, 102, 923–933. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Montanaro, G.; Xiloyannis, C. Shade Effect on Photosynthesis and Photoinhibition in Olive during Drought and Rewatering. Agric. Water Manag. 2009, 96, 1201–1206. [Google Scholar] [CrossRef]
- Díaz-Barradas, M.C.; Zunzunegui, M.; Alvarez-Cansino, L.; Esquivias, M.P.; Valera, J.; Rodríguez, H. How Do Mediterranean Shrub Species Cope with Shade? Ecophysiological Response to Different Light Intensities. Plant Biol. 2018, 20, 296–306. [Google Scholar] [CrossRef]
- Pallardy, S. Physiology of Woody Plants, 3rd ed.; Elsevier Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- Prider, J.; Facelli, M. Interactive Effects of Drought and Shade on Three Arid Zone Chenopod Shrubs with Contrasting Distributions in Relation to Tree Canopies. Funct. Ecol. 2004, 18, 67–76. [Google Scholar] [CrossRef]
- Oliet, J.A.; Puértolas, J.; Valenzuela, P.; Vázquez de Castro, A. Light Transmissivity of Tree Shelters Interacts with Site Environment and Species Ecophysiology to Determine Outplanting Performance in Mediterranean Climates. Land 2021, 10, 753. [Google Scholar] [CrossRef]
- Rojas-Arévalo, N.; Ovalle, J.F.; Oliet, J.A.; Piper, F.I.; Valenzuela, P.; Ginocchio, R.; Arellano, E.C. Solid Shelter Tubes Alleviate Summer Stresses during Outplanting in Drought-Tolerant Species of Mediterranean Forests. New For. 2022, 53, 555–569. [Google Scholar] [CrossRef]
- Martínez-Tillería, K.; Loayza, A.P.; Sandquist, D.R.; Squeo, F.A. No Evidence of a Trade-off between Drought and Shade Tolerance in Seedlings of Six Coastal Desert Shrub Species in North-Central Chile. J. Veg. Sci. 2012, 23, 1051–1061. [Google Scholar] [CrossRef]
- Amissah, L.; Mohren, G.M.J.; Kyereh, B.; Poorter, L. The Effects of Drought and Shade on the Performance, Morphology and Physiology of Ghanaian Tree Species. PLoS ONE 2015, 10, e0121004. [Google Scholar] [CrossRef]
- Amigo, J.; Flores-Toro, L. Sclerophyllous Forests and Preforests of Central Chile: Lithraeion Causticae Alliance. Int. J. Geobot. Res. 2013, 3, 47–67. [Google Scholar] [CrossRef]
- Spiers, J.M. Substrate Temperatures Influence Root and Shoot Growth of Southern Highbush and Rabbiteye Blueberries. HortScience 1995, 30, 1029–1030. [Google Scholar] [CrossRef]
- Markham, J.W.; Bremer, D.J.; Boyer, C.R.; Schroeder, K.R. Effect of Container Color on Substrate Temperatures and Growth of Red Maple and Redbud. HortScience 2011, 46, 721–726. [Google Scholar] [CrossRef]
- Witcher, A.L.; Pickens, J.M.; Blythe, E.K. Container Type and Substrate Affect Root Zone Temperature and Growth of ‘Green Giant’ Arborvitae. Horticulturae 2020, 6, 22. [Google Scholar] [CrossRef]
- González-Villagra, J.; Rodrigues-Salvador, A.; Nunes-Nesi, A.; Cohen, J.D.; Reyes-Díaz, M.M. Age-Related Mechanism and Its Relationship with Secondary Metabolism and Abscisic Acid in Aristotelia chilensis Plants Subjected to Drought Stress. Plant Physiol. Biochem. 2018, 124, 136–145. [Google Scholar] [CrossRef]
- Montenegro, G.; Riveros de la Puente, F. Comparison of Differential Environmental Responses of Colliguaja Odorifera. Flora 1977, 166, 125–135. [Google Scholar] [CrossRef]
- Peña-Rojas, K.; Donoso, S.; Pacheco, C.; Riquelme, A.; Gangas, R.; Guajardo, A.; Durán, S.; Peña-Rojas, K.; Donoso, S.; Pacheco, C.; et al. Respuestas Morfo-Fisiológicas de Plantas de Lithraea Caustica (Anacardiaceae) Sometidas a Restricción Hídrica Controlada. Bosque (Valdivia) 2018, 39, 27–36. [Google Scholar] [CrossRef]
- Vogel, H.; González, B.; Razmilic, I. Boldo (Peumus boldus) Cultivated under Different Light Conditions, Soil Humidity and Plantation Density. Ind. Crops Prod. 2011, 34, 1310–1312. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Xu, L. What limits evaporation from Mediterranean oak woodlands—the supply of moisture in the soil, physiological control by plants or the demand by the atmosphere? Adv. Water Resour. 2007, 30, 2113–2122. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Stomatal response to drought at a Mediterranean site: A comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol. 2003, 23, 978–996. [Google Scholar] [CrossRef]
- Luis, V.C.; Puértolas, J.; Climent, J.; Peters, J.; González-Rodríguez, Á.M.; Morales, D.; Jiménez, M.S. Nursery fertilization enhances survival and physiological status in Canary Island pine (Pinus canariensis) seedlings planted in a semiarid environment. Eur. J. For. Res. 2009, 128, 221–229. [Google Scholar] [CrossRef]
- Giliberto, J.; Estay, H. Seasonal water stress in some chilean matorral shrubs. Bot. Gaz. 1978, 139, 236–240. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kummerow, J. Root Studies in the Chilean Matorral. Oecologia 1978, 69, 57–69. [Google Scholar] [CrossRef]
- Ovalle, J.F.; Arellano, E.C.; Ginocchio, R. Trade-Offs between Drought Survival and Rooting Strategy of Two South American Mediterranean Tree Species: Implications for Dryland Forests Restoration. Forests 2015, 6, 3733–3747. [Google Scholar] [CrossRef]
- Yáñez, M.A.; Gómez, P.; Gajardo, J.; Espinoza, S. Growth and Physiological Acclimation to Shade in Young Plants of Adesmia Bijuga Phil., a Critically Endangered Species in Central Chile. iForest Biogeosciences For. 2021, 14, 307–312. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism Underlying the Shading-Induced Chlorophyll Accumulation in Tea Leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Marchin, R.; Backes, D.; Ossola, A.; Leishman, M.; Tjoelker, M.; Ellsworth, D. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Change Biol. 2021, 28, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.A.; Urzua, J.I.; Espinoza, S.E.; Peña, V.L. Limited Phenotypic Variation in Vulnerability to Cavitation and Stomatal Sensitivity to Vapor Pressure Deficit among Clones of Aristotelia chilensis from Different Climatic Origins. Plants 2021, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.; González, B.; Doll, U.; Yuri, J.A.; Vogel, H. Different Covers Affect Growth and Development of Three Maqui Clones (Aristotelia chilensis [Molina] Stuntz). J. Berry Res. 2019, 9, 449–458. [Google Scholar] [CrossRef]
- Quiroz, I.A.; Espinoza, S.E.; Yáñez, M.A.; Magni, C.R. The Use of Shelters Improves Growth and Survival of the Endangered Nothofagus alessandrii after 5 Years in a Mediterranean Drought-Prone Site. Ecol. Eng. 2021, 164, 106220. [Google Scholar] [CrossRef]
- Mohsin, F.; Arias, M.; Albrecht, C.; Wahl, K.; Fierro-Cabo, A.; Christoffersen, B. Species-Specific Responses to Restoration Interventions in a Tamaulipan Thornforest. For. Ecol. Manag. 2021, 491, 119154. [Google Scholar] [CrossRef]
- Martínez de Azagra Paredes, A.; Del Río San José, J.; Reque Kilchenmann, J.; Diez Hernández, J.M.; Sanz Ronda, F.J. Methods for Watering Seedlings in Arid Zones. Forests 2022, 13, 351. [Google Scholar] [CrossRef]
- Apostol, K.G.; Jacobs, D.F.; Dumroese, R.K. Root desiccation and drought stress responses of bareroot Quercus rubra seedlings treated with a hydrophilic polymer root dip. Plant Soil 2019, 315, 229–240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).