Flowering Periods, Seed Yield Components, Seed Quality, and Patterns of Seed Shattering in Paspalum: Effect of Taxonomy and Nitrogen Fertilization
Abstract
:1. Introduction
2. Results
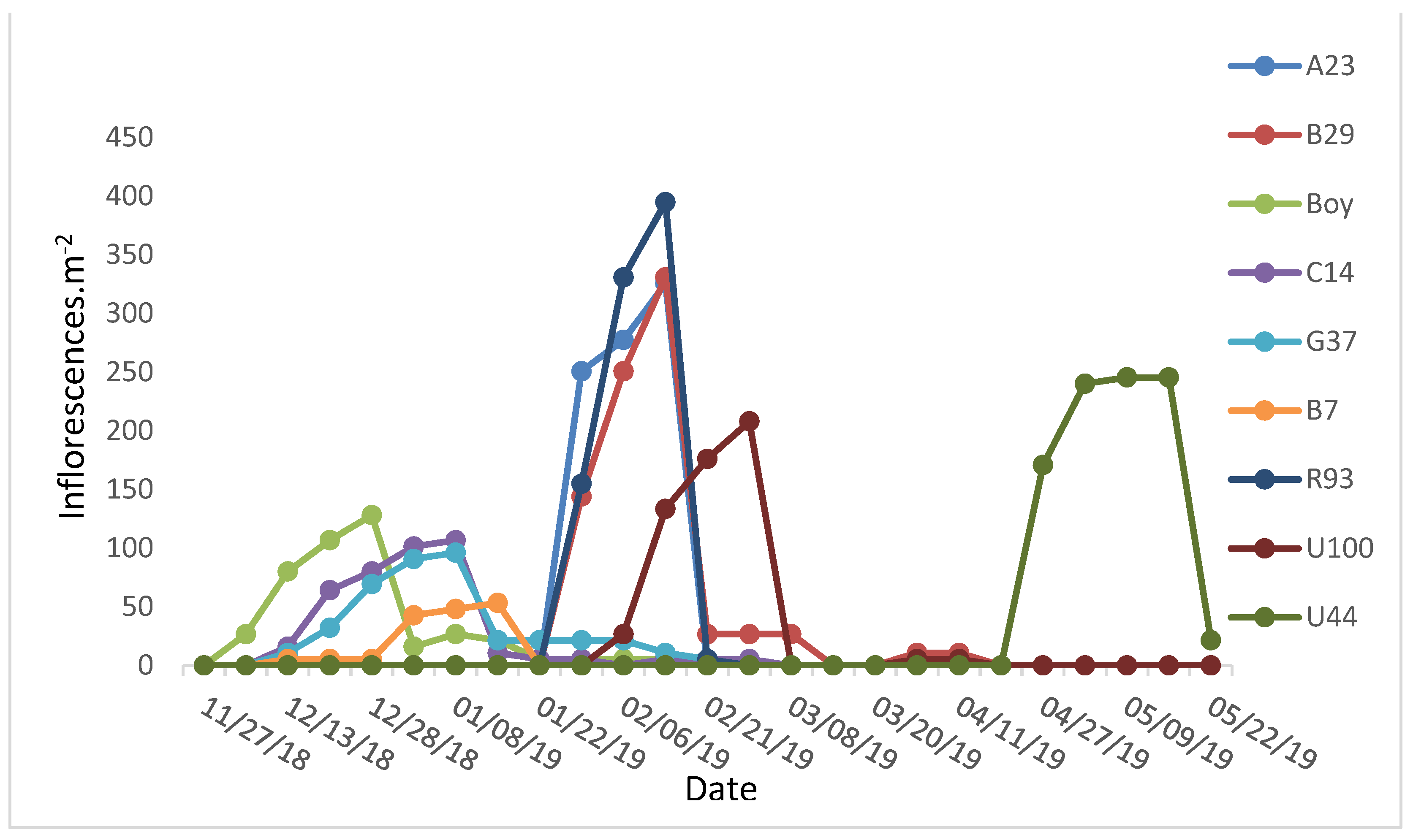
2.1. Flowering Period
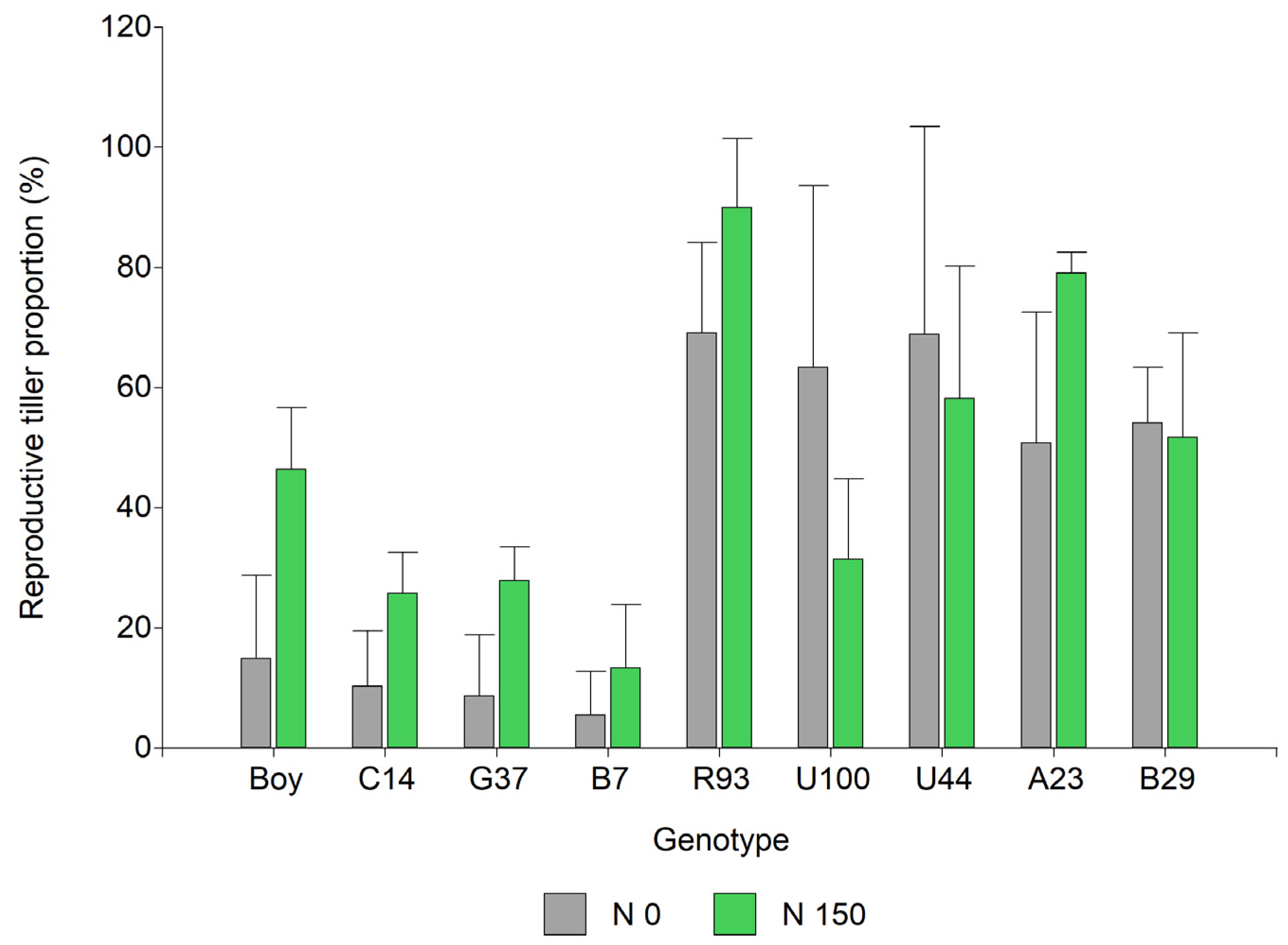
2.2. Seed Yield and Yield Components
2.3. Seed Dimensions and Quality
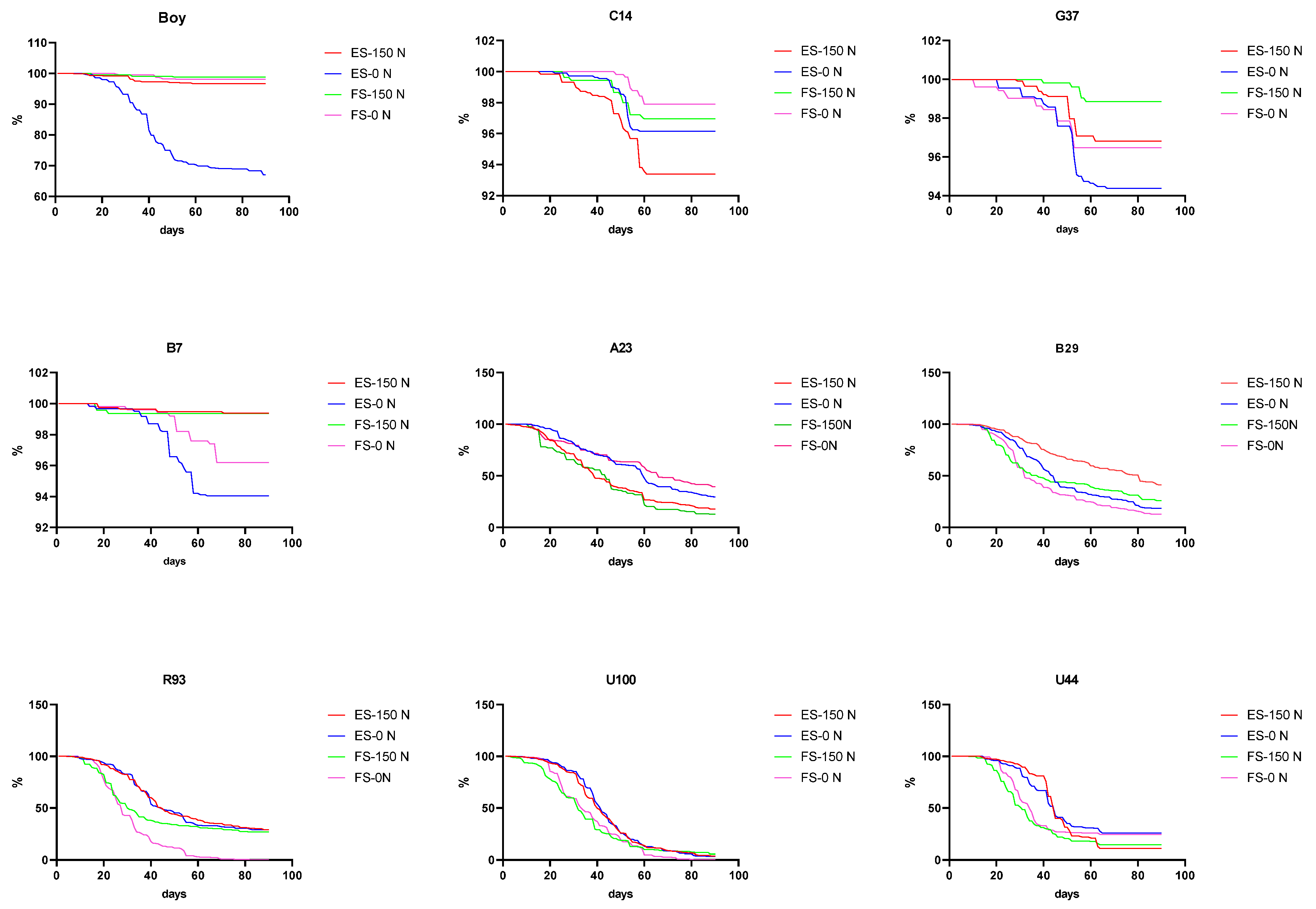
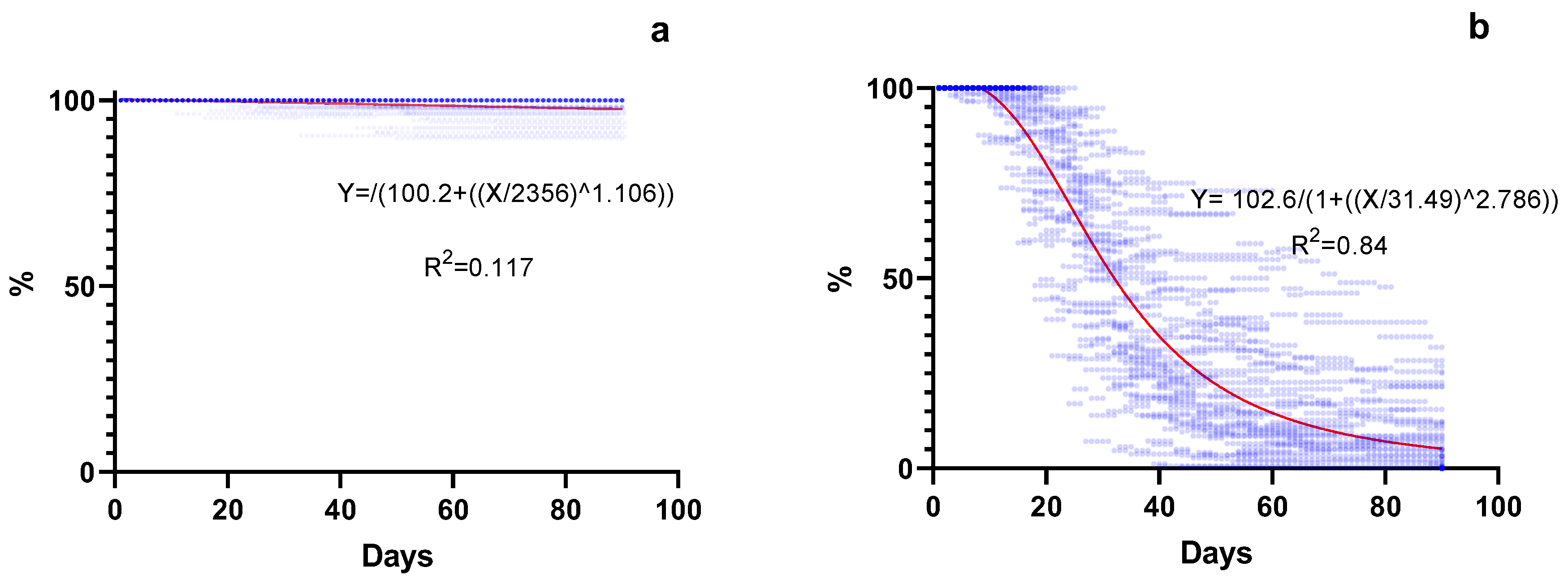
2.4. Seed Shattering
2.5. Relationship among Evaluated Variables
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Evaluated Variables
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moser, L.E.; Burson, B.L.; Sollenberger, L.E. Warm-season (C4) grass overview. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; Volume 45, pp. 1–14. [Google Scholar]
- Andrade, R.P. Pasture seed production technology in Brazil. In Proceedings of the XIX International Grassland Congress: Grassland ecosystems, São Pedro, São Paulo, Brazil, 11–21 February 2001. [Google Scholar]
- Vogel, K.P.; Burson, B.L. Breeding and genetics. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; Volume 45, pp. 51–96. [Google Scholar]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Loch, D.S.; Adkins, S.W.; Heslehurst, M.R.; Paterson, M.F.; Bellairs, S.M. Seed formation, development, and germination. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; Volume 45, pp. 95–144. [Google Scholar]
- Acuña, C.A.; Martínez, E.J.; Zilli, A.L.; Brugnoli, E.A.; Espinoza, F.; Marcón, F.; Urbani, M.H.; Quarin, C.L. Reproductive systems in Paspalum: Relevance for germplasm collection and conservation, breeding techniques, and adoption of released cultivars. Front. Plant Sci 2019, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Hare, M.D.; Wongpichet, K.; Saengkham, M.; Thummasaeng, K.; Suriyajantratong, W. Juvenility and long-short day requirement in relation to flowering of Paspalum atratum in Thailand. Trop. Grassl. 2001, 35, 139–143. [Google Scholar]
- Evers, G.W.; Burson, B.L. Dallisgrass and other Paspalum species. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; Volume 45, pp. 681–713. [Google Scholar]
- Zilli, A.L.; Brugnoli, E.A.; Marcón, F.; Billa, M.B.; Rios, E.F.; Martínez, E.J.; Acuña, C.A. Heterosis and expressivity of apospory in tetraploid bahiagrass hybrids. Crop Sci. 2015, 55, 1189–1201. [Google Scholar] [CrossRef]
- Novo, P.E.; Acuña, C.A.; Quarin, C.L.; Urbani, M.H.; Marcón, F.; Espinoza, F. Hybridization and heterosis in the Plicatula group of Paspalum. Euphytica 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Rufty, T.W. Nitrogen and water resources commonly limit crop yield increases, not necessarily plant genetics. Glob. Food Secur. 2012, 1, 94–98. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- Rios, E.F.; Zilli, A.; Kenworthy, K.E.; Mackowiak, C.; Quesenberry, K.; Blount, A. Managing forage and turf-type bahiagrass for seed production. Crop Sci. 2020, 60, 1569–1579. [Google Scholar] [CrossRef]
- Souza, C.H.L.; Motta, E.A.M.; Brunes, A.P.; Weiler, R.L.; Simioni, C.; Sampaio, R.; Rios, E.F.; Dall’Agnol, M. Seed yield and quality of Paspalum notatum Flügge intraspecific hybrids. Acta Sci. Agron. 2023, 46, e62530. [Google Scholar] [CrossRef]
- Schulz, R.R.; Zilli, A.L.; Brugnoli, E.A.; Marcón, F.; Acuña, C.A. Structural and morphogenetic characteristics in Paspalum notatum: Responses to nitrogen fertilization, season, and genotype. Plants 2023, 12, 2633. [Google Scholar] [CrossRef] [PubMed]
- Loch, D.S.; Souza, F.H.D. Seed harvesting and drying: Grasses. In Forage Seed Production; Volume 2: Tropical and Subtropical Species; Loch, D.S., Ferguson, J.E., Eds.; CAB International: Wallingford, UK, 1999; pp. 191–212. [Google Scholar]
- Miles, J.W.; Valle, C.B.; Rao, I.M.; Euclídes, V.P.B. Brachiariagrasses. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; Volume 45, pp. 754–783. [Google Scholar]
- Tomás, M.A.; Maina, M.; Lifschitz, M.E.; Armando, L.V.; Giordano, M.C. Seed yield potential improvement through breeding in Panicum coloratum var. makarikariense. Crop Pasture Sci. 2022, 74, 194–203. [Google Scholar] [CrossRef]
- Gates, R.N.; Quarin, C.L.; Pedreira, C.G.S. Bahiagrass. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; Volume 45, pp. 651–680. [Google Scholar]
- Boonman, J.G. Experimental studies on seed production of tropical grasses in Kenya. 2. Tillering and heading in seed crops of eight grasses. Neth. J. Agri. Sci. 1971, 19, 237–249. [Google Scholar] [CrossRef]
- Adjei, M.B.; Mislevy, P.; Chason, W. Seed yield of bahiagrass in response to sward management by phenology. Agron. J. 1992, 82, 599–603. [Google Scholar] [CrossRef]
- Adjei, M.B.; Mislevy, P.; Chason, W. Timing, defoliation management, and nitrogen effects on seed yield of ‘Argentine’ bahiagrass. Agron. J. 2000, 92, 36–41. [Google Scholar]
- Gates, R.N.; Burton, G.W. Seed yield and seed quality response of Pensacola and improved Bahiagrasses to fertilization. Agron. J. 1998, 90, 607–611. [Google Scholar] [CrossRef]
- West, S.H.; Marousky, F. Mechanism of dormancy in Pensacola Bahiagrass. Crop Sci. 1989, 29, 787–791. [Google Scholar] [CrossRef]
- Scienza, C.; Antoniolli, J.; Longhi, J.; Dias, L.W.; Silveira, D.C.; Sampaio, R.; de Ávila, V.S.; Escosteguy, L.A.; Simioni, C.; Weiler, R.L.; et al. Seed yield and quality of interspecific hybrids of Paspalum plicatulum × P. guenoarum at different harvest times. Crop Sci. 2024, 64, 697–709. [Google Scholar] [CrossRef]
- Urbani, M.H.; Acuña, C.A.; Doval, D.W.; Sartor, M.E.; Galdeano, F.; Blount, A.R.; Quesenberry, K.H.; Mackowiak, C.L.; Quarin, C.L. Registration of ‘Boyero UNNE’ Bahiagrass. J. Plant Regist. 2017, 11, 26–32. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. Available online: https://www.infostat.com.ar/ (accessed on 17 July 2024).



| ID | N Level | Seed Yield (g/m2) | N° Flowers/Raceme | N° F/i | 1000 Seed Weight (g) | Tillers /m2 | Vegetative Tillers/m2 | Reproductive Tillers/m2 | Seed Set (%) | G (%) | Rate G | Seed Length (mm) | Seed Width (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boy | 0 | 8.7 | 93 | 185 | 3.17 | 837 | 709 | 192 | 26 | 2.7 | 0.09 | 3.8 | 2.5 |
| 150 | 31.6 * | 91 | 181 | 2.86 | 1221 * | 651 | 571 * | 25 | 18 | 0.81 | 3.8 | 2.6 | |
| C14 | 0 | 3.8 | 84 | 168 | 3.49 | 1008 | 901 | 160 | 34 | 6 | 0.27 | 3.9 | 2.8 |
| 150 | 25 * | 89 | 180 | 3.52 | 1371 | 1003 | 464 * | 34 | 12.7 | 0.64 | 3.8 | 2.9 * | |
| G37 | 0 | 3.8 | 89 | 177 | 3.36 | 981 | 885 | 144 | 32 | 16.7 | 0.66 | 3.7 | 2.8 |
| 150 | 10.5 | 95 | 189 | 3.80 | 1050 | 763 | 288 | 32 | 15.3 | 0.56 | 3.9 * | 2.9 | |
| B7 | 0 | 1.5 | 86 | 171 | 3.34 | 944 | 891 | 80 | 29 | 4 | 0.20 | 3.9 | 2.6 |
| 150 | 4.5 * | 91 | 182 | 3.37 | 1173 | 1013 | 160 | 29 | 8.7 | 0.33 | 3.9 | 2.6 | |
| A23 | 0 | 37.6 | 115 | 376 | 2.99 | 619 | 293 | 325 | 22 | 21.3 | 1.59 | 3.5 | 2.0 |
| 150 | 41.5 | 115 | 372 | 3.13 | 1130 * | 240 | 890 * | 29 | 27.3 | 2.01 | 3.6 * | 2.0 | |
| B29 | 0 | 54.4 | 117 | 423 | 2.96 | 603 | 272 | 330 | 27 | 28.7 | 2.07 | 3.1 | 3.1 |
| 150 | 63.4 * | 121 | 485 | 2.98 | 661 | 315 | 347 | 27 | 30.7 | 2.49 | 3.1 | 3.1 | |
| R93 | 0 | 47.6 | 113 | 510 | 2.65 | 587 | 192 | 347 | 35 | 39.3 | 2.75 | 3.2 | 2.0 |
| 150 | 48.3 | 117 | 607 | 2.53 | 720 | 101 | 736 * | 23 | 30.3 | 2.35 | 3.2 | 2.0 | |
| U100 | 0 | 5.7 | 74 | 486 | 3.44 | 331 | 123 | 208 | 12 | 47.3 | 4.13 | 3.5 | 2.1 * |
| 150 | 13.1 | 84 | 549 | 3.36 | 848 * | 586 | 261 | 19 | 45.3 | 4.06 | 3.4 | 2.0 | |
| U44 | 0 | 54.9 | 77 | 438 | 3.15 | 544 | 245 | 299 | 36 | 44 | 3.72 | 2.0 | 3.0 |
| 150 | 68.4 | 74 | 458 | 3.20 | 880 | 432 | 448 | 42 | 39.3 | 3.27 | 2.0 | 3.0 | |
| MSD † | 0 | 30.1 | 13 | 76 | 0.65 | 577 | 524 | 299 | 22 | 20 | 1.4 | 0.14 | 0.12 |
| 150 | 24.4 | 12 | 88 | 0.60 | 714 | 524 | 393 | 12 | 28 | 2.1 | 0.12 | 0.11 |
| ID | N Level | E/S Retention 20 Days (%) | F/S Retention 20 Days (%) | E/S Retention 40 Days (%) | F/S Retention 40 Days (%) | E/S Retention 60 Days (%) | F/S Retention 60 Days (%) | E/S Retention 90 Days (%) | F/S Retention 90 Days (%) |
|---|---|---|---|---|---|---|---|---|---|
| Boy | 0 | 98 a† | 100 a | 81 abc | 100 a | 97 a | 98 a | 97 a | 98 a |
| 150 | 99 a | 100 a | 97 ab | 99 a | 99 a | 99 a | 99 a | 99 a | |
| C14 | 0 | 100 a | 100 a | 100 a | 100 a | 96 a | 98 a | 96 a | 98 a |
| 150 | 100 a | 100 a | 98 a | 100 a | 93 a | 97 a | 93 a | 97 a | |
| G37 | 0 | 100 a | 100 a | 99 a | 98 a | 95 a | 96 a | 95 a | 96 a |
| 150 | 100 a | 100 a | 99 a | 100 a | 97 a | 99 a | 98 a | 99 a | |
| B7 | 0 | 100 a | 100 a | 99 a | 100 a | 94 a | 98 a | 94 a | 96 a |
| 150 | 100 a | 100 a | 100 a | 100 a | 100 a | 99 a | 99 a | 99 a | |
| A23 | 0 | 96 ab | 86 a | 72 abc | 73 ab | 50 bcd | 62 b | 13 bc | 12 c |
| 150 | 86 b | 77 a | 49 c | 55 b | 28 cde | 23 cde | 19 cd | 13 c | |
| B29 | 0 | 93 ab | 89 a | 56 abc | 40 bcd | 31 cde | 26 cd | 8 de | 6 d |
| 150 | 95 ab | 80 a | 75 abc | 48 bcd | 59 bc | 40 c | 32 b | 19 b | |
| R93 | 0 | 95 ab | 78 a | 60 abc | 17 d | 42 bcde | 10 de | 5 cd | 3 de |
| 150 | 92 ab | 82 a | 59 abc | 35 cd | 35 cde | 28 cd | 4 cd | 2 de | |
| U100 | 0 | 95 ab | 85 a | 54 abc | 36 cd | 12 e | 5 e | 2 d | 1 de |
| 150 | 92 ab | 81 a | 53 bc | 30 cd | 16 de | 11 de | 3 d | 2 de | |
| U44 | 0 | 96 ab | 96 a | 67 abc | 33 cd | 31 cde | 25 cde | 0 d | 0 e |
| 150 | 97 ab | 88 a | 81 abc | 31 cd | 21 de | 18 de | 1 d | 1 de |
| ID | Origen | Flowering Period |
|---|---|---|
| Boy | P. notatum cv Boyero UNNE [26] | Early |
| C14 | Intraspecific hybrid of P. notatum [9] | |
| G37 | Intraspecific hybrid of P. notatum [9] | |
| B7 | Intraspecific hybrid of P. notatum [9] | |
| B29 | Hybrid between P. plicatulum and P. genoarum Baio [10] | Intermediate |
| R93 | Hybrid between P. plicatulum and P. genoarum Rojas GR19 [10] | |
| A23 | Hybrid between P. plicatulum and P. genoarum Azulao [10] | |
| U100 | P. guenoarum ecotype | |
| U44 | P. atratum ecotype | Late |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, L.L.; Brugnoli, E.A.; Zilli, A.L.; Schulz, R.R.; Marcón, F.; Acuña, C.A. Flowering Periods, Seed Yield Components, Seed Quality, and Patterns of Seed Shattering in Paspalum: Effect of Taxonomy and Nitrogen Fertilization. Plants 2024, 13, 2411. https://doi.org/10.3390/plants13172411
Chamorro LL, Brugnoli EA, Zilli AL, Schulz RR, Marcón F, Acuña CA. Flowering Periods, Seed Yield Components, Seed Quality, and Patterns of Seed Shattering in Paspalum: Effect of Taxonomy and Nitrogen Fertilization. Plants. 2024; 13(17):2411. https://doi.org/10.3390/plants13172411
Chicago/Turabian StyleChamorro, Luis Leandro, Elsa Andrea Brugnoli, Alex Leonel Zilli, Roberto Ramón Schulz, Florencia Marcón, and Carlos Alberto Acuña. 2024. "Flowering Periods, Seed Yield Components, Seed Quality, and Patterns of Seed Shattering in Paspalum: Effect of Taxonomy and Nitrogen Fertilization" Plants 13, no. 17: 2411. https://doi.org/10.3390/plants13172411






