Impact of Plant Community Diversity on Greenhouse Gas Emissions in Riparian Zones
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Greenhouse Gas (GHG) Emission and Global Warming Potential Measurement
2.3. Soil Samples Collection and Soil Parameters Measurement
2.4. Statistical Analysis
3. Results
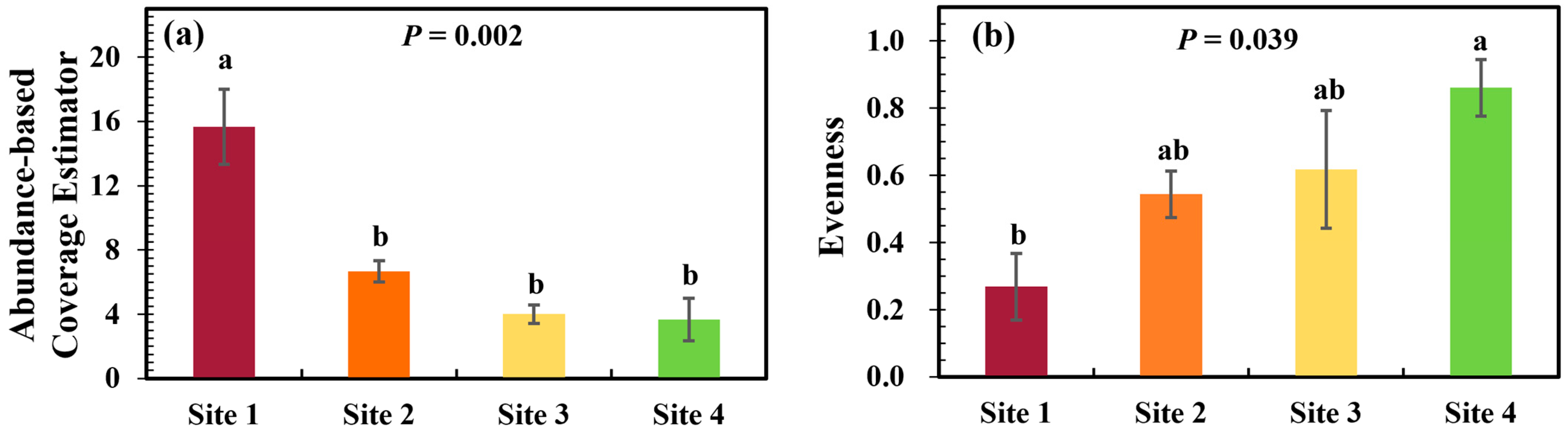
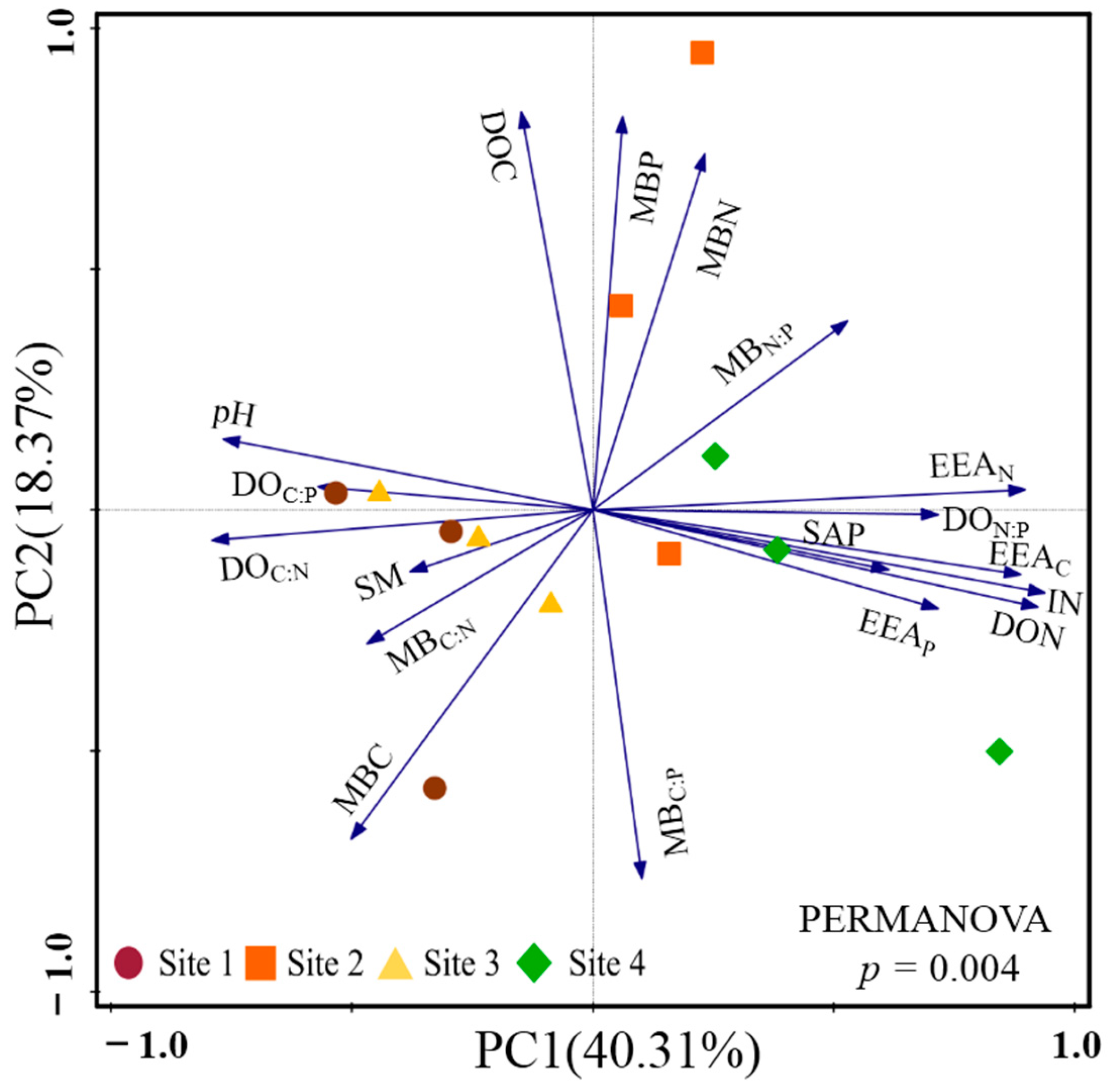
3.1. Plant Community Diversity and Soil Parameters
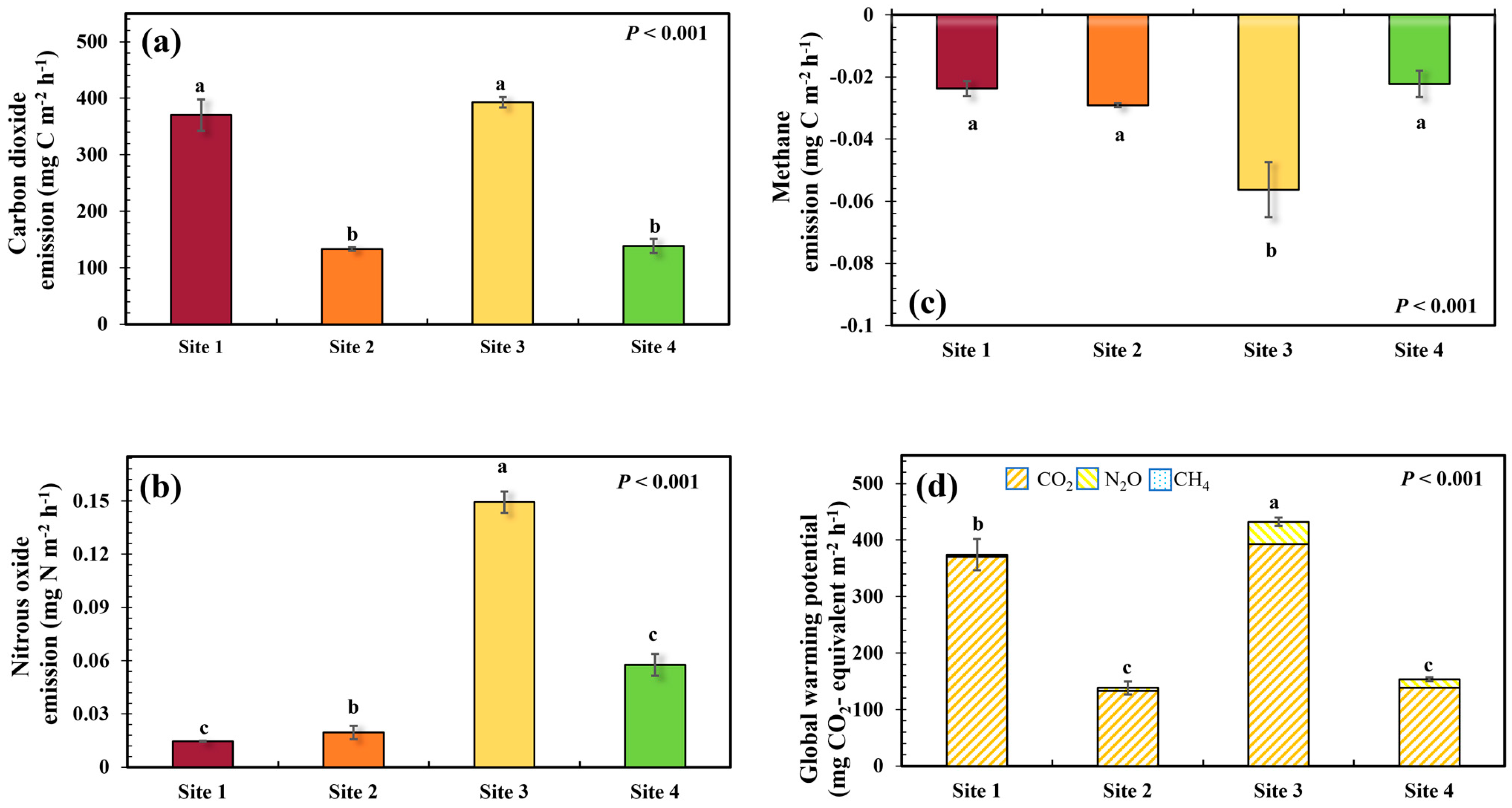
3.2. Soil GHG Emissions and Global Warming Potential
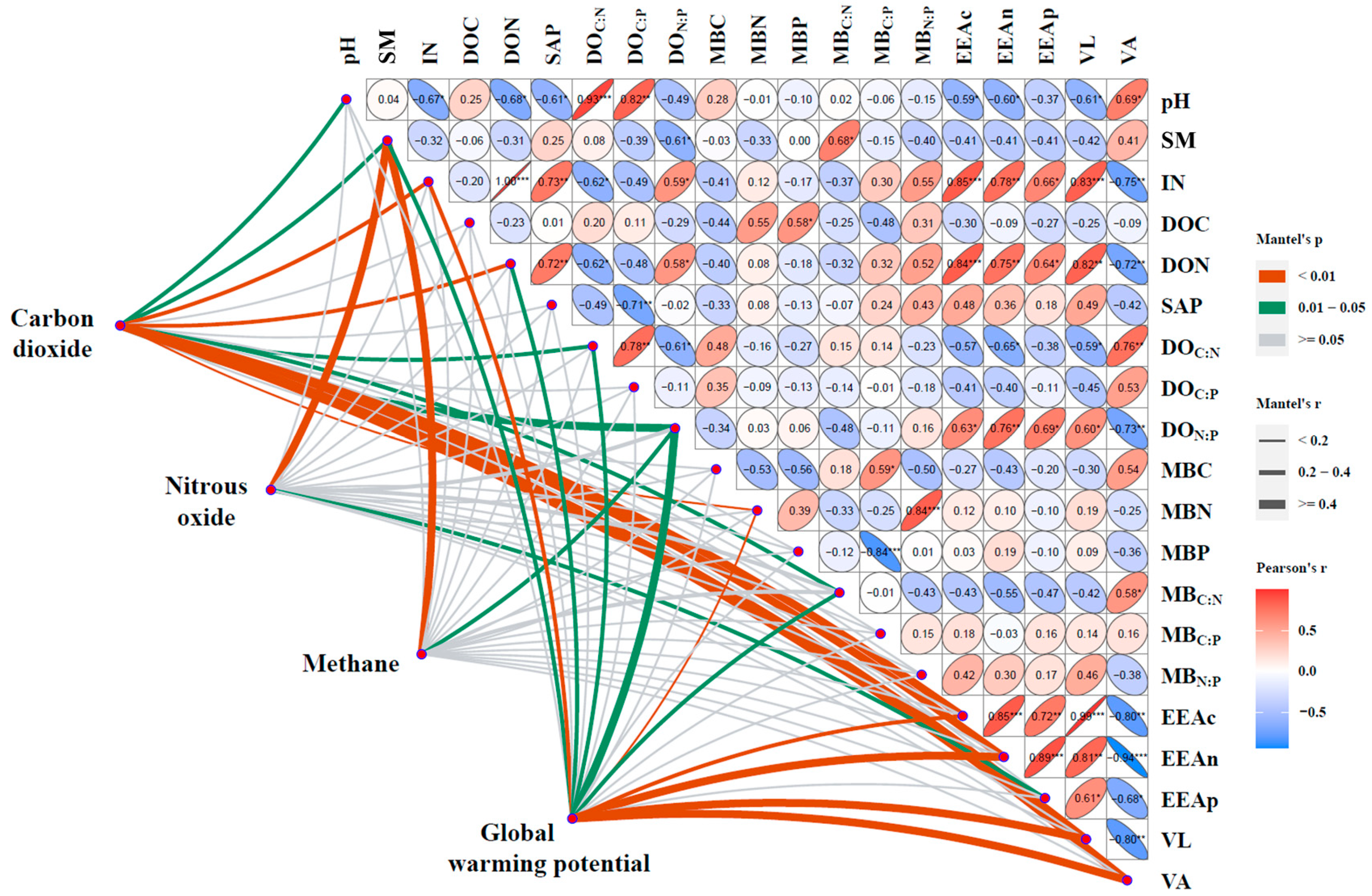
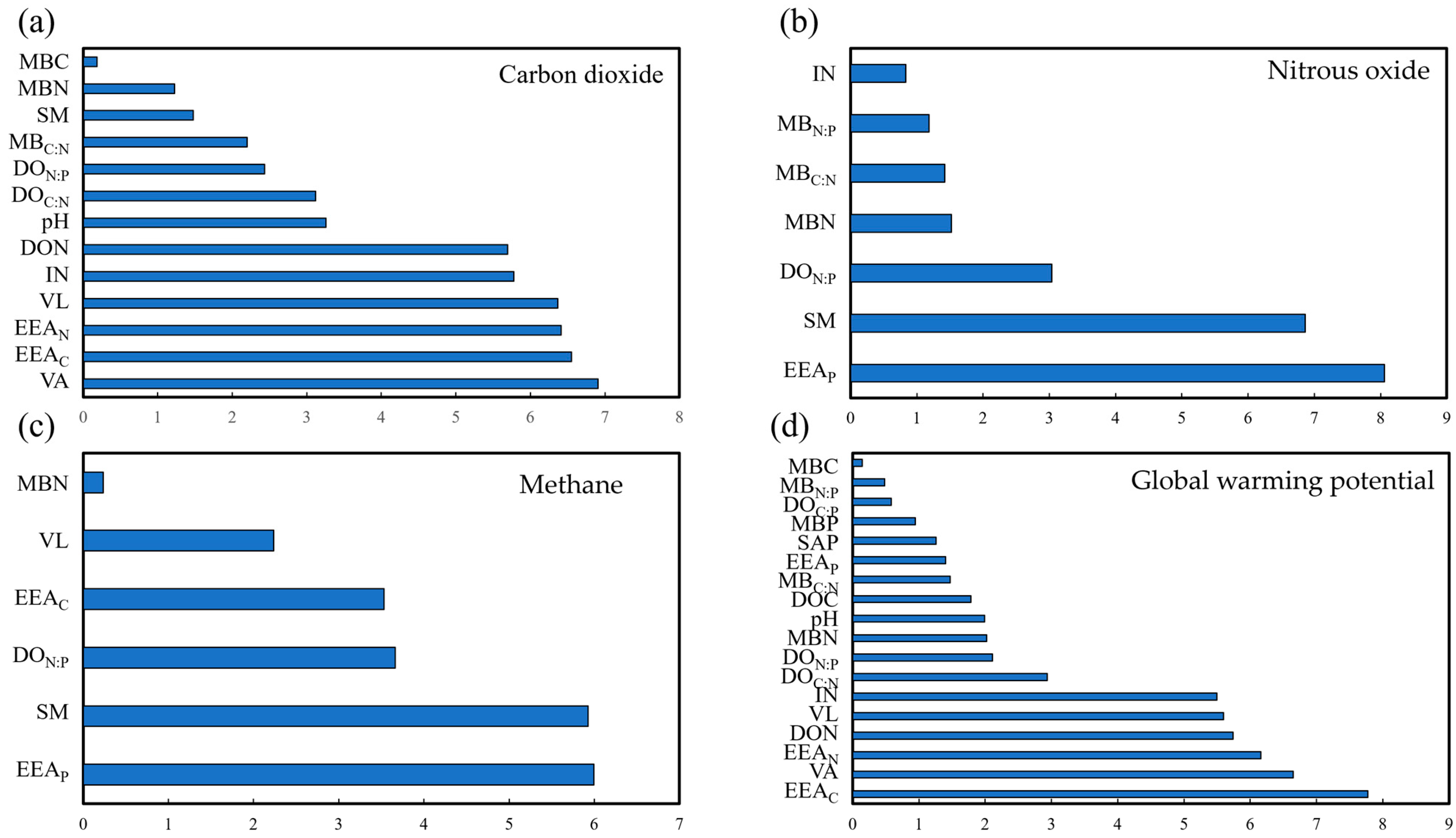
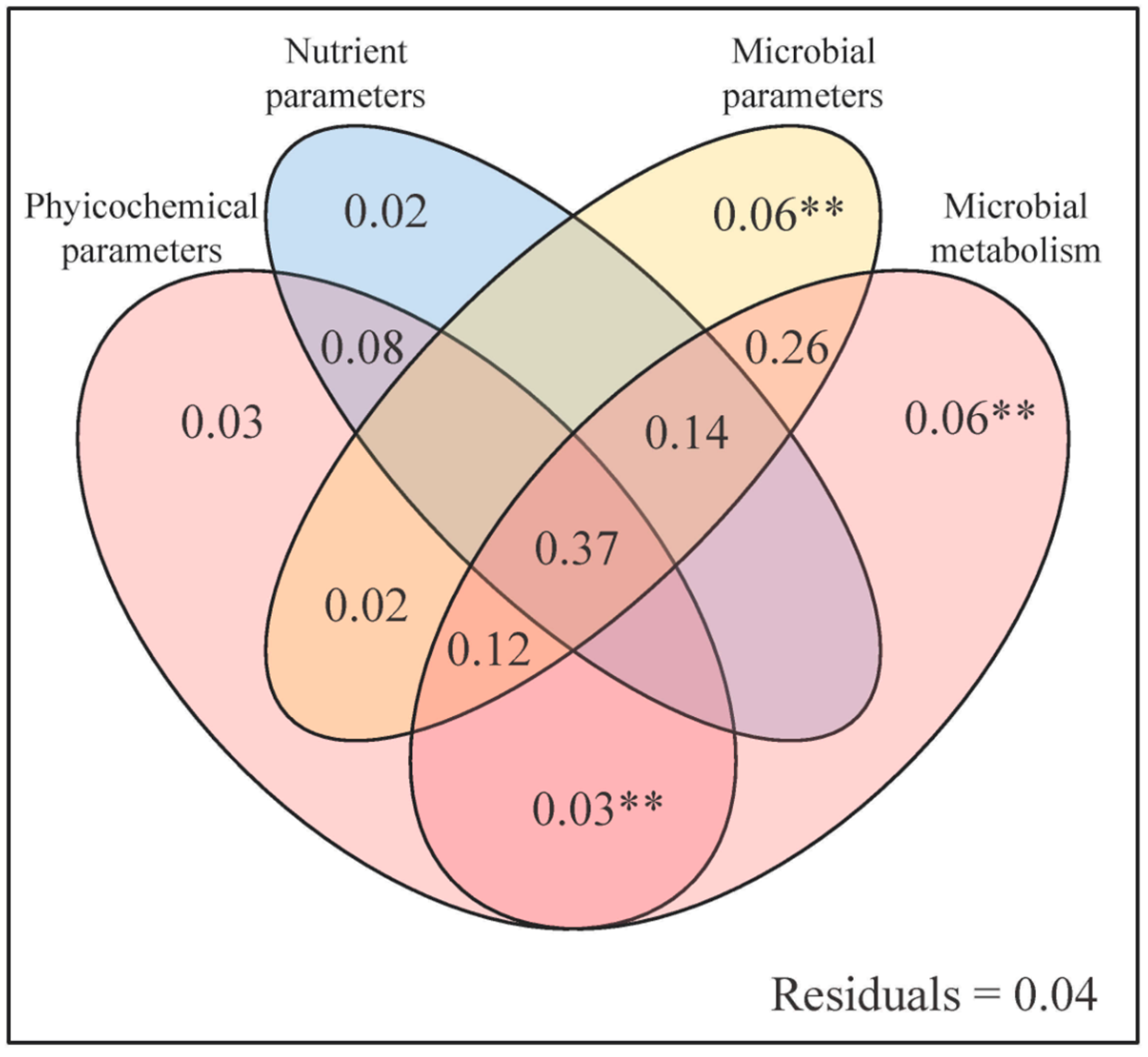
3.3. Response of Soil GHG Emissions to Soil Parameter
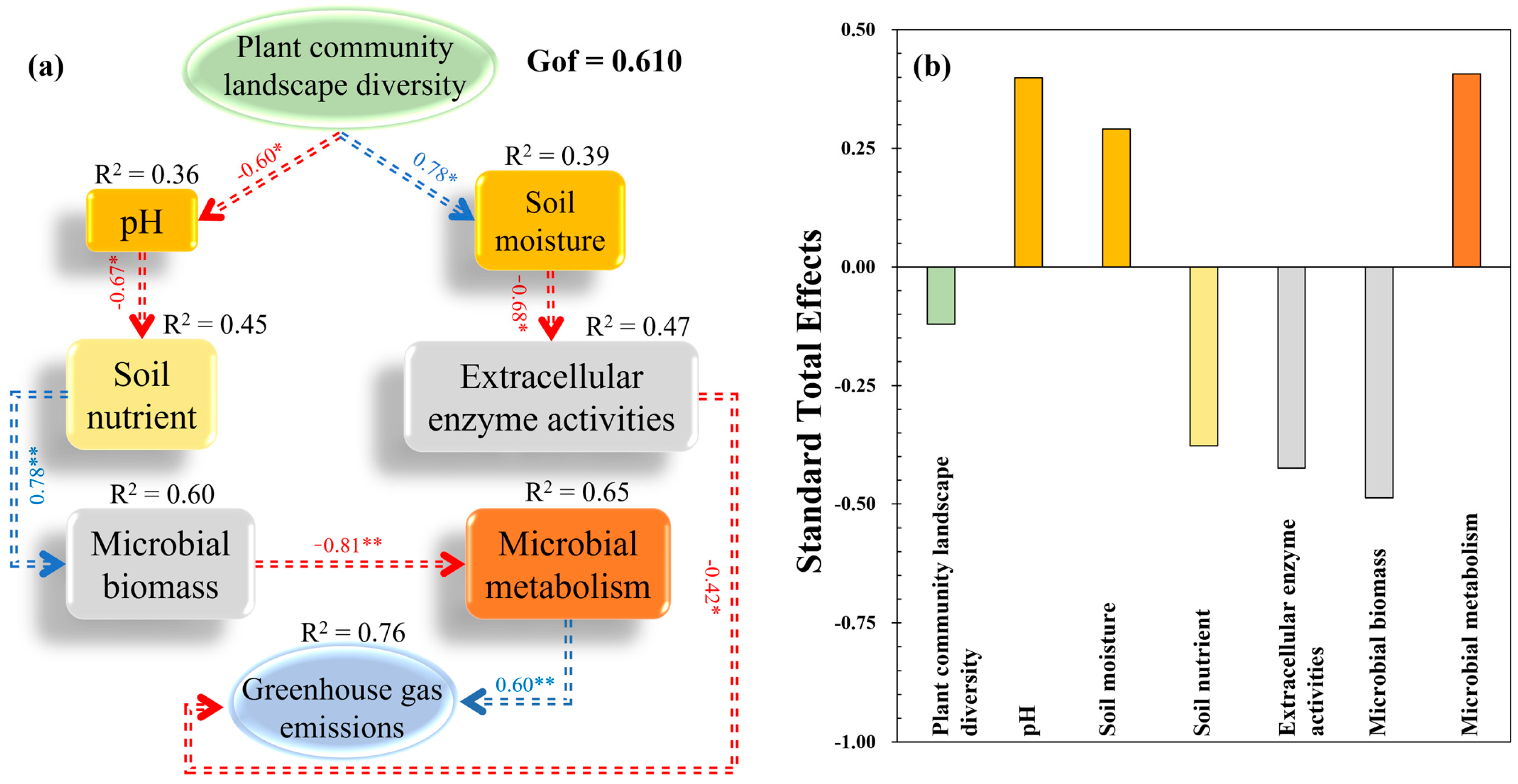
3.4. Influence Path of Plant Community Diversity on Soil GHG Emissions
4. Discussion
4.1. Direct Effect of Plant Community Landscape Diversity on Soil GHG Emissions
4.2. Plant Community Landscape Diversity Altered GHG Emissions through Changing Soil Parameters
4.3. Implications for Managing Plant Community Diversity to Mitigate Soil GHG Emissions in Riparian Zones
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate change 2021: The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Zhai, V.P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Huang, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Cao, X.; Tang, S.; Liu, H.; Wen, L.; Kou, X.; Yu, X.; Wang, Q.; Wang, J.; Liu, D.; Zhuo, Y.; et al. Effects of plant communities on the emission of soil greenhouse gases in riparian wetlands during spring thaw. Ecohydrology 2022, 10, 1002. [Google Scholar] [CrossRef]
- Hooper, D.; Adair, E.; Cardinale, B.; Byrnes, J.; Hungate, B.; Matulich, K.; Gonzalez, A.; Duffy, J.; Gamfeldt, L.; O’Connor, M. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 10, 1038. [Google Scholar] [CrossRef]
- Cui, M.; Wang, J.; Zhang, X.; Wang, C.; Li, G.; Wan, J.S.H.; Du, D. Warming significantly inhibited the competitive advantage of native plants in interspecific competition under phosphorus deposition. Plant Soil 2023, 486, 503–518. [Google Scholar] [CrossRef]
- Nazir, M.; Li, G.; Nazir, M.; Zulfiqar, F.; Siddique, K.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Till. Res. 2024, 237, 105–959. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Le Roux, X.; Poly, F.; Buchmann, N.; Scherer-Lorenzen, M.; Weigelt, A.; Barnard, R.L. Plant species diversity affects soil–atmosphere fluxes of methane and nitrous oxide. Oecologia 2016, 10, 1007. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Yuan, C.S.; Ie, I.R.; Yang, L.; Lin, H.J.; Hsueh, M.L. In-situ measurement of greenhouse gas emissions from a coastal estuarine wetland using a novel continuous monitoring technology: Comparison of indigenous and exotic plant species. J. Environ. Manag. 2021, 281, 111–905. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Hernandez, M.E.; Moreno-Casasola, P. Greenhouse gas emissions from coastal fresh water wetlands in Veracruz Mexico: Effect of plant community and seasonal dynamics. Atmos. Environ. 2015, 107, 107–117. [Google Scholar] [CrossRef]
- Vroom, R.J.E.; van den Berg, M.; Pangala, S.R.; van der Scheer, O.E.; Sorrell, B.K. Physiological processes affecting methane transport by wetland vegetation—A review. Aquat. Bot. 2022, 182, 103–547. [Google Scholar] [CrossRef]
- Yao, H.; Peng, H.; Hong, B.; Ding, H.; Hong, Y.; Zhu, Y.; Wang, J.; Cai, C. Seasonal and diurnal variation in ecosystem respiration and environmental controls from an alpine wetland in arid northwest China. J. Plant Ecol. 2022, 15, 933–946. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.; Manzoni, S.; Frey, S.; Schmidt, M.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Buragienė, S.; Šarauskis, E.; Romaneckas, K.; Adamavičienė, A.; Kriaučiūnienė, Z.; Avižienytė, D.; Marozas, V.; Naujokienė, V. Relationship between CO2 emissions and soil properties of differently tilled soils. Sci. Total Environ. 2019, 662, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Tariq, M.; Alabbosh, K.; Rehman, A.; Jalal, A.; Khan, A.; Farooq, M.; Li, G.; Iqbal, B.; Ahmad, N.; et al. Soil microplastics: Impacts on greenhouse gasses emissions, carbon cycling, microbial diversity, and soil characteristics. Appl. Soil Ecol. 2024, 197, 105–343. [Google Scholar] [CrossRef]
- Han, W.; Shi, M.; Chang, J.; Ren, Y.; Xu, R.; Zhang, C.; Ge, Y. Plant species diversity reduces N2O but not CH4 emissions from constructed wetlands under high nitrogen levels. Environ. Sci. Pollut. Res. 2017, 10, 1007. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Jiang, K.; Wei, M.; Wang, S. Effects of different concentrations and types of Cu and Pb on soil N-fixing bacterial communities in the wheat rhizosphere. Appl. Soil Ecol. 2019, 144, 51–59. [Google Scholar] [CrossRef]
- Khan, K.; Tang, Y.; Cheng, P.; Song, Y.; Li, X.; Lou, J.; Iqbal, B.; Zhao, X.; Hameed, R.; Li, G.; et al. Effects of degradable and non-degradable microplastics and oxytetracycline co-exposure on soil N2O and CO2 emissions. Appl. Soil Ecol. 2024, 197, 105–331. [Google Scholar] [CrossRef]
- Guenet, B.; Gabrielle, B.; Chenu, C.; Arrouays, D.; Balesdent, J.; Bernoux, M.; Bruni, E.; Caliman, J.; Cardinael, R.; Chen, S.; et al. Can N2O emissions offset the benefits from soil organic carbon storage? Glob. Change Biol. 2021, 27, 237–256. [Google Scholar] [CrossRef]
- Philippot, L.; Hallin, S.; Borjesson, G.; Baggs, E.M. Biochemical cycling in the rhizosphere having an impact on global change. Plant Soil 2009, 10, 1007. [Google Scholar] [CrossRef]
- Chang, J.; Fan, X.; Sun, H.; Zhang, C.; Song, C.; Chang, S.X.; Gu, B.; Liu, Y.; Li, D.; Wang, Y.; et al. Plant species richness enhances nitrous oxide emissions in microcosms of constructed wetlands. Ecol. Eng. 2014, 64, 108–115. [Google Scholar] [CrossRef]
- Maucieri, C.; Borin, M.; Milani, M.; Cirelli, G.L.; Barbera, A.C. Plant species effect on CO2 and CH4 emissions from pilot constructed wetlands in Mediterranean area. Ecol. Eng. 2019, 134, 112–117. [Google Scholar] [CrossRef]
- Mueller, P.; Mozdzer, T.J.; Langley, J.A.; Aoki, L.R.; Noyce, G.L.; Megonigal, J.P. Plant species determine tidal wetland methane response to sea level rise. Nat. Commun. 2020, 11, 5154. [Google Scholar] [CrossRef]
- Chen, M.; Chang, L.; Zhang, J.; Guo, F.; Vymazal, J.; He, Q.; Chen, Y. Global nitrogen input on wetland ecosystem: The driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 2020, 4, 100063. [Google Scholar] [CrossRef]
- Das, N.; Mandal, S. Microbial populations regulate greenhouse gas emissions in Sundarban mangrove ecosystem, India. Acta Ecol. Sin. 2022, 42, 641–652. [Google Scholar] [CrossRef]
- Ding, H.; Liu, T.; Hu, Q.; Liu, M.; Cai, M.; Jiang, Y.; Cao, C. Effect of microbial community structures and metabolite profile on greenhouse gas emissions in rice varieties. Environ. Pollut. 2022, 306, 119–365. [Google Scholar] [CrossRef] [PubMed]
- Han, W.J.; Chang, J.; Fan, X.; Du, Y.Y.; Chang, S.X.; Zhang, C.B.; Ge, Y. Plant species diversity impacts nitrogen removal and nitrous oxide emissions as much as carbon addition in constructed wetland microcosms. Ecol. Eng. 2016, 93, 144–151. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Chang, J.; Han, W.J.; Wang, M.; Ma, D.P.; Du, Y.Y.; Qu, Z.L.; Chang, S.X.; Ge, Y. Effects of plant diversity and sand particle size on methane emission and nitrogen removal in microcosms of constructed wetlands. Ecol. Eng. 2016, 95, 390–398. [Google Scholar] [CrossRef]
- Luo, B.; Du, Y.Y.; Han, W.J.; Geng, Y.; Wang, Q.; Duan, Y.Y.; Ren, Y.; Liu, D.; Chang, J.; Ge, Y. Reduce health damage cost of greenhouse gas and ammonia emissions by assembling plant diversity in floating constructed wetlands treating wastewater. J. Clean. Prod. 2020, 244, 118–927. [Google Scholar] [CrossRef]
- Wang, L.; Luo, B.; Du, Y.; Jiang, H.; Chang, S.X.; Fan, X.; Chang, J.; Ge, Y. Carbon nanotubes and plant diversity reduce greenhouse gas emissions and improve nitrogen removal efficiency of constructed wetlands. J. Clean. Prod. 2022, 380, 135023. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Shi, K.; Ren, G.; Dai, Z.; Sun, J.; Zheng, X.; Zhou, Y.; Zhang, J.; Li, G.; et al. Effects of Canada goldenrod invasion on soil extracellular enzyme activities and ecoenzymatic stoichiometry. Sustainability 2021, 13, 3768. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Du, Y.; Shi, K.; Ren, G.; Iqbal, B.; Dai, Z.; Li, G.; Du, D. Substrate availability regulates the suppressive effects of Canada goldenrod invasion on soil respiration. J. Plant Ecol. 2022, 15, 509–523. [Google Scholar] [CrossRef]
- Xu, S.; Li, K.; Li, G.; Hu, Z.; Zhang, J.; Iqbal, B.; Du, D. Canada Goldenrod invasion regulates the effects of soil moisture on soil respiration. Int. J. Environ. Res. Public Health 2022, 19, 15446. [Google Scholar] [CrossRef]
- Jiang, H.; Du, Y.; Han, W.; Wang, L.; Xiang, C.; Ge, Y.; Chang, J. Assembling plant diversity mitigates greenhouse gas emissions and achieves high nitrogen removal when treating the low-C/N wastewater by constructed wetlands. Environ. Sci. Pollut. R 2022, 30, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.; Stirling, G. Species richness and evenness respond in a different manner to propagule density in developing prairie microcosm communities. Plant Ecol. 2007, 190, 259–273. [Google Scholar] [CrossRef]
- Cheng, X.; Peng, R.; Chen, J.; Luo, Y.; Zhang, Q.; An, S.; Chen, J.; Li, B. CH4 and N2O emissions from Spartina alterniflora and Phragmites australis in experimental mesocosms. Chemosphere 2007, 68, 420–427. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Li, G.; Tang, Y.; Son, Y.; Zhao, X.; Iqbal, B.; Khan, K.; Guo, R.; Yin, W.; Zhao, X.; Du, D. Divergent responses in microbial metabolic limitations and carbon use efficiency to variably sized polystyrene microplastics in soil. Land Degrad. Dev. 2024, 35, 2658–2671. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Song, Y.; Alabbosh, K.; Li, K.; Lou, J.; Hameed, R.; Iqbal, B.; Li, G.; Du, D. Nitrogen deposition enhanced the effect of Solidago Canadensis invasion on soil microbial metabolic limitation and carbon use efficiency. Pol. J. Environ. Stud. 2023, 10, 15244. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.; Iqbal, B.; Tariq, M.; Rehman, A.; Khan, I.; Du, D. Soil nutrient levels regulate the effect of soil microplastic contamination on microbial element metabolism and carbon use efficiency. Ecotox. Environ. Saf. 2023, 267, 115–640. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013; Available online: https://www.R-project.org (accessed on 1 January 2013).
- Schmid, M.W.; van Moorsel, S.J.; Hahl, T.; De Luca, E.; De Deyn, G.B.; Wagg, C.; Niklaus, P.; Schmid, B. Effects of plant community history, soil legacy and plant diversity on soil microbial communities. J. Ecol. 2021, 109, 3007–3023. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Hu, H.; Sun, C.; Zhao, X.; Wei, C. Dynamics and controls of CO2 and CH4 emissions in the wetland of a montane permafrost region, Northeast China. Atmos. Environ. 2015, 122, 454–462. [Google Scholar] [CrossRef]
- Bansal, S.; Johnson, O.F.; Meier, J.; Zhu, X. Vegetation affects timing and location of wetland methane emissions. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005777. [Google Scholar] [CrossRef]
- Ma, W.; Alhassan, A.R.M.; Wang, Y.; Li, G.; Wang, H.; Zhao, J. Greenhouse gas emissions as influenced by wetland vegetation degradation along a moisture gradient on the eastern Qinghai-Tibet Plateau of North-West China. Nutr. Cycl. Agroecosys. 2018, 112, 335–354. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Son, Y. Effect of soil moisture on the response of soil respiration to open-field experimental warming and precipitation manipulation. Forests 2017, 8, 56. [Google Scholar] [CrossRef]
- Duan, J.; Cao, P.; Shu, T.; Zhou, B.; Xue, L.; Yang, L. Effects of cellulosic carbon addition on nitrogen removal from simulated dry land drainage, and its environmental effects. Agronomy 2023, 13, 304–412. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, G.; Zhou, X.; Zhou, L.; Shao, J.; Liu, R.; Gao, J.; He, Y.; Du, Z.; Tang, J. Effects of tree mycorrhizal type on soil respiration and carbon stock via fine root biomass and litter dynamic in tropical plantations. J. Plant Ecol. 2023, 10, 1093. [Google Scholar] [CrossRef]
- Olefeldt, D.; Turetsky, M.; Crill, P.; David, M. Environmental and physical controls on northern terrestrial methane emissions across permafrost zones. Glob. Change Biol. 2013, 10, 1111. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, J.; Wang, D.; Li, P.; Yin, Q. Effects of soil moisture on Phragmites australis (cav.) allelochemicals in soil and on growth of Phalaris arundinacea L. in Chinese wetland. Allelopath. J. 2020, 10, 26651. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, S.; Li, Y.; Riaz, L.; Yu, F.; Yang, Q.; Han, J.; Ma, J. Effects and mechanisms of land-types conversion on greenhouse gas emissions in the Yellow River floodplain. wetland. Sci. Total Environ. 2022, 813, 152–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.J.; Xiao, R.; Deng, H.; DeLaune, R.D. Effect of salinity on greenhouse gas production and emission in marsh soils during the decomposition of wetland plants. J. Soils Sediments 2022, 23, 133–144. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhao, R.; Guo, Y.; Hao, L. Aboveground net primary productivity and soil respiration display different responses to precipitation changes in desert grassland. J. Plant Ecol. 2022, 15, 57–70. [Google Scholar] [CrossRef]
- Voigt, C.; Marushchak, M.E.; Abbott, B.W.; Biasi, C.; Elberling, B.; Siciliano, S.D.; Sonnentag, O.; Stewart, K.J.; Yang, Y.; Martikainen, P.J. Nitrous oxide emissions from permafrost affected soils. Nat. Rev. Earth Environ. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Diao, H.; Chen, X.; Wang, G.; Ning, Q.; Hu, S.; Sun, W.; Dong, K.; Wang, C. The response of soil respiration to different n compounds addition in a saline-alkaline grassland of northern China. J. Plant Ecol. 2022, 15, 897–910. [Google Scholar] [CrossRef]
- Troxler, T.G.; Ikenaga, M.; Scinto, L.; Boyer, J.N.; Condit, R.; Perez, R.; Gann, G.D.; Childers, D.L. Patterns of soil bacteria and canopy community structure related to tropical peatland development. Wetlands 2012, 32, 769–782. [Google Scholar] [CrossRef]
- Zhang, H.; Goncalves, P.; Copeland, E.; Qi, S.; Dai, Z.; Li, G.; Wang, C.; Du, D.; Thomas, T. Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil. Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Sun, H.; Xin, Q.; Ma, Z.; Lan, S. Effects of plant diversity on carbon dioxide emissions and carbon removal in laboratory-scale constructed wetland. Environ. Sci. Pollut. Res. 2019, 10, 1007. [Google Scholar] [CrossRef]
- Huo, L.; Zou, Y.; Lyu, X.; Zhang, Z.; Wang, X.; An, Y. Effect of wetland reclamation on soil organic carbon stability in peat mire soil around Xingkai Lake in Northeast China. Chin. Geogr. Sci. 2018, 28, 325–336. [Google Scholar] [CrossRef]

| Parameters | F | p | Site 1 | Site 2 | Site 3 | Site 4 |
|---|---|---|---|---|---|---|
| pH | 2.7 | ns | 8.30 ± 0.05 a | 8.18 ± 0.01 ab | 8.21 ± 0.05 ab | 8.15 ± 0.02 b |
| SM (w/w%) | 10.02 | ** | 0.21 ± 0.01 b | 0.20 ± 0.01 b | 0.24 ± 0.01 a | 0.21 ± 0.01 b |
| IN (×10−1 μg−1 N g soil) | 4.28 | * | 0.54 ± 0.07 b | 6.08 ± 0.44 ab | 2.53 ± 1.25 b | 11.63 ± 4.51 a |
| DOC (×10−1 mg C g−1 soil) | 1.33 | ns | 0.25 ± 0.03 | 0.32 ± 0.04 | 0.27 ± 0.01 | 0.24 ± 0.025 |
| DON (mg N g−1 soil) | 3.65 | ns | 0.04 ± 0.01 | 0.24 ± 0.01 | 0.11 ± 0.06 | 0.46 ± 0.18 |
| SAP (mg P g−1 soil) | 2.12 | ns | 0.03 ± 0.01 | 0.10 ± 0.02 | 0.13 ± 0.03 | 0.13 ± 0.06 |
| DOC:N | 3.57 | ns | 7.27 ± 2.70 | 1.33 ± 0.22 | 4.19 ± 1.64 | 0.71 ± 0.26 |
| DOC:P | 4.81 | * | 10.13 ± 3.18 a | 3.29 ± 0.36 b | 2.22 ± 0.54 b | 2.60 ± 1.06 b |
| DON:P | 5.39 | * | 1.55 ± 0.48 bc | 2.76 ± 0.84 ab | 0.79 ± 0.37 c | 3.52 ± 0.17 a |
| MBC (×10−1 mg C g−1 soil) | 2.98 | ns | 0.83 ± 0.10 | 0.53 ± 0.08 | 0.66 ± 0.06 | 0.59 ± 0.053 |
| MBN (×10−1 mg N g−1 soil) | 1.03 | ns | 0.03 ± 0.02 | 0.37 ± 0.33 | 0.02 ± 0.02 | 0.06 ± 0.02 |
| MBP (×10−1 mg P g−1 soil) | 1.44 | ns | 0.03 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 |
| MBC:N (×10−1) | 3.2 | ns | 3.75 ± 1.44 | 0.93 ± 0.45 | 8.84 ± 3.82 | 1.12 ± 0.30 |
| MBC:P (×10−1) | 0.96 | ns | 3.22 ± 1.29 | 0.96 ± 0.41 | 1.64 ± 0.31 | 2.45 ± 1.43 |
| MBN:P | 0.66 | ns | 1.13 ± 0.51 | 4.99 ± 4.07 | 0.68 ± 0.57 | 3.66 ± 2.91 |
| EEAC (×10−3 nmol h−1 g−1 soil) | 7.1 | * | 2.75 ± 1.33 b | 4.55 ± 2.06 b | 2.65 ± 1.46 b | 7.26 ± 1.59 a |
| EEAN (×10−2 nmol h−1 g−1 soil) | 414.21 | *** | 2.94 ± 0.02 c | 4.31 ± 0.07 b | 2.93 ± 0.12 c | 6.05 ± 0.04 a |
| EEAP (×10−1 nmol h−1 g−1 soil) | 129.1 | *** | 2.97 ± 0.02 b | 2.93 ± 0.01 b | 2.80 ± 0.02 c | 3.59 ± 0.05 a |
| VL (×10−2) | 5.02 | * | 0.93 ± 0.05 a | 1.56 ± 0.06 ab | 0.95 ± 0.05 b | 2.04 ± 0.47 a |
| VA (°) | 101.59 | *** | 5.78 ± 0.09 a | 3.88 ± 0.08 ab | 5.45 ± 0.19 b | 3.40 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xu, J.; Tang, Y.; Wang, Y.; Lou, J.; Xu, S.; Iqbal, B.; Li, Y.; Du, D. Impact of Plant Community Diversity on Greenhouse Gas Emissions in Riparian Zones. Plants 2024, 13, 2412. https://doi.org/10.3390/plants13172412
Li G, Xu J, Tang Y, Wang Y, Lou J, Xu S, Iqbal B, Li Y, Du D. Impact of Plant Community Diversity on Greenhouse Gas Emissions in Riparian Zones. Plants. 2024; 13(17):2412. https://doi.org/10.3390/plants13172412
Chicago/Turabian StyleLi, Guanlin, Jiacong Xu, Yi Tang, Yanjiao Wang, Jiabao Lou, Sixuan Xu, Babar Iqbal, Yingnan Li, and Daolin Du. 2024. "Impact of Plant Community Diversity on Greenhouse Gas Emissions in Riparian Zones" Plants 13, no. 17: 2412. https://doi.org/10.3390/plants13172412
APA StyleLi, G., Xu, J., Tang, Y., Wang, Y., Lou, J., Xu, S., Iqbal, B., Li, Y., & Du, D. (2024). Impact of Plant Community Diversity on Greenhouse Gas Emissions in Riparian Zones. Plants, 13(17), 2412. https://doi.org/10.3390/plants13172412









