Biochemical and Antioxidant Characteristics of Chlorococcum oleofaciens (Chlorophyceae, Chlorophyta) under Various Cultivation Conditions
Abstract
:1. Introduction
2. Results
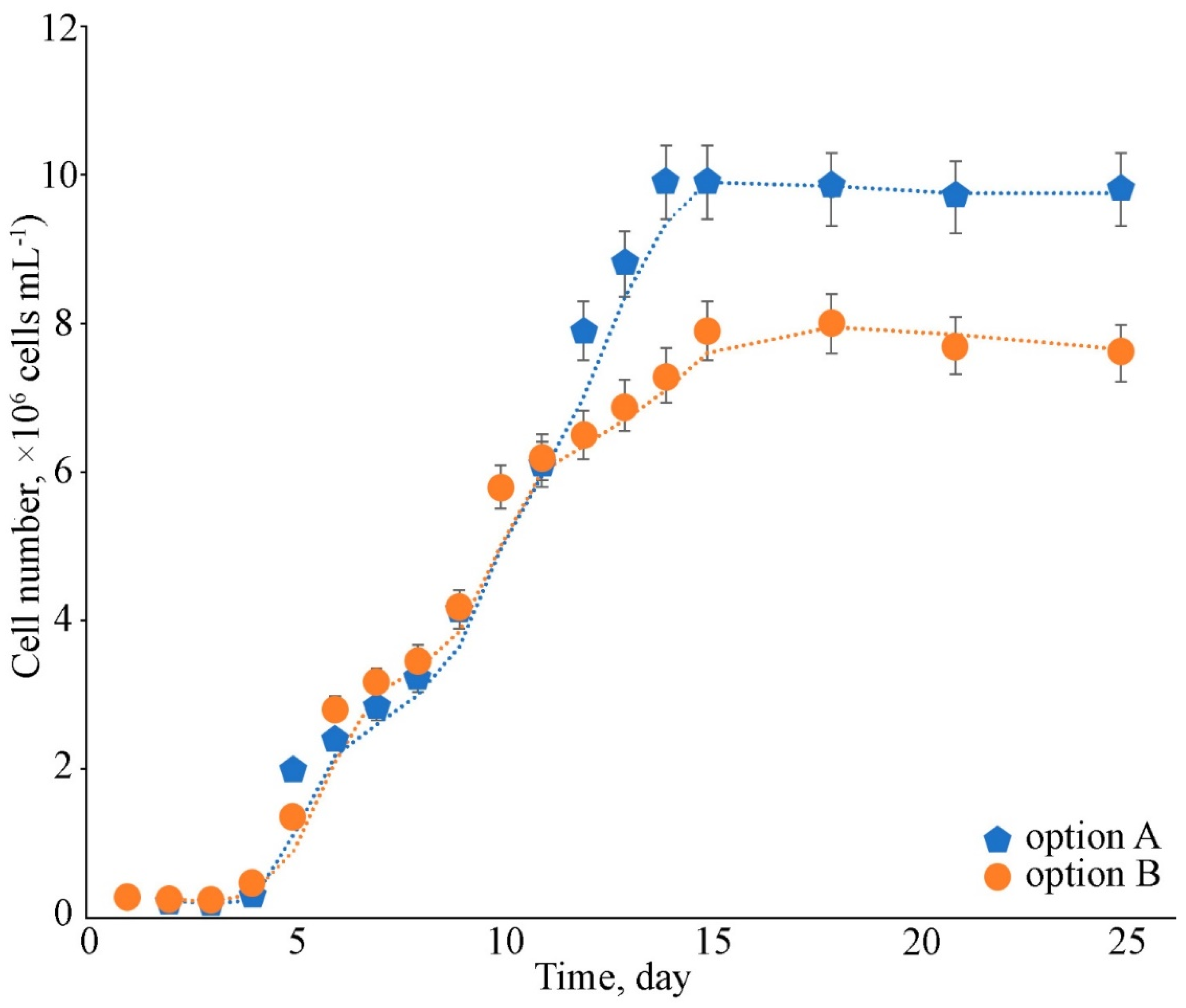
2.1. Growth Assessment
2.2. Biochemical Parameters of Strain Cc. oleofaciens CAMU MZ–Ch4 under Different Aeration Conditions
2.2.1. Biomass
2.2.2. Pigment Composition
2.2.3. Lipid and Protein Contents
2.2.4. Fatty- Soluble and Hydrophilic Vitamin Contents
2.2.5. Succinate Dehydrogenase Activity
2.2.6. Antioxidant Enzyme Activity
2.2.7. Products of Lipid Peroxidation
2.2.8. Antioxidant Activity Coefficient
2.3. Change in Biochemical Parameters and Antioxidant Status of Strain Cc. oleofaciens CAMU MZ–Ch4 in Prolonged Culture
2.4. Change in Biochemical Parameters and Antioxidant Status of Strain Cc. oleofaciens CAMU MZ–Ch4 in Experiments with Nitrogen and Phosphorus Deficiencies
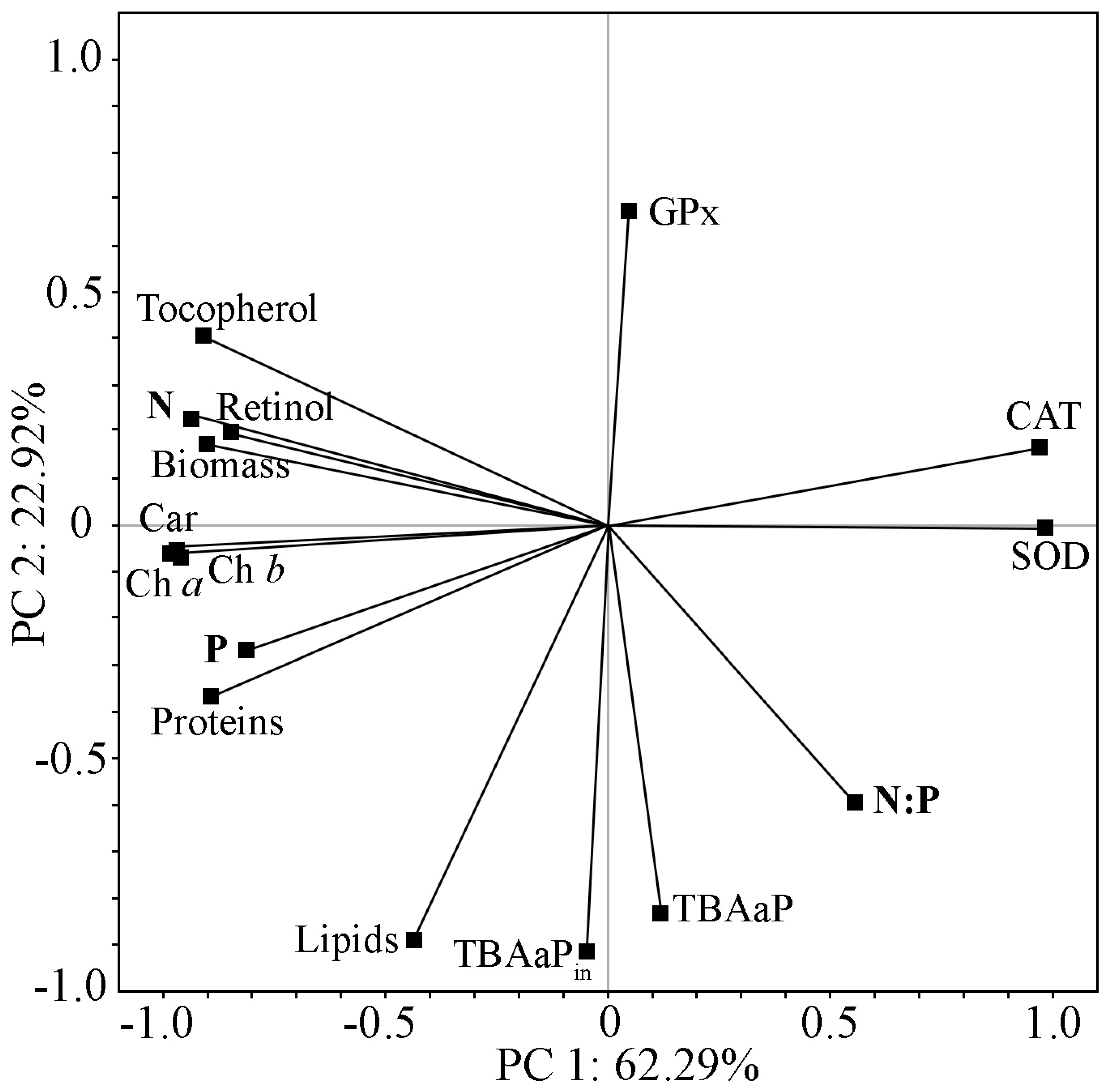
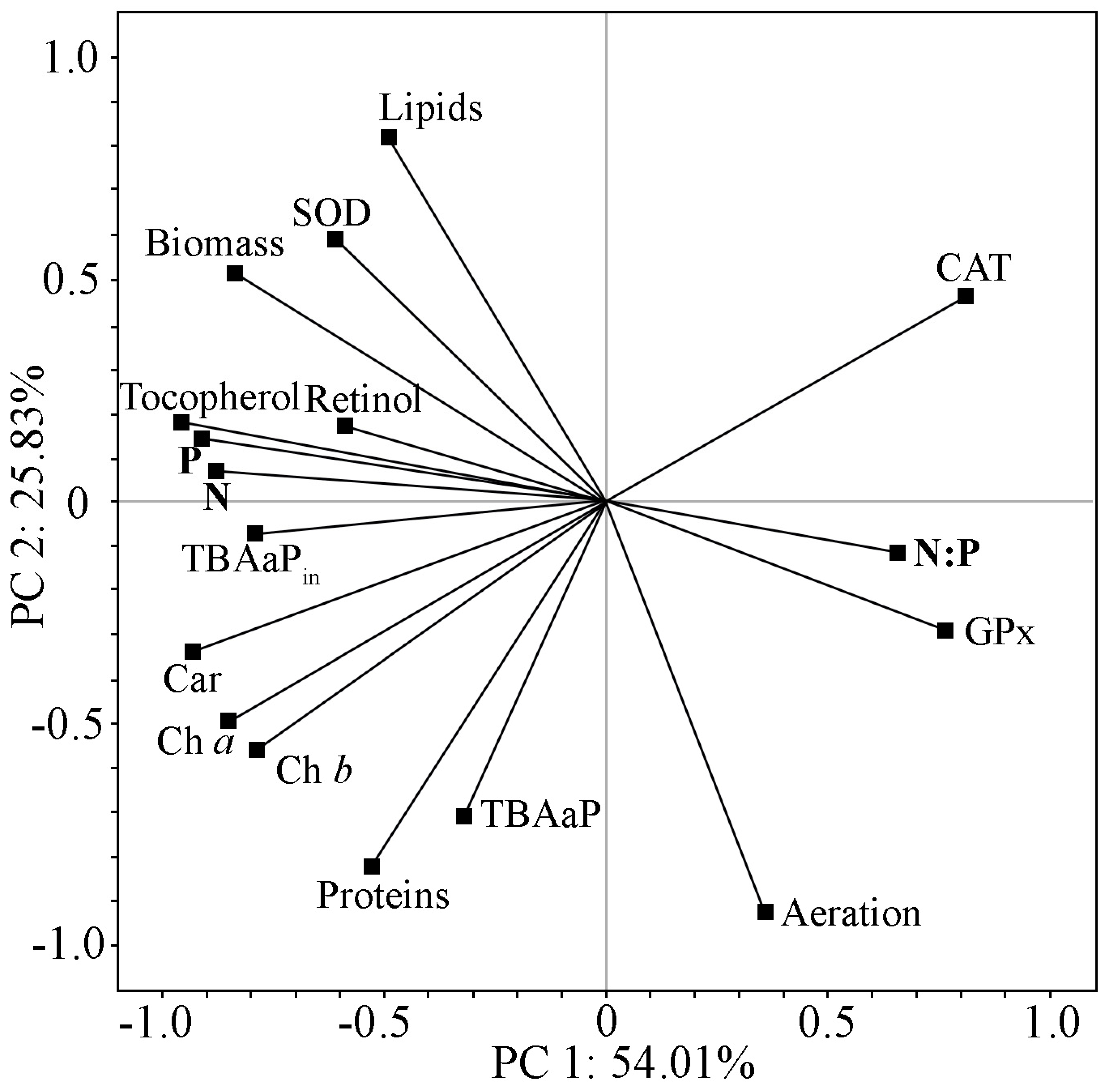
2.5. Comprehensive Analysis of Biochemical Parameters and Antioxidant Status of Strain Cc. oleofaciens CAMU MZ–Ch4 under Different Culture Conditions
3. Discussion
3.1. Growth and Biomass Production
3.2. Biochemical Parameters
3.2.1. Lipids, Proteins
3.2.2. Pigments
3.2.3. Vitamins
3.2.4. Antioxidant Defence System Enzymes
4. Material and Methods
4.1. Microalgal Material
4.2. Experimental Design
4.3. Growth Assessment
4.4. Biochemical Parameters
4.4.1. Measurement of Chlorophyll Content
4.4.2. Measurement of Carotenoid Content
4.4.3. Vitamin A (Retinol) and E (α-Tocopherol) Content Measurement
4.4.4. Vitamin C (Ascorbic Acid) and P (Phenolic Compounds) Content Measurement
4.4.5. Succinate Dehydrogenase Activity Measurement
4.4.6. Antioxidant Enzyme Activity Measurement
4.4.7. Measurement of TBA-Active Product Content
4.4.8. Antioxidant Activity Coefficient Calculation
4.4.9. Lipid Content Measurement
4.4.10. Measurement of Protein Content
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging technologies in algal biotechnology: Toward the establishment of a sustainable, algae-based bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, I.; Maltseva, S.; Kociolek, J.P.; Kulikovskiy, M. A new species of freshwater algae Nephrochlamys yushanlensis sp. nov. (Selenastraceae, Sphaeropleales) and its lipid accumulation during nitrogen and phosphorus starvation. J. Phycol. 2021, 57, 606–618. [Google Scholar] [CrossRef]
- Maltsev, Y.; Krivova, Z.; Maltseva, S.; Maltseva, K.; Gorshkova, E.; Kulikovskiy, M. Lipid accumulation by Coelastrella multistriata (Scenedesmaceae, Sphaeropleales) during nitrogen and phosphorus starvation. Sci. Rep. 2021, 11, 19818. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Sero, E.T.; Siziba, N.; Bunhu, T.; Shoko, R. Isolation and screening of microalgal species, native to Zimbabwe, with potential use in biodiesel production. All Life 2021, 14, 256–264. [Google Scholar] [CrossRef]
- Maltsev, Y.; Kulikovskiy, M.; Maltseva, S. Nitrogen and phosphorus stress as a tool to induce lipid production in microalgae. Microb. Cell Fact. 2023, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Lan, C.Q.; Liao, D. Effects of sodium bicarbonate on cell growth, lipid accumulation, and morphology of Chlorella vulgaris. Microb. Cell Fact. 2018, 17, 111. [Google Scholar] [CrossRef]
- Borowitzka, M.A. The ‘stress’ concept in microalgal biology—Homeostasis, acclimation and adaptation. J. Appl. Phycol. 2018, 30, 2815–2825. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae–mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Abdalla, A.L.; Alvarez, C.; Arndt, C.; Becquet, P.; Benchaar, C.; Berndt, A.; Mauricio, R.M.; McAllister, T.A.; et al. Invited review: Current enteric methane mitigation options. J. Dairy Sci. 2022, 105, 9297–9326. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Vigara, J.; Funk, C. Exploring Nordic microalgae as a potential novel source of antioxidant and bioactive compounds. New Biotechnol. 2023, 73, 1–8. [Google Scholar] [CrossRef]
- López-Hernández, J.F.; García-Alamilla, P.; Palma-Ramírez, D.; Álvarez-González, C.A.; Paredes-Rojas, J.C.; Márquez-Rocha, F.J. Continuous microalgal cultivation for antioxidants production. Molecules 2020, 25, 4171. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.S.; Yeong, Y.S.; Zakeri, H.A.; Wahid, M.E.A.; Ghafar, S.N.A.; Yusuf, N. Photoperiod influenced the growth and antioxidative responses of Chlorella vulgaris, Isochrysis galbana, and Tetraselmis chuii. J. Appl. Pharm. Sci. 2021, 11, 125–134. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Chen, H.; He, C.-L.; Wang, Q. Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS ONE 2013, 8, e69225. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Nong, Q.-Y.; Liu, Y.-A.; Qin, L.-T.; Liu, M.; Mo, L.-Y.; Liang, Y.-P.; Zeng, H.-H. Toxic mechanism of three azole fungicides and their mixture to green alga Chlorella pyrenoidosa. Chemosphere 2021, 262, 127793. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, W.; Zhang, Y.; Zhou, G.-J.; Bi, Y. Response of antioxidant enzyme activities of the green microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ stress. Sustainability 2022, 14, 10320. [Google Scholar] [CrossRef]
- Tang, W.; He, M.; Chen, B.; Ruan, G.; Xia, Y.; Xu, P.; Song, G.; Bi, Y.; Hu, B. Investigation of toxic effect of mercury on Microcystis aeruginosa: Correlation between intracellular mercury content at single cells level and algae physiological responses. Sci. Total Environ. 2023, 858, 159894. [Google Scholar] [CrossRef]
- Maltsev, Y.I.; Konovalenko, T.V. New finding of green algae with potential for algal biotechnology, Chlorococcum oleofaciens and its molecular investigation. Regul. Mech. Biosyst. 2017, 8, 532–539. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, I. The influence of forest-forming tree species on diversity and spatial distribution of algae in forest litter. Folia Oecologica 2018, 45, 72–81. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Nakada, T.; Tomita, M. Taxonomic revision of oil-producing green algae, Chlorococcum oleofaciens (Volvocales, Chlorophyceae), and its relatives. J. Phycol. 2015, 51, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft und Flechtenalgen; Gustav Fischer Verlag: Stuttgart, Germany, 1995. [Google Scholar]
- Scherbina, V.V.; Maltseva, I.A.; Solonenko, A.N. Peculiarities of postpyrogene development of algae in steppe biocenoses at Askania Nova Biospheric National Park. Contemp. Probl. Ecol. 2014, 7, 187–191. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, S.; Maltseva, I. Diversity of cyanobacteria and algae during primary succession in iron ore tailing dumps. Microb. Ecol. 2022, 83, 408–423. [Google Scholar] [CrossRef]
- Trainor, F.R.; Bold, H.C. Three new unicellular Chlorophyceae from soil. Am. J. Bot. 1953, 40, 758–767. [Google Scholar] [CrossRef]
- Rayati, M.; Rajabi Islami, H.; Shamsaie Mehrgan, M. Light intensity improves growth, lipid productivity, and fatty acid profile of Chlorococcum oleofaciens (Chlorophyceae) for biodiesel production. BioEnergy Res. 2020, 13, 1235–1245. [Google Scholar] [CrossRef]
- Rajabi Islami, H.; Assareh, R. Enhancement effects of ferric ion concentrations on growth and lipid characteristics of freshwater microalga Chlorococcum oleofaciens KF584224.1 for biodiesel production. Renew. Energy 2020, 149, 264–272. [Google Scholar] [CrossRef]
- Del Río, E.; García-Gómez, E.; Moreno, J.; Guerrero, M.G.; García-González, M. Microalgae for oil. Assessment of fatty acid productivity in continuous culture by two high-yield strains, Chlorococcum oleofaciens and Pseudokirchneriella subcapitata. Algal Res. 2017, 23, 37–42. [Google Scholar] [CrossRef]
- Pauline, J.M.N.; Achary, A. Novel media for effective lipid production of Chlorococcum oleofaciens: A RSM approach. Acta Protozool. 2019, 58, 31–41. [Google Scholar] [CrossRef]
- Bouzidi, N.E.; Grama, S.B.; Khelef, A.E.; Yang, D.; Li, J. Inhibition of antioxidant enzyme activities enhances carotenogenesis in microalga Dactylococcus dissociatus MT1. Front. Bioeng. Biotechnol. 2022, 10, 1014604. [Google Scholar] [CrossRef]
- Qi, F.; Pei, H.; Mu, R.; Ma, G.; Wu, D.; Han, Q. Characterization and optimization of endogenous lipid accumulation in Chlorella vulgaris SDEC-3M ability to rapidly accumulate lipid for reversing nightly lipid loss. Biotechnol. Biofuels 2019, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.B.; Udhaya, R.; Sandhya, S. Biomass and lipid productivity by four fresh water microalgae in photoreactor. J. Mod. Biotechnol. 2013, 2, 82–88. [Google Scholar]
- Brinkert, K.; De Causmaecker, S.; Krieger-Liszkay, A.; Fantuzzi, A.; Rutherford, A.W. Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 12144–12149. [Google Scholar] [CrossRef]
- Sonkar, S.; Mallick, N. Development of a single phase nitrate feeding strategy for enhanced lipid productivity from green microalgae for biodiesel production. Environ. Prog. Sustain. Energy 2017, 36, 222–231. [Google Scholar] [CrossRef]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresour. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef]
- Juntila, D.J.; Bautista, M.A.; Monotilla, W. Biomass and lipid production of a local isolate Chlorella sorokiniana under mixotrophic growth conditions. Bioresour. Technol. 2015, 191, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Mtaki, K.; Kyewalyanga, M.S.; Mtolera, M.S.P. Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Ann. Microbiol. 2021, 71, 7. [Google Scholar] [CrossRef]
- Thangavel, K.; Krishnan, P.R.; Nagaiah, S.; Kuppusamy, S.; Chinnasamy, S.; Rajadorai, J.S.; Olaganathan, G.N.; Dananjeyan, B. Growth and metabolic characteristics of oleaginous microalgal isolates from Nilgiri biosphere Reserve of India. BMC Microbiol. 2018, 18, 1. [Google Scholar] [CrossRef]
- Yilancioğlu, K.; Tekin, H.O.; Cetiner, S. Nitrogen source, an important determinant of fatty acid accumulation and profile in Scenedesmus obliquus. Acta Phys. Pol. A 2016, 130, 428–433. [Google Scholar] [CrossRef]
- Cordeiro, R.S.; Vaz, I.C.D.; Magalhães, S.M.S.; Barbosa, F.A.R. Effects of nutritional conditions on lipid production by cyanobacteria. An. Acad. Bras. Ciênc. 2017, 89, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Sassi, K.K.B.; Da Silva, J.A.; Calixto, C.D.; Sassi, R.; Da Costa Sassi, C.F. Metabolites of interest for food technology produced by microalgae from the Northeast Brazil. Rev. Ciênc. Agron. 2019, 50, 54–65. [Google Scholar] [CrossRef]
- Fatini, M.A.; Basri, E.M.; Wan Maznah, W.O. Effect of different nitrogen sources on cell growth and biochemical compositions of Chlorococcum sp. cultivated under laboratory conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 711, 012010. [Google Scholar] [CrossRef]
- Correia, N.; Pereira, H.; Schulze, P.S.C.; Costa, M.M.; Santo, G.E.; Guerra, I.; Trovão, M.; Barros, A.; Cardoso, H.; Silva, J.L.; et al. Heterotrophic and photoautotrophic media optimization using response surface methodology for the novel microalga Chlorococcum amblystomatis. Appl. Sci. 2023, 13, 2089. [Google Scholar] [CrossRef]
- Chia, M.A.; Lombardi, A.T.; Da Graça, M.G.M. Growth and biochemical composition of Chlorella vulgaris in different growth media. An. Acad. Bras. Ciênc. 2013, 85, 1427–1438. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, J.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Physiological changes of Parachlorella kessleri TY02 in lipid accumulation under nitrogen stress. Int. J. Environ. Res. Public Health 2019, 16, 1188. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal carotenoids: Chemistry, sources, and application. Foods 2023, 12, 2768. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Sirohi, P.; Verma, H.; Singh, S.K.; Singh, V.K.; Pandey, J.; Khusharia, S.; Kumar, D.; Kaushalendra; Teotia, P.; Kumar, A. Microalgal carotenoids: Therapeutic application and latest approaches to enhance the production. Curr. Issues Mol. Biol. 2022, 44, 6257–6279. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Babadi, F.E.; Boonnoun, P.; Nootong, K.; Powtongsook, S.; Goto, M.; Shotipruk, A. Identification of carotenoids and chlorophylls fromgreen algae Chlorococcum humicola and extraction by liquefied dimethyl ether. Food Bioprod. Process. 2020, 123, 296–303. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Lauceri, R.; Faraloni, C.; Benavides, A.M.S.; Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2023, 22, 1733–1789. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. The prospects of algae-derived vitamins and their precursors for sustainable cosmeceuticals. Processes 2023, 11, 587. [Google Scholar] [CrossRef]
- Carballo-Cardenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Brown, M.R.; Miller, K.A. The ascorbic acid content of eleven species of microalgae used in mariculture. J. Appl. Phycol. 1992, 4, 205–215. [Google Scholar] [CrossRef]
- Canelli, G.; Tevere, S.; Jaquenod, L.; Dionisi, F.; Rohfritsch, Z.; Bolten, C.J.; Neutsch, L.; Mathys, A. A novel strategy to simultaneously enhance bioaccessible lipids and antioxidants in hetero/mixotrophic Chlorella vulgaris as functional ingredient. Bioresour. Technol. 2022, 347, 126744. [Google Scholar] [CrossRef]
- Durmaz, Y. Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture 2007, 272, 717–722. [Google Scholar] [CrossRef]
- Singh, R.; Paliwal, C.; Nesamma, A.A.; Narula, A.; Jutur, P.P. Nutrient deprivation mobilizes the production of unique tocopherols as a stress-promoting response in a new indigenous isolate Monoraphidium sp. Front. Mar. Sci. 2020, 7, 575817. [Google Scholar] [CrossRef]
- Mokrosnop, V.M.; Zolotareva, E.K. Microalgae as tocopherol producers. Biotechnol. Acta 2014, 7, 26–33. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a source of valuable phenolic compounds and carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulos, V.; Gkioni, M.D.; Koutra, E.; Mastropetros, S.G.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Total phenolic content, biomass composition, and antioxidant activity of selected marine microalgal species with potential as aquaculture feed. Antioxidants 2022, 11, 1320. [Google Scholar] [CrossRef]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of antioxidant capacities of green microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Hemalatha, A.; Parthiban, C.; Saranya, C.; Girija, K.; Anantharaman, P. Evaluation of antioxidant activities and total phenolic contents of different solvent extracts of selected marine diatoms. Indian. J. Mar. Sci. 2015, 44, 1630–1636. [Google Scholar]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef]
- Patova, E.N.; Novakovskaya, I.V. Soil algae of the Northeastern European Russia. Nov. Sist. Nizshikh Rastenii 2018, 52, 311–353. [Google Scholar] [CrossRef]
- Ignatenko, M.E.; Yatsenko-Stepanova, T.N.; Khlopko, Y.A. Halotolerant strain of Chlorococcum oleofaciens from the Lake Elton Biosphere Reserve. Biosyst. Divers. 2019, 27, 244–249. [Google Scholar] [CrossRef]
- Mikhailyuk, T.; Glaser, K.; Tsarenko, P.; Demchenko, E.; Karsten, U. Composition of biological soil crusts from sand dunes of the Baltic Sea coast in the context of an integrative approach to the taxonomy of microalgae and cyanobacteria. Eur. J. Phycol. 2019, 54, 263–290. [Google Scholar] [CrossRef]
- Tan, K.A.; Lalung, J.; Morad, N.; Ismail, N.; Wan Omar, W.M.; Khan, M.A.; Sillanpää, M.; Rafatullah, M. Post-treatment of palm oil mill effluent using immobilised green microalgae Chlorococcum oleofaciens. Sustainability 2021, 13, 11562. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Giordano, E.; Visioli, F. Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 1–4. [Google Scholar] [CrossRef]
- Huang, S.; Millar, A.H. Succinate dehydrogenase: The complex roles of a simple enzyme. Curr. Opin. Plant Biol. 2013, 16, 344–349. [Google Scholar] [CrossRef]
- Moosavi, B.; Berry, E.A.; Zhu, X.L.; Yang, W.C.; Yang, G.F. The assembly of succinate dehydrogenase: A key enzyme in bioenergetics. Cell. Mol. Life Sci. 2019, 76, 4023–4042. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Santiago-Morales, I.S.; Trujillo-Valle, L.; Márquez-Rocha, F.J.; López Hernández, J.F. Tocopherols, Phycocyanin and Superoxide Dismutase from Microalgae: As Potential Food Antioxidants. Appl. Food Biotechnol. 2018, 5, 19–27. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical Applications of Twenty-Five Species of Rapid-Growing Green-Microalgae as Indicated by Their Antibacterial, Antioxidant and Mineral Content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Smerilli, A.; Orefice, I.; Corato, F.; Gavalás Olea, A.; Ruban, A.V.; Brunet, C. Photoprotective and Antioxidant Responses to Light Spectrum and Intensity Variations in the Coastal Diatom Skeletonema marinoi. Environ. Microbiol. 2017, 19, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Yakoviichuk, A.; Krivova, Z.; Maltseva, S.; Kochubey, A.; Kulikovskiy, M.; Maltsev, Y. Antioxidant status and biotechnological potential of new Vischeria vischeri (Eustigmatophyceae) soil strains in enrichment cultures. Antioxidants 2023, 12, 654. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Phycological studies IV. In Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas Publication: Austin, TX, USA, 1963. [Google Scholar]
- Wong, Y.K.; Yung, K.K.; Tsang, Y.F.; Xia, Y.; Wang, L.; Ho, K.C. Scenedesmus quadricauda for nutrient removal and lipid production in wastewater. Water Environ. Res. 2015, 87, 2037–2044. [Google Scholar] [CrossRef]
- Polat, E.; Yüksel, E.; Altınbaş, M. Mutual effect of sodium and magnesium on the cultivation of microalgae Auxenochlorella protothecoides. Biomass Bioenergy 2020, 132, 105441. [Google Scholar] [CrossRef]
- Dere, Ş.; Güneş, T.; Sivaci, R. Spectrophotometric determination of chlorophyll—A, B and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–16. [Google Scholar]
- Hodisan, T.; Casoni, D.; Beldean-Galea, M.S.; Cimpoiu, C. Identification and quantification of tocopherols in vegetable oils by thin-layer chromatography. JPC-J. Planar Chromatogr. 2008, 21, 213–215. [Google Scholar] [CrossRef]
- Fung, Y.K.; Rahwan, R.G.; Sams, R.A. Separation of vitamin A compounds by thin-layer chromatography. J. Chromatogr. A 1978, 147, 528–531. [Google Scholar] [CrossRef]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the presence and contents of phytochemicals in honey samples: Phenolic compounds as indicators to identify their botanical origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Munujos, P.; Collcanti, J.; Gonzalezsastre, F.; Gella, F.J. Assay of succinate dehydrogenase activity by a colorimetric-continuous method using iodonitrotetrazolium chloride as electron acceptor. Anal. Biochem. 1993, 212, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Razygraev, A.V.; Arutiunian, A.V. Determination of human serum glutathione peroxidase activity, by using hydrogen peroxide and 5,5′-dithio-bis (2-nitrobenzoic acid). Klin. Lab. Diagn. 2006, 6, 13–16. [Google Scholar]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Fried, R. Enzymatic and non-enzymatic assay of superoxide dismutase. Biochimie 1975, 57, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Ullah, F. A Simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.J.S.C. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2016, 73, A.3A.1–A.3A.32. [Google Scholar] [CrossRef]
- De Sá, J.P.M. Applied Statistics Using SPSS, STATISTICA, MATLAB and R; Springer: Heidelberg, Germany, 2007. [Google Scholar]


| Indicator | Option A | Option B | Option C |
|---|---|---|---|
| Early Stationary Growth Phase (18 Days) | Early Stationary Growth Phase (18 Days) | Late Stationary Growth Phase (74 Days) | |
| Biomass concentration, g L−1 | 1.35 ± 0.034 | 1.93 ± 0.061 | 2.21 ± 0.085 *# |
| Ch a, mg g−1 DW | 4.85 ± 0.24 | 1.75 ± 0.09 * | 0.36 ± 0.04 *# |
| Ch b, mg g−1 DW | 1.52 ± 0.10 | 0.48 ± 0.01 * | 0.13 ± 0.01 *# |
| Carotenoids, mg g−1 DW | 2.12 ± 0.06 | 1.30 ± 0.08 * | 0.64 ± 0.09 *# |
| Ch a/Car | 1:0.44 | 1:0.74 | 1:1.78 |
| Protein, mg g−1 DW | 283.56 ± 2.12 | 131.40 ± 12.26 * | 87.80 ± 10.26 *# |
| Lipids, mg g−1 DW | 137.39 ± 4.51 | 290.71 ± 1.66 * | 381.03 ± 3.96 *# |
| Ascorbic acid, mg g−1 DW | 0.19 ± 0.57 | 0.08 ± 0.02 * | 0.02 ± 0.01 * |
| Phenolics, mg routine g−1 DW | 0.32 ± 0.05 | 0.23 ± 0.04 * | 0.13 ± 0.03 *# |
| Retinol, mg g−1 DW | 0.08 ± 0.01 | 0.11 ± 0.02 * | 0.16 ± 0.011 *# |
| Tocopherol, mg g−1 DW | 0.49 ± 0.03 | 0.43 ± 0.05 | 0.68 ± 0.06 *# |
| SDH, µmol mg−1 min−1 | 0.04 ± 0.01 | 0.14 ± 0.02 * | 0.08 ± 0.02 *# |
| GPx, nMol mg−1 min−1 | 0.31 ± 0.01 | 0.55 ± 0.02 * | 0.74 ± 0.08 * |
| CAT, pmol mg−1 min−1 | 25.13 ± 0.37 | 57.43 ± 0.98 * | 88.10 ± 4.69 *# |
| SOD, 10 u.unit mg−1 min−1 | 2.59 ± 0.17 | 3.40 ± 0.53 * | 2.87 ± 0.23 |
| TBAaP, nMol g−1 DW | 94.28 ± 6.68 | 57.74 ± 10.63 * | 33.65 ± 9.38 *# |
| TBAaPin, nMol g−1 DW | 192.8 ± 8.64 | 132.9 ± 5.54 * | 55.46 ± 10.93 *# |
| KAAC | 0.49 ± 0.01 | 0.43 ± 0.06 | 0.61 ± 0.4 |
| pH of medium | 10.05 ± 0.04 | 9.92 ± 0.04 * | 9.11 ± 0.06 * |
| O2 disolv. in medium | 20.57 ± 2.70 | 14.48 ± 0.28 * | 12.2 ± 0.15 * |
| CO2 in medium | 0.072 ± 0.001 | 0.069 ± 0.001 | 0.044 ± 0.003 |
| Indicator | Experiment Options | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | EO1 | EO2 | EO3 | EO4 | EO5 | EO6 | EO7 | EO8 | EO9 | |
| Total nitrogen, g L−1 | 0.0413 | 0.0384 | 0.0258 | 0.0129 | 0.0064 | 0.0026 | 0.00129 | 0.00077 | 0.00038 | 0.00026 |
| Total phosphorus, g L−1 | 0.0534 | 0.0072 | 0.0072 | 0.0072 | 0.0005 | 0.0005 | 0.0005 | 0.0002 | 0.0002 | 0.0002 |
| N/P ratio | 0.77:1 | 5.33:1 | 3.58:1 | 1.79:1 | 12.8:1 | 5.2:1 | 2.58:1 | 3.85:1 | 1.9:1 | 1.3:1 |
| Parameters | Control | EO1 | EO2 | EO3 | EO4 | EO5 | EO6 | EO7 | EO8 | EO9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pigments | ||||||||||
| Ch a, mg g−1 DW | 3.78 ± 0.21 | 1.88 ± 0.08 * | 2.66 ± 0.11 *# | 0.3 ± 0.01 *# | 0.65 ± 0.16 *# | 0.95 ± 0.04 * | 2.03 ± 0.21 *# | 1.54 ± 0.01 *# | 0.76 ± 0.01 *# | 0.55 ± 0.09 * |
| Ch b, mg g−1 DW | 1.34 ± 0.08 | 0.67 ± 0.01 * | 0.95 ± 0.03 *# | 0.1 ± 0.01 *# | 0.22 ± 0.05 *# | 0.33 ± 0.01 *# | 0.62 ± 0.06 *# | 0.48 ± 0.01 *# | 0.25 ± 0.01 *# | 0.18 ± 0.04 * |
| Car, mg g−1 DW | 1.61 ± 0.07 | 1.06 ± 0.04 * | 1.18 ± 0.03 * | 0.39 ± 0.01 *# | 0.55 ± 0.13 * | 0.66 ± 0.04 * | 1.3 ± 0.11 *# | 1.03 ± 0.01 *# | 0.52 ± 0.01 *# | 0.4 ± 0.07 * |
| Ch a/Car | 1:0.43 | 1:0.57 | 1:0.44 | 1:1.29 | 1:0.85 | 1:0.69 | 1:0.64 | 1:0.67 | 1:0.68 | 1:0.74 |
| First metabolites | ||||||||||
| Prot., mg g−1 DW | 220.19 ± 12.01 * | 184.51 ± 3.69 * | 197.81 ± 3.94 *# | 156.87 ± 4.33 *# | 181.32 ± 2.34 *# | n/a | n/a | n/a | n/a | n/a |
| Lipids, mg g−1 DW | 141.4 ± 4.7 | 109.1 ± 7.2 | 110.2 ± 9.3 | 92.3 ± 9.4 * | 132.8 ± 18.9 # | n/a | n/a | n/a | n/a | n/a |
| Second metabolites | ||||||||||
| Retinol, mg g−1 DW | 0.11 ± 0.04 | 0.13 ± 0.04 * | 0.08 ± 0.01 *# | 0.02 ± 0.002 *# | 0.027 ± 0.08 * | n/a | n/a | n/a | n/a | n/a |
| Tocopherol, mg g−1 DW | 0.25 ± 0.03 | 0.2 ± 0.07 | 0.2 ± 0.02 | 0.13 ± 0.04 *# | 0.04 ± 0.004 *# | n/a | n/a | n/a | n/a | n/a |
| Antioxidant system | ||||||||||
| GPx, nMol mg−1 min−1 | 2.09 ± 0.15 | 1.85 ± 0.31 | 2.46 ± 0.01 # | 2.53 ± 0.15 | 1.73 ± 0.05 # | n/a | n/a | n/a | n/a | n/a |
| CAT, pmol mg−1 min−1 | 26.0 ± 2.0 | 49.0 ± 2.0 * | 48.0 ± 8.1 * | 85.0 ± 6.0 *# | 70.0 ± 3.4 *# | 94.0 ± 6.0 *# | n/a | n/a | n/a | n/a |
| SOD, 10 u.un mg−1 min−1 | 0.24 ± 0.06 | 0.42 ± 0.15 | 0.55 ± 0.08 | 0.93 ± 0.14 * | 0.87 ± 0.12 * | 1.44 ± 0.31 *# | n/a | n/a | n/a | n/a |
| TBAaP, nMol g−1 DW | 76.35 ± 6.98 | 66.36 ± 4.97 | 63.27 ± 4.93 | 71.57 ± 4.17 | 77.4 ± 6.08 | n/a | n/a | n/a | n/a | n/a |
| TBAaPin, nMol g−1 DW | 101.03 ± 7.45 | 85.46 ± 5.23 * | 90.94 ± 4.37 | 90.94 ± 4.39 | 102.15 ± 6.48 | n/a | n/a | n/a | n/a | n/a |
| KAAC | 0.76 | 0.78 | 0.7 | 0.79 | 0.76 | n/a | n/a | n/a | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltseva, I.; Yakoviichuk, A.; Maltseva, S.; Cherkashina, S.; Kulikovskiy, M.; Maltsev, Y. Biochemical and Antioxidant Characteristics of Chlorococcum oleofaciens (Chlorophyceae, Chlorophyta) under Various Cultivation Conditions. Plants 2024, 13, 2413. https://doi.org/10.3390/plants13172413
Maltseva I, Yakoviichuk A, Maltseva S, Cherkashina S, Kulikovskiy M, Maltsev Y. Biochemical and Antioxidant Characteristics of Chlorococcum oleofaciens (Chlorophyceae, Chlorophyta) under Various Cultivation Conditions. Plants. 2024; 13(17):2413. https://doi.org/10.3390/plants13172413
Chicago/Turabian StyleMaltseva, Irina, Aleksandr Yakoviichuk, Svetlana Maltseva, Svetlana Cherkashina, Maxim Kulikovskiy, and Yevhen Maltsev. 2024. "Biochemical and Antioxidant Characteristics of Chlorococcum oleofaciens (Chlorophyceae, Chlorophyta) under Various Cultivation Conditions" Plants 13, no. 17: 2413. https://doi.org/10.3390/plants13172413





