Leaf Functional Traits and Their Influencing Factors in Six Typical Vegetation Communities
Abstract
1. Introduction
2. Results
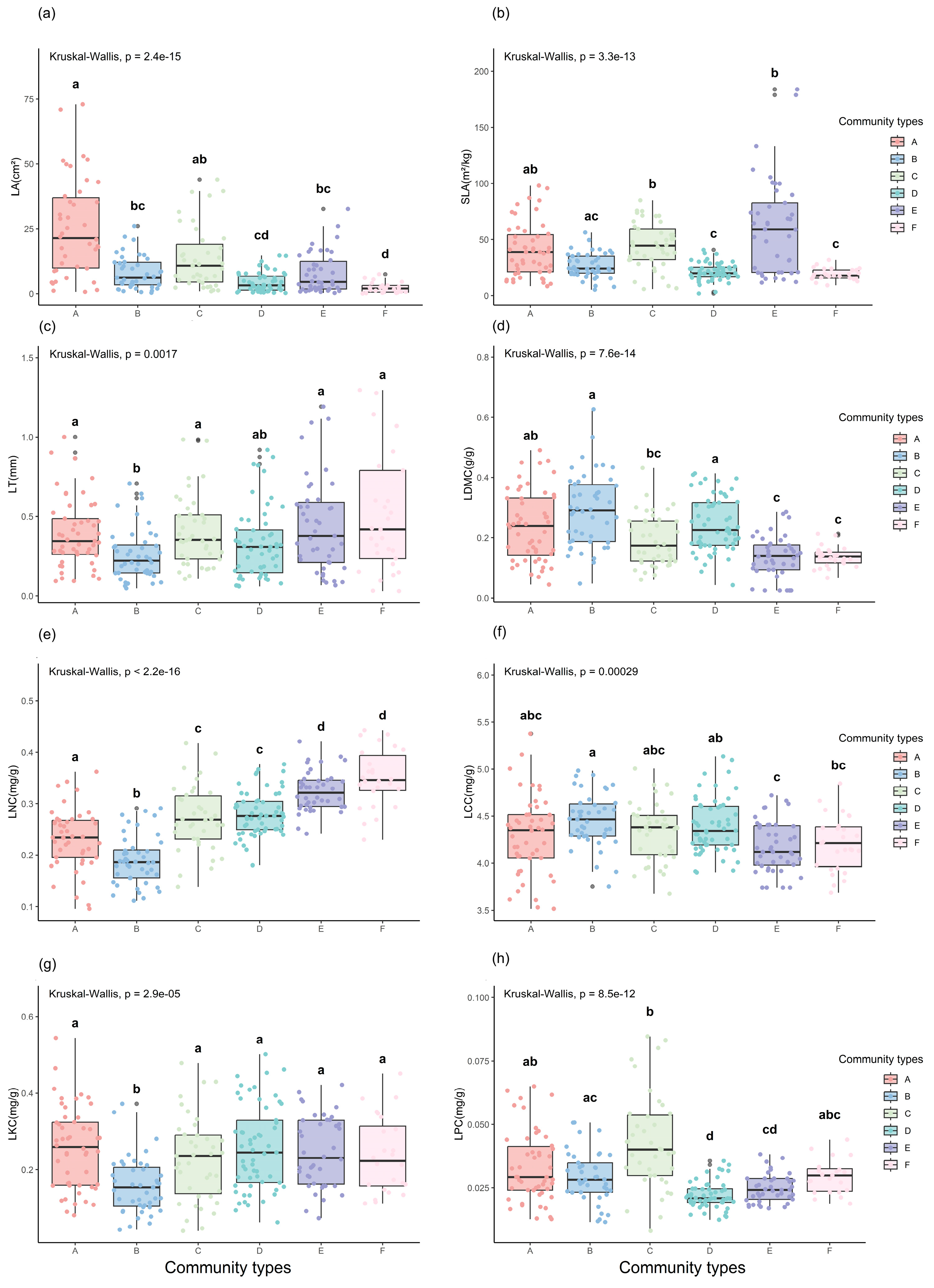
2.1. Comparative Analysis of LFTs among Plant Communities
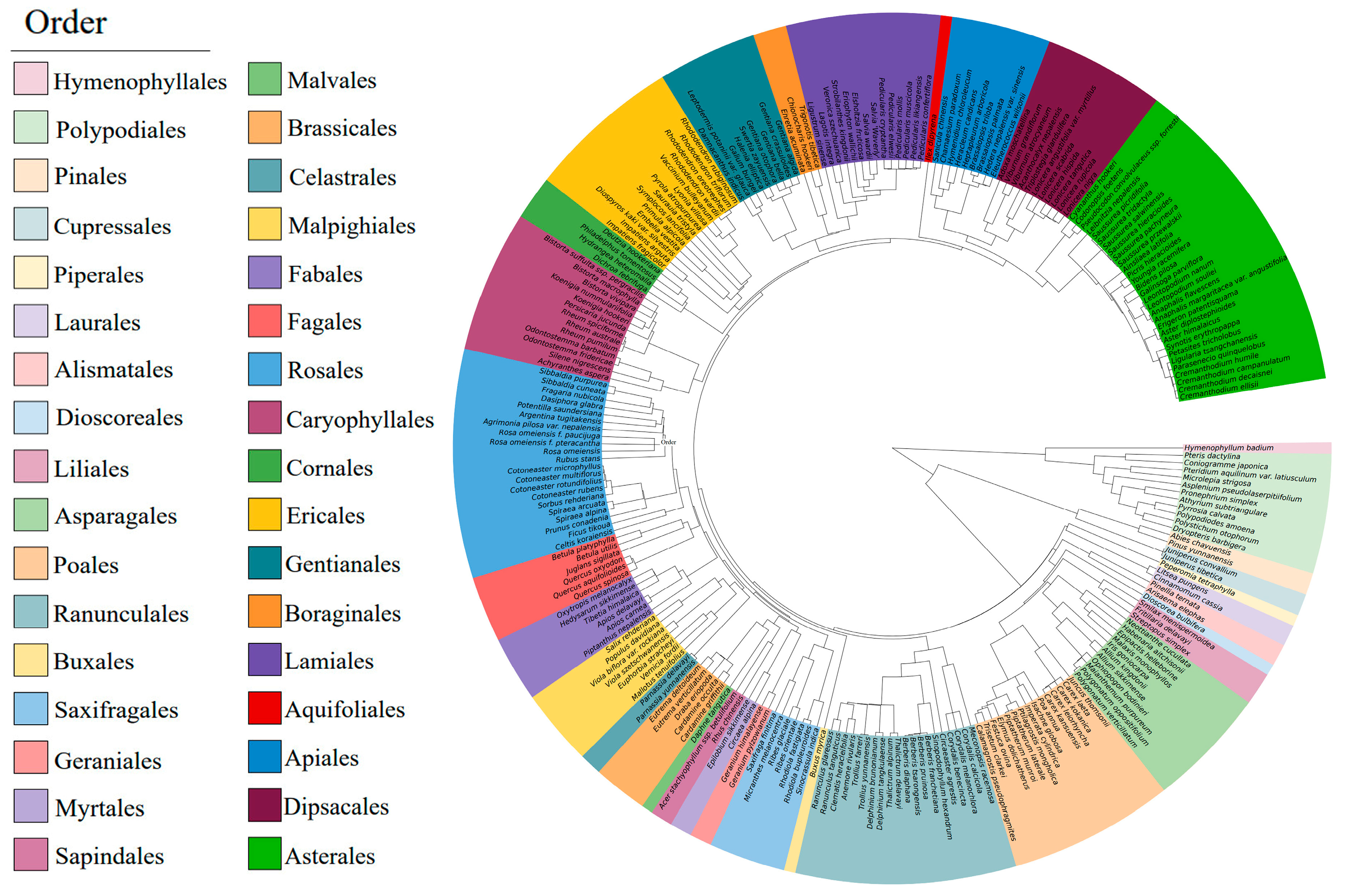
2.2. Phylogenetic Composition of the Plant Community
2.3. The Phylogenetic Signals of the LFTs
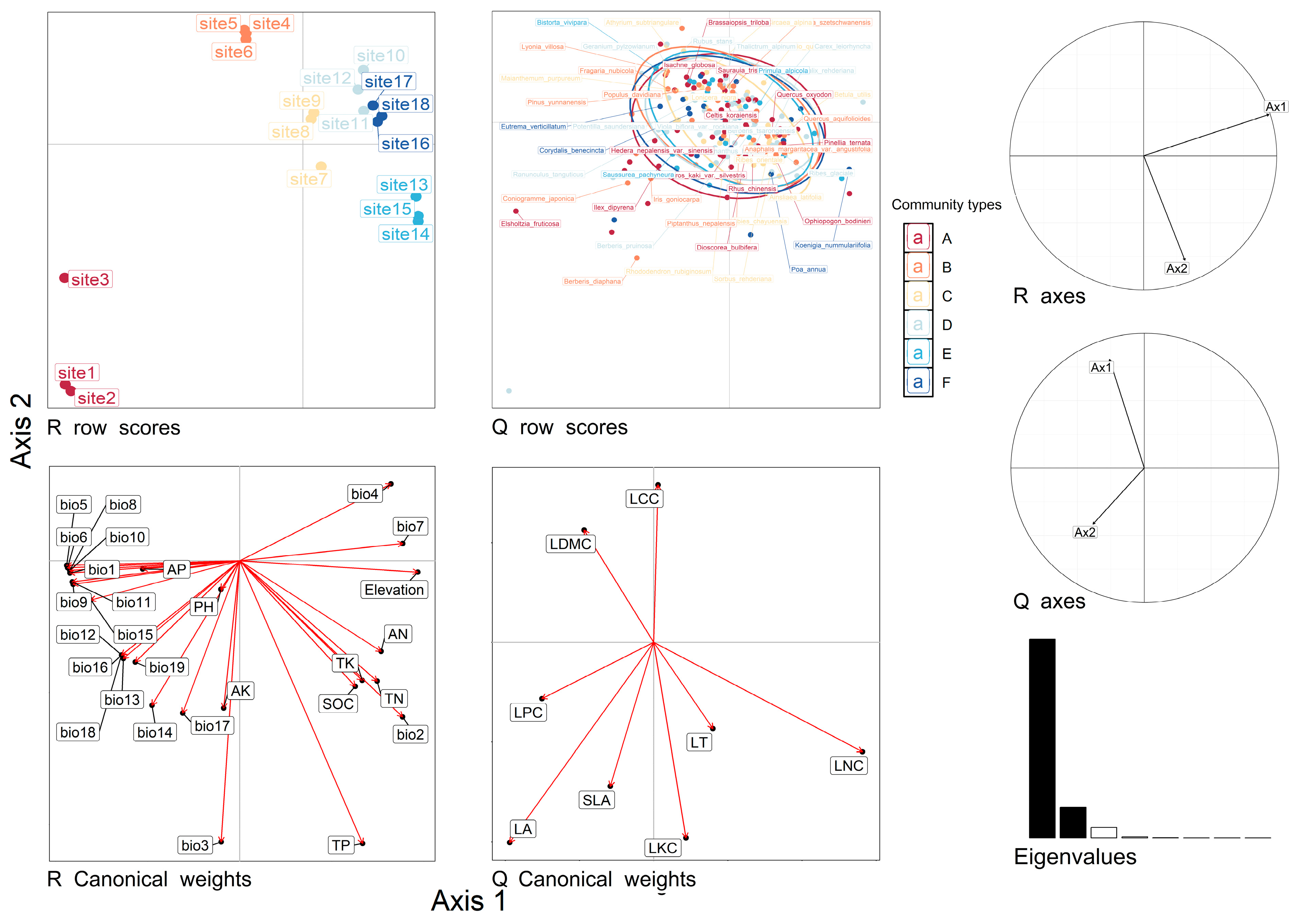
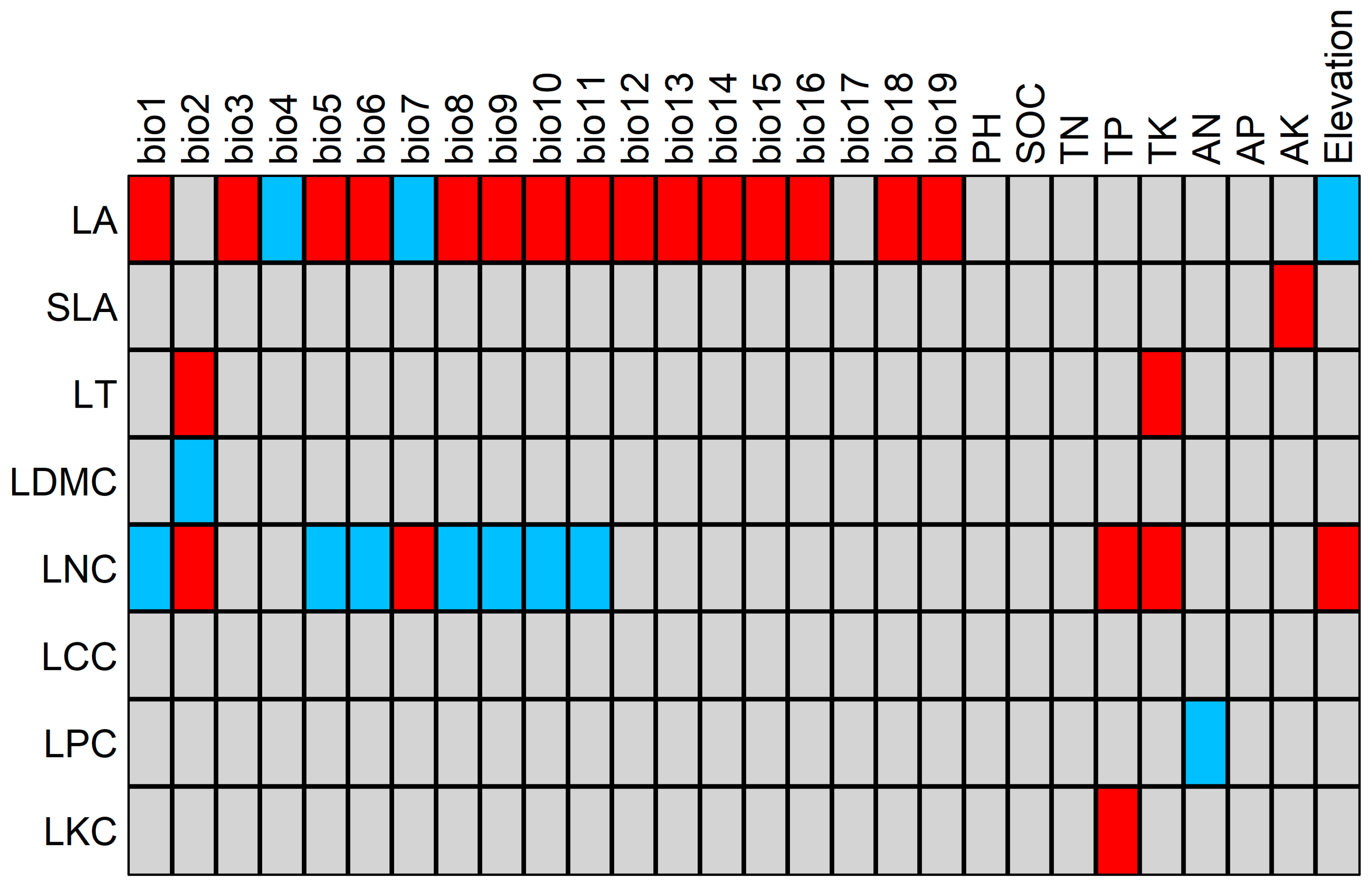
2.4. Relationships between Functional Traits and Environmental Factors
3. Discussion
3.1. Differences in LFTs among Plant Communities
3.2. Relationships between Functional Traits and Phylogenetic Signals
3.3. Response of Functional Traits to Environmental Factors
4. Materials and Methods
4.1. Study Area
4.2. Field Investigation
4.3. Acquisition of Environmental Variable Data
4.4. Measurement of Leaf Functional Traits
4.5. Analysis of Differences in LFTs among Communities
4.6. Construction of the Phylogenetic Tree
4.7. Detection of Phylogenetic Signals
4.8. Analysis of the Relationships between LFTs and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Wang, T.; Wang, H.; Yang, J.; Xie, T.; Zhang, Z.; He, C.; Shan, L. Variation and Correlation among Fine Root Traits of Desert Plants in Arid Areas of Northwest China. Forests 2024, 15, 476. [Google Scholar] [CrossRef]
- Luo, W.; Valverde-Barrantes, O.J.; Weemstra, M.; Cahill, J.F., Jr.; Wang, Z.; He, D.; Chen, Y.; Chu, C.; Wang, Y. Leaf and root traits are partially coordinated but they show contrasting multi-trait-based community trait dispersion patterns in a subtropical forest. J. Plant Ecol. 2024, 17, rtad045. [Google Scholar] [CrossRef]
- Huxley, J.D.; White, C.T.; Humphries, H.C.; Weber, S.E.; Spasojevic, M.J. Plant functional traits are dynamic predictors of ecosystem functioning in variable environments. J. Ecol. 2023, 111, 2597–2613. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Wang, S.; Lv, P.; Yue, P.; Chen, M.; Zuo, X. Patterns and driving factors of functional traits of desert species with different elevational distributions in the Tibetan Plateau and adjacent areas. BMC Plant Biol. 2024, 24, 371. [Google Scholar] [CrossRef]
- Liu, H.; Yin, D.; He, P.; Cadotte, M.W.; Ye, Q. Linking plant functional traits to biodiversity under environmental change. Biol. Divers. 2024, 1, 22–28. [Google Scholar] [CrossRef]
- Eker, T.; Sari, H.; Sari, D.; Canci, H.; Arslan, M.; Aydinoglu, B.; Ozay, H.; Toker, C. Advantage of multiple pods and compound leaf in kabuli chickpea under heat stress conditions. Agronomy 2022, 12, 557. [Google Scholar] [CrossRef]
- Leister, D. Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef]
- Sandoval-Granillo, V.; Meave, J.A. Leaf functional diversity and environmental filtering in a tropical dry forest: Comparison between two geological substrates. Ecol. Evol. 2023, 13, e104912023. [Google Scholar] [CrossRef]
- Puglielli, G.; Bricca, A.; Chelli, S.; Petruzzellis, F.; Acosta, A.T.; Bacaro, G.; Bolpagni, R.; Boscutti, F.; Calvia, G.; Campetella, G.; et al. Intraspecific variability of leaf form and function across habitat types. Ecol. Lett. 2024, 27, e143962024. [Google Scholar] [CrossRef]
- Jin, N.; Yu, X.; Dong, J.; Duan, M.; Mo, Y.; Feng, L.; Bai, R.; Zhao, J.L.; Song, J.; Dossa, G.G.O.; et al. Vertical variation in leaf functional traits of Parashorea chinensis with different canopy layers. Front. Plant Sci. 2024, 15, 1335524. [Google Scholar] [CrossRef]
- Sharma, P.; Rathee, S.; Ahmad, M.; Siddiqui, M.H.; Alamri, S.; Kaur, S.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Leaf functional traits and resource use strategies facilitate the spread of invasive plant Parthenium hysterophorus across an elevational gradient in western Himalayas. BMC Plant Biol. 2024, 24, 234. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Wang, X.; Hu, W.; Xiong, J.; Zhang, Y.; Deng, Y.; Ran, J.; Deng, J. Convergent variations in the leaf traits of desert plants. Plants 2020, 9, 990. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Ji, Y.; Gao, J. Climate factors determine the utilization strategy of forest plant resources at large scales. Front. Plant Sci. 2022, 13, 990441. [Google Scholar] [CrossRef]
- Reich, P.; Wright, I.; Cavender-Bares, J.; Craine, J.; Oleksyn, J.; Westoby, M.; Walters, M. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Gong, H.; Song, W.; Wang, J.; Wang, X.; Ji, Y.; Zhang, X.; Gao, J. Climate factors affect forest biomass allocation by altering soil nutrient availability and leaf traits. J. Integr. Plant Biol. 2023, 65, 2292–2303. [Google Scholar] [CrossRef]
- Firn, J.; McGree, J.M.; Harvey, E.; Flores-Moreno, H.; Schütz, M.; Buckley, Y.M.; Borer, E.T.; Seabloom, E.W.; La Pierre, K.J.; MacDougall, A.M.; et al. Leaf nutrients, not specific leaf area, are consistent indicators of elevated nutrient inputs. Nat. Ecol. Evol. 2019, 3, 400–406. [Google Scholar] [CrossRef]
- Tan, Q.; Jia, Y.; Wang, G. Decoupling of soil nitrogen and phosphorus dynamics along a temperature gradient on the Qinghai-Tibetan plateau. Geoderma 2021, 396, 115084. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, L.; Lv, C.; Liu, L.; Zhao, H.; Gao, J. Climatic factors determine the distribution patterns of leaf nutrient traits at large scales. Plants 2022, 11, 2171. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Peng, S.; Chen, Y.; Cao, Y. The coupling of leaf, litter, and soil nutrients in warm temperate forests in northwestern China. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Yao, L.; Wu, C.; Wang, Z.; Jiang, B. Alpha and beta diversity of functional traits in subtropical evergreen broad-leaved secondary forest communities. Front. Plant Sci. 2024, 15, 1223351. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Niu, K. Biodiversity in mosaic communities: Predicting soil microbial diversity using plant functional traits in alpine meadow. Eur. J. Soil Biol. 2024, 120, 103599. [Google Scholar] [CrossRef]
- Tüfekcioğlu, İ.; Tavşanoğlu, Ç. Growth form, regeneration mode, and vegetation type explain leaf trait variability at the species and community levels in Mediterranean woody vegetation. Ecol. Evol. 2024, 14, e111452024. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, S.; Hu, J.; Zou, X.; Tie, L.; Li, Y.; Cui, X.L.; Huang, C.D.; Sardans, J.; Peñuelas, J. Variations and trade-offs in leaf and culm functional traits among 77 woody bamboo species. BMC Plant Biol. 2024, 24, 387. [Google Scholar] [CrossRef]
- Kelly, R.; Healy, K.; Anand, M.; Baudraz, M.E.; Bahn, M.; Cerabolini, B.E.; Cornelissen, J.H.C.; Dwyer, J.M.; Jackson, A.L.; Kattge, J.; et al. Climatic and evolutionary contexts are required to infer plant life history strategies from functional traits at a global scale. Ecol. Lett. 2021, 24, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Galmán, A.; Abdala-Roberts, L.; Covelo, F.; Rasmann, S.; Moreira, X. Parallel increases in insect herbivory and defenses with increasing elevation for both saplings and adult trees of oak (Quercus) species. Am. J. Bot. 2019, 106, 1558–1565. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, R.; Yang, L.; Tan, T.; Li, H.; Liu, M. Elevation gradient distribution of indices of tree population in a montane forest: The role of leaf traits and the environment. For. Ecosyst. 2022, 9, 100012. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Rumpf, S.B.; Giejsztowt, J.; Millen, J.; Morgan, J.W.; Nicotra, A.B.; Venn, S.; Zong, S.; Dickinson, K.J.; et al. Intraspecific trait variation in alpine plants relates to their elevational distribution. J. Ecol. 2022, 110, 860–875. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Liang, E.; Rokaya, M.B.; Rai, S.; Dyola, N.; Sun, J.; Zhang, L.; Zhu, H.; Chettri, N.; Chaudhary, R.P.; et al. Functional traits of a plant species fingerprint ecosystem productivity along broad elevational gradients in the Himalayas. Funct. Ecol. 2023, 37, 383–394. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugeanan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture; the multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Schmull, M.; Thomas, F.M. Morphological and physiological reactions of young deciduous trees (Quercus robur L., Q. petraea [Matt.] Liebl., Fagus sylvatica L.) to waterlogging. Plant Soil 2000, 225, 227–242. [Google Scholar] [CrossRef]
- Li, F.; Yang, G.; Xie, Y.; Chen, X.; Deng, Z.; Hu, J. Competition between two wetland macrophytes under different levels of sediment saturation. J. Limnol. 2015, 74, 623–630. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Umer, M.; Guo, Y.; Tan, Q.; Kang, L.; Fang, Z.Y.; Shen, K.P.; Xia, T.T.; Wu, P.; et al. Strategic differentiation of subcommunities composed of evergreen and deciduous woody species associated with leaf functional traits in the subtropical mixed forest. Ecol. Indic. 2023, 150, 110281. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Wu, J.X.; Du, Z.Y.; Chen, Y.J.; Wang, Y.; Liu, M.; Li, W.C.; Liang, E.Y. Plant community traits and functions mediate the biomass trade-off of alpine grasslands along precipitation gradients on the Tibetan Plateau. J. Plant Ecol. 2023, 16, rtad009. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Li, X.E.; Song, X.; Zhao, J.; Lu, H.; Qian, C.; Zhao, X. Shifts and plasticity of plant leaf mass per area and leaf size among slope aspects in a subalpine meadow. Ecol. Evol. 2021, 11, 14042–14055. [Google Scholar] [CrossRef]
- Ji-Shi, A.; Tian, L.; Zhao, W.; Zhao, J. Elevational variations of leaf morphological traits and its responses to simulated climate warming in Tibetan alpine meadows. Glob. Ecol. Conserv. 2024, 49, e027882024. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Cornelissen, J.H.; Pérez-Harguindeguy, N.; Díaz, S.; Grime, J.P.; Marzano, B.; Cabido, M.; Vendramini, F.; Cerabolini, B. Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol. 1999, 143, 191–200. [Google Scholar] [CrossRef]
- Qin, S.Y.; Zuo, Z.Y.; Guo, C.; Du, X.Y.; Liu, S.Y.; Yu, X.Q.; Xiang, X.G.; Rong, J.; Liu, B.; Liu, Z.F.; et al. Phylogenomic insights into the origin and evolutionary history of evergreen broadleaved forests in East Asia under Cenozoic climate change. Mol. Ecol. 2023, 32, 2850–2868. [Google Scholar] [CrossRef]
- Wangda, P.; Ohsawa, M. Structure and regeneration dynamics of dominant tree species along altitudinal gradient in a dry valley slopes of the Bhutan Himalaya. For. Ecol. Manag. 2006, 230, 136–150. [Google Scholar] [CrossRef]
- Nguyen, L.X.; Lambertini, C.; Sorrell, B.K.; Eller, F.; Achenbach, L.; Brix, H. Photosynthesis of co-existing Phragmites haplotypes in their non-native range: Are characteristics determined by adaptations derived from their native origin? AoB Plants 2013, 5, plt016. [Google Scholar] [CrossRef]
- Mount, H.E.; Smith, M.D.; Knapp, A.K.; Griffin-Nolan, R.J.; Collins, S.L.; Atkins, D.H.; Stears, A.E.; Laughlin, D.C. Drought-tolerant grassland species are generally more resistant to competition. J. Ecol. 2024, 112, 416–426. [Google Scholar] [CrossRef]
- Sedej, T.T.; Erznožnik, T.; Rovtar, J. Effect of UV radiation and altitude characteristics on the functional traits and leaf optical properties in Saxifraga hostii at the alpine and montane sites in the Slovenian Alps. Photochem. Photobiol. Sci. 2020, 19, 180–192. [Google Scholar] [CrossRef]
- Zhou, Y.; Kitudom, N.; Fauset, S.; Slot, M.; Fan, Z.; Wang, J.; Liu, W.W.; Lin, H. Leaf thermal regulation strategies of canopy species across four vegetation types along a temperature and precipitation gradient. Agric. For. Meteorol. 2023, 343, 109766. [Google Scholar] [CrossRef]
- Liu, M.X.; Che, Y.D.; Li, L.R.; Jiao, J.; Xiao, W. Redundancy analysis of leaf traits and environmental factors of alpine meadow in Southern Gansu Province. Chin. J. Ecol. 2017, 36, 2473. (In Chinese) [Google Scholar]
- Zhou, T.; Hou, G.; Sun, J.; Zong, N.; Shi, P. Degradation shifts plant communities from S-to R-strategy in an alpine meadow, Tibetan Plateau. Sci. Total Environ. 2021, 800, 149572. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Osnas, J.L.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Wang, H.; Atkin, O.K.; Bloomfield, K.J.; Domingues, T.F.; Gleason, S.M.; Maire, V.; Onoda, Y.; et al. Leaf nitrogen from the perspective of optimal plant function. J. Ecol. 2022, 110, 2585–2602. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Haslehurst, T.; Ares, A.; Baruch, Z. Leaf trait relationships of native and invasive plants: Community-and global-scale comparisons. New Phytol. 2007, 176, 635–643. [Google Scholar] [CrossRef]
- Montesinos, D. Fast invasives fastly become faster: Invasive plants align largely with the fast side of the plant economics spectrum. J. Ecol. 2022, 110, 1010–1014. [Google Scholar] [CrossRef]
- Zhao, W.; Mao, Q.; Liu, G.; Li, Y.; Xia, J.; Zhang, Y.J. Patterns of compound-leaf form and deciduous-leaf habit across forests in China: Their association and key climatic factors. Sci. Total Environ. 2022, 851, 158108. [Google Scholar] [CrossRef]
- Liu, M.; Xu, L.; Mu, R.; Zhang, G.; Yu, R.; Li, L. Plant community assembly of alpine meadow at different altitudes in Northeast Qinghai-Tibet Plateau. Ecosphere 2023, 14, e4354. [Google Scholar] [CrossRef]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.J.; Sun, Y.; Li, F.; Deng, J. Phylogenetic independence in the variations in leaf functional traits among different plant life forms in an arid environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef]
- An, N.; Lu, N.; Fu, B.; Wang, M.; He, N. Distinct responses of leaf traits to environment and phylogeny between herbaceous and woody angiosperm species in China. Front. Plant Sci. 2021, 12, 799401. [Google Scholar] [CrossRef]
- E-Vojtkó, A.; de Bello, F.; Lososová, Z.; Götzenberger, L. Phylogenetic diversity is a weak proxy for functional diversity but they are complementary in explaining community assembly patterns in temperate vegetation. J. Ecol. 2023, 111, 2218–2230. [Google Scholar] [CrossRef]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.H.; Hou, Q.Q.; Hu, W.G.; Ran, J.Z.; et al. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Zhang, J.; Li, M.; Hou, J.; He, N. Significant phylogenetic signal and climate-related trends in leaf caloric value from tropical to cold-temperate forests. Sci. Rep. 2016, 6, 36674. [Google Scholar] [CrossRef]
- Shui, W.; Feng, J.; Li, H.; Jiang, C.; Sun, X.; Liu, Y.; Zhang, Y.; Sun, X. Phylogeny and functional traits structure of plant communities with different slope aspects in the degraded karst tiankeng. Acta Ecol. Sin. 2022, 42, 8050–8060. (In Chinese) [Google Scholar]
- Cavender-Bares, J.; Keen, A.; Miles, B. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology 2006, 87, S109–S122. [Google Scholar] [CrossRef] [PubMed]
- Swenson, N.G.; Enquist, B.J.; Pither, J.; Thompson, J.; Zimmerman, J.K. The problem and promise of scale dependency in community phylogenetics. Ecology 2006, 87, 2418–2424. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental fltering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Atkinson, L.J.; Campbell, C.D.; Zaragoza-Castells, J.; Hurry, V.; Atkin, O.K. Impact of growth temperature on scaling relationships linking photosynthetic metabolism to leaf functional traits. Funct. Ecol. 2010, 24, 1181–1191. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, N. Leaf trait networks based on global data: Representing variation and adaptation in plants. Front. Plant Sci. 2021, 12, 710530. [Google Scholar] [CrossRef]
- Islam, T.; Hamid, M.; Nawchoo, I.A.; Khuroo, A.A. Leaf functional traits vary among growth forms and vegetation zones in the Himalaya. Sci. Total Environ. 2024, 906, 167274. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.A.; Schwalm, C.R.; Arcus, V.L.; Koch, G.W.; Liang, L.L.; Schipper, L.A. How close are we to the temperature tipping point of the terrestrial biosphere? Sci. Adv. 2021, 7, eaay1052. [Google Scholar] [CrossRef]
- Miller, B.D.; Carter, K.R.; Reed, S.C.; Wood, T.E.; Cavaleri, M.A. Only sun-lit leaves of the uppermost canopy exceed both air temperature and photosynthetic thermal optima in a wet tropical forest. Agric. For. Meteorol. 2021, 301, 108347. [Google Scholar] [CrossRef]
- An, H.; Zhao, Y.; Ma, M. Precipitation controls seed bank size and its role in alpine meadow community regeneration with in-creasing altitude. Glob. Chang. Biol. 2020, 26, 5767–5777. [Google Scholar] [CrossRef]
- Wang, R.; Dijkstra, F.A.; Han, X.; Jiang, Y. Root Nitrogen Reallocation: What Makes It Matter? Trends Plant Sci. 2024. [Google Scholar] [CrossRef]
- Meng, X.; Lou, H.; Zhai, S.; Zhang, R.; Liu, G.; Xu, W.; Yu, J.Z.; Zhang, Y.F.; Ni, Z.F.; Sun, Q.X.; et al. TaNAM-6A is essential for nitrogen remobilisation and regulates grain protein content in wheat (Triticum aestivum L.). Plant Cell Environ. 2024, 47, 2310–2321. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Su, J.; Xu, J.; Zhang, X.; Shang, J.; Gao, J. Leaf nutrient traits of planted forests demonstrate a heightened sensitivity to environmental changes compared to natural forests. Front. Plant Sci. 2024, 15, 1372530. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Prentice, I.C.; Harrison, S.P.; Wang, G.; Sun, X. Predictability of leaf traits with climate and elevation: A case study in Gongga Mountain, China. Tree Physiol. 2021, 41, 1336–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; He, N.; Wang, Q.; Zhang, X.; Wang, R.; Xu, Z.; Yu, G. The altitudinal patterns of leaf C:N:P stoichiometry are regulated by plant growth form, climate and soil on Changbai Mountain, China. PLoS ONE 2014, 9, e951962014. [Google Scholar]
- Ruan, X.; Galy, A. On the significance of periglacial conditions in active mountain belts for chemical weathering processes: Insights from the Chayu area, SE Tibet. Chem. Geol. 2021, 585, 120581. [Google Scholar] [CrossRef]
- Xing, Y.; Ree, R.H. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2017, 114, E3444–E3451. [Google Scholar] [CrossRef]
- Sun, H. Tethys retreat and Himalayas-Hengduanshan Mountains uplift and their significance on the origin and development of the Sino-Himalayan elements and alpine flora. Acta Bot. Yunnanica 2002, 24, 273–288. (In Chinese) [Google Scholar]
- Sun, H.; Zhang, J.; Deng, T.; Boufford, D.E. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers. 2017, 39, 161. [Google Scholar] [CrossRef]
- Gu, W.Q.; Yang, X.X.; Zhang, S.J.; Luo, J. Study on the flora of seed plants in Zayü River Basin, southeast Qinghai-Tibet Plateau. Acta Bot. Boreali-Occident. Sin. 2022, 42, 1749–1759. (In Chinese) [Google Scholar]
- Wang, X.; Liu, J.; He, Z.; Xing, C.; Zhu, J.; Gu, X.; Lan, Y.Q.; Wu, Z.Y.; Liao, P.C.; Zhu, D. Forest gaps mediate the structure and function of the soil microbial community in a Castanopsis kawakamii forest. Ecol. Indic. 2021, 122, 107288. [Google Scholar] [CrossRef]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.R.; Ling, N. Soil carbon, nitrogen, and phosphorus cycling microbial populations and their resistance to global change depend on soil C: N: P stoichiometry. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Xiong, K.; Yu, Y.; Min, X. Changes of leaf functional traits in karst rocky desertification ecological environment and the driving factors. Glob. Ecol. Conserv. 2020, 24, e01381. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Yao, L.; Ding, Y.; Xu, H.; Deng, F.; Yao, L.; Ai, X.; Zang, R. Patterns of diversity change for forest vegetation across different climatic regions-A compound habitat gradient analysis approach. Glob. Ecol. Conserv. 2020, 23, e01106. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Zhu, Y.; Tu, Y.; Wang, D.; Indree, T. Plant diversity mediates the response of ecosystem multifunctionality to climate factor in Eastern Eurasian Steppe. Glob. Ecol. Conserv. 2024, 50, e02827. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Montès, N.; Tosini, L.; Laffont-Schwob, I.; Le Bagousse-Pinguet, Y.; Folzer, H. FAMeLeS: A multispecies and fully automated method to measure morphological leaf traits. Methods Ecol. Evol. 2024, 15, 484–492. [Google Scholar] [CrossRef]
- Li, Y.; Zou, D.; Shrestha, N.; Xu, X.; Wang, Q.; Jia, W.; Wang, Z. Spatiotemporal variation in leaf size and shape in response to climate. J. Plant Ecol. 2020, 13, 87–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Lu, Q.; Ma, J.; Shen, Y.; Wang, G. Different responses of leaf and root economics spectrum to grazing time at the community level in desert steppe, China. Sci. Total Environ. 2024, 909, 168547. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: https://www.R-project.org/ (accessed on 16 May 2024).
- Zhang, J.; Qian, H.U. Taxonstand: An R package for standardizing scientific names of plants and animals. Plant Divers. 2023, 45, 1–5. [Google Scholar]
- Jin, Y.; Qian, H.U. PhyloMaker: An R package that can generate large phylogenetic trees for plants and animals. Plant Divers. 2023, 45, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T., Jr.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar]
- Keck, F.; Rimet, F.; Bouchez, A.; Franc, A. phylosignal: An R package to measure, test, and explore the phylogenetic signal. Ecol. Evol. 2016, 6, 2774–2780. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Deosaran, R.; Carvalho, F.; Nunes, A.; Köbel, M.; Serafim, J.; Hooda, P.S.; Waller, M.; Branquinho, C.; Brown, K.A. Response of soil carbon and plant diversity to grazing and precipitation in High Nature Value farmlands. For. Ecol. Manag. 2024, 555, 121734. [Google Scholar] [CrossRef]


| Leaf Traits | K | p | Leaf Traits | K | p |
|---|---|---|---|---|---|
| LA | 0.071137 | 0.243 | LNC | 0.041542 | 0.072 |
| SLA | 0.08277 | 0.607 | LCC | 0.072946 | 0.059 |
| LT | 0.090912 | 0.01 * | LPC | 0.065856 | 0.006 * |
| LDMC | 0.033216 | 0.463 | LKC | 0.054758 | 0.003 * |
| Climate Variables | Description |
|---|---|
| Bio1 | Annual Mean Temperature |
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) |
| Bio3 | Isothermality (BIO2/BIO7) (×100) |
| Bio4 | Temperature Seasonality (standard deviation × 100) |
| Bio5 | Max Temperature of Warmest Month |
| Bio6 | Min Temperature of Coldest Month |
| Bio7 | Temperature Annual Range (BIO5–BIO6) |
| Bio8 | Mean Temperature of Wettest Quarter |
| Bio9 | Mean Temperature of Driest Quarter |
| Bio10 | Mean Temperature of Warmest Quarter |
| Bio11 | Mean Temperature of Coldest Quarter |
| Bio12 | Annual Precipitation |
| Bio13 | Precipitation of Wettest Month |
| Bio14 | Precipitation of Driest Month |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) |
| Bio16 | Precipitation of Wettest Quarter |
| Bio17 | Precipitation of Driest Quarter |
| Bio18 | Precipitation of Warmest Quarter |
| Bio19 | Precipitation of Coldest Quarter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Deng, S.; Bai, Y.; Wu, Z.; Luo, J. Leaf Functional Traits and Their Influencing Factors in Six Typical Vegetation Communities. Plants 2024, 13, 2423. https://doi.org/10.3390/plants13172423
Xing Y, Deng S, Bai Y, Wu Z, Luo J. Leaf Functional Traits and Their Influencing Factors in Six Typical Vegetation Communities. Plants. 2024; 13(17):2423. https://doi.org/10.3390/plants13172423
Chicago/Turabian StyleXing, Yuting, Shiqin Deng, Yuanyin Bai, Zhengjie Wu, and Jian Luo. 2024. "Leaf Functional Traits and Their Influencing Factors in Six Typical Vegetation Communities" Plants 13, no. 17: 2423. https://doi.org/10.3390/plants13172423
APA StyleXing, Y., Deng, S., Bai, Y., Wu, Z., & Luo, J. (2024). Leaf Functional Traits and Their Influencing Factors in Six Typical Vegetation Communities. Plants, 13(17), 2423. https://doi.org/10.3390/plants13172423





