Landscape, Human Disturbance, and Climate Factors Drive the Species Richness of Alien Invasive Plants on Subtropical Islands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plant Species Data
2.3. Environmental Factors
2.4. Statistics and Analysis
3. Results
3.1. Analysis of Species Composition and Distribution Patterns
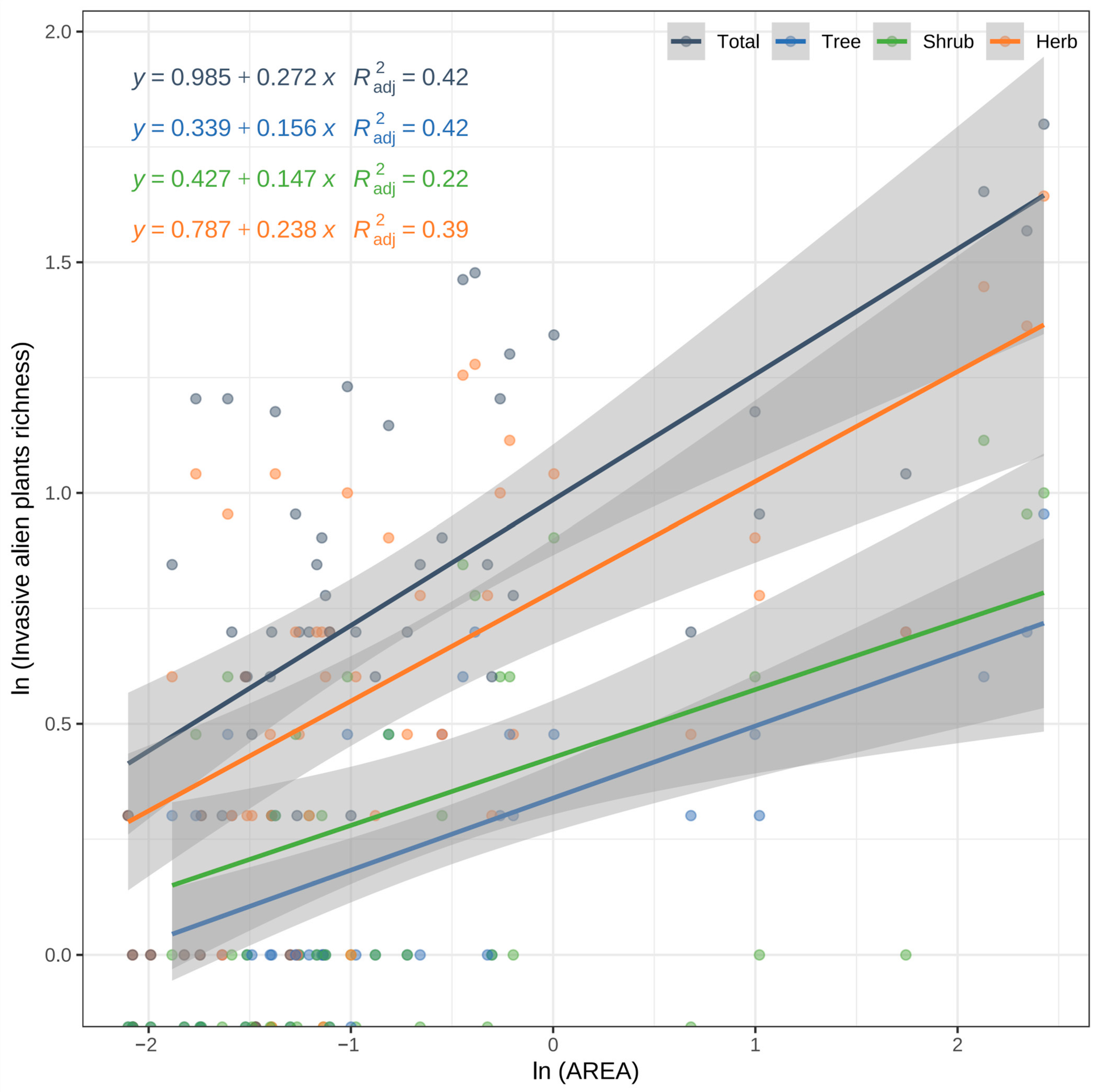
3.2. Species–Area Relationship
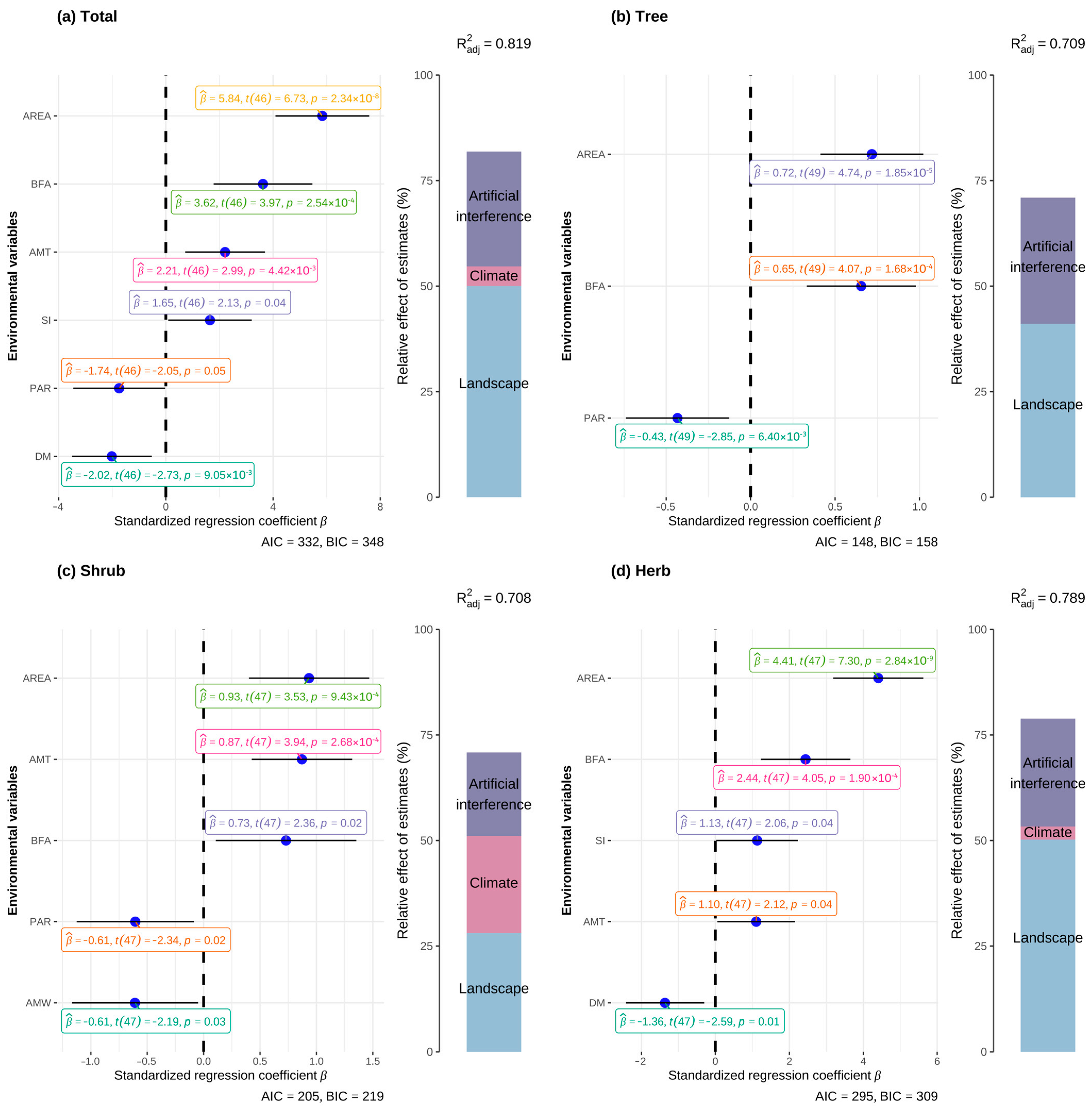
3.3. IAPs and Island Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Islands Name | Latitude and Longitude | Species Richness | No. of Forest Investigation Plots | No. of Shrub Investigation Plots | No. of Herb Investigation Plots | Area (km2) | Elevation (m) | Perimeter–area Ratio | Shape Index | Annual Mean Wind (m/s) | Annual Mean Temperature (°C) | Annual Precipitation (mm) | Distance to the Mainland (km) | Distance to the Nearest Large Island (km) | Proportion of Buildings and Farmland Area (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baijiangdao | 119°815′ E–25°425′ N | 2 | 4 | 16 | 20 | 0.05 | 33.6 | 27.5 | 1.81 | 8.86 | 19.94 | 1258 | 17 | 3.92 | - |
| Beiguanyu | 119°687′ E–25°349′ N | 4 | 6 | 24 | 30 | 0.13 | 40.90 | 12.94 | 1.33 | 8.22 | 19.94 | 1258 | 3.29 | 8.42 | - |
| Beixiangluyu | 119°690′ E–25°616′ N | 5 | 3 | 12 | 15 | 0.04 | 15.6 | 20.83 | 1.18 | 8.44 | 19.84 | 1257 | 9.79 | 0.6 | - |
| Chixietedao | 119°789′ E–25°646′ N | 2 | 2 | 8 | 10 | 0.02 | 28.9 | 31.89 | 1.37 | 7.88 | 19.84 | 1257 | 19.77 | 1.14 | - |
| Dahuaiyu | 119°705′ E–25°545′ N | 3 | 3 | 12 | 15 | 0.03 | 26.80 | 31.78 | 1.61 | 6.79 | 19.84 | 1257 | 9.17 | 0.63 | - |
| Daisongdao | 117°957′ E–24°174′ N | 29 | 12 | 48 | 60 | 0.36 | 27.30 | 7.71 | 1.3 | 4.81 | 20.96 | 1142 | 0.4 | 1.4 | 0.79 |
| Daliandao | 119°689′ E–25°649′ N | 16 | 30 | 120 | 150 | 9.96 | 238.5 | 2.03 | 1.81 | 5.96 | 19.84 | 1257 | 7.88 | 1.18 | 0.17 |
| Dashengdao | 119°020′E–25°081′ N | 1 | 5 | 20 | 25 | 0.07 | 442 | 17.78 | 136 | 7.9 | 20.32 | 1131 | 2.54 | 1.28 | - |
| Dasongdao | 119°799′ E–25°649′ N | 9 | 10 | 40 | 50 | 0.28 | 51.80 | 8.86 | 1.33 | 9.06 | 19.84 | 1257 | 20.14 | 1.16 | - |
| Dayudao | 119°663′ E–25°451′ N | 7 | 8 | 32 | 40 | 0.22 | 45.10 | 12.38 | 1.64 | 7.76 | 19.94 | 1211 | 2.47 | 1.05 | 0.13 |
| Dayuzai | 119°398′ E–25°254′ N | 1 | 2 | 8 | 10 | 0.01 | 13.98 | 17.67 | 0.45 | 8.30 | 20.30 | 1190 | 3.33 | 4.38 | - |
| Dazuidao | 118°771′ E–24°830′ N | 20 | 16 | 64 | 80 | 0.61 | 103.10 | 8.39 | 1.85 | 7.19 | 20.57 | 1085 | 2.33 | 40.97 | 0.04 |
| Dongshandao | 117°424′ E–23°698′ N | 37 | 80 | 320 | 400 | 220.18 | 274.30 | 0.51 | 2.12 | 3.07 | 21.28 | 1296 | 0.31 | 23.13 | 0.24 |
| Dongxiangdao | 119°885′ E–25°594′ N | 5 | 24 | 96 | 120 | 4.8 | 134.60 | 4.1 | 2.53 | 5.56 | 19.75 | 1280 | 26.5 | 2.3 | 0.46 |
| Dongyuzai | 119°400′ E–25°254′ N | 1 | 2 | 8 | 10 | 0.02 | 16.00 | 29.8 | 1.03 | 8.28 | 20.3 | 1190 | 3.31 | 4.11 | - |
| Dongzhuodao | 119°828′ E–25°669′ N | 0 | 3 | 12 | 15 | 0.03 | 36.00 | 27.73 | 1.44 | 7.95 | 20.03 | 1259 | 21.27 | 4.11 | - |
| Duimiandao | 117°524′ E–23°748′ N | 17 | 6 | 24 | 30 | 0.10 | 25.80 | 20.71 | 1.81 | 6.3 | 21.22 | 1239 | 5.78 | 0.49 | 0.01 |
| Geshaqingyu | 119°738′ E–26°278′ N | 2 | 2 | 8 | 10 | 0.02 | 21.05 | 42.14 | 1.60 | 4.53 | 18.81 | 1436 | 0.66 | 1.49 | - |
| Guangyouyu | 119°834′ E–25°581′ N | 5 | 4 | 16 | 20 | 0.06 | 24.20 | 17.30 | 1.22 | 8.14 | 19.75 | 1280 | 22.57 | 0.32 | 0.03 |
| Guluoyu | 119°813′ E–25°671′ N | 1 | 2 | 8 | 10 | 0.01 | 33.2 | 41.84 | 1.19 | 7.88 | 20.03 | 1259 | 20.33 | 2.64 | - |
| Haitandao | 119°766′ E–25°544′ N | 63 | 104 | 416 | 520 | 267.13 | 438.2 | 0.48 | 2.23 | 4.67 | 19.84 | 1257 | 3.59 | 1.19 | 0.62 |
| Hengyu | 119°704′ E–25°533′ N | 4 | 3 | 12 | 15 | 0.03 | 22.4 | 31.23 | 1.53 | 7.06 | 19.84 | 1257 | 9.04 | 0.47 | - |
| Hongshanyu | 119°841′ E–25°578′ N | 4 | 3 | 12 | 15 | 0.03 | 20.8 | 33.09 | 1.63 | 7.98 | 19.75 | 1280 | 23.05 | 0.67 | - |
| Huanggandao | 119°024′ E–25°037′ N | 16 | 16 | 64 | 80 | 0.55 | 72.4 | 8.27 | 1.73 | 6.34 | 20.32 | 1131 | 0.72 | 3.99 | - |
| Huangmendao | 119°680′ E–25°461′ N | 8 | 4 | 16 | 20 | 0.07 | 34.7 | 19.73 | 1.49 | 6.85 | 19.94 | 1258 | 3.89 | 0.15 | - |
| Huangwanyu | 119°823′ E–26°287′ N | 7 | 5 | 20 | 25 | 0.07 | 26.9 | 28.88 | 2.12 | 6.46 | 18.81 | 1436 | 1.2 | 1.15 | 0.01 |
| Huchudao | 119°656′ E–26°146′ N | 6 | 5 | 20 | 25 | 0.07 | 33.72 | 22.56 | 1.74 | 5.5 | 19.35 | 1352 | 5.2 | 0.38 | - |
| Huiyu | 118°995′ E–25°183′ N | 30 | 14 | 56 | 70 | 0.41 | 59.9 | 13.14 | 2.38 | 6.58 | 20.04 | 1196 | 1.11 | 1.28 | 0.55 |
| Huoqingyu | 119°363′ E–25°331′ N | 4 | 16 | 64 | 80 | 0.5 | 54 | 9.32 | 1.86 | 8.67 | 20.3 | 1190 | 5.79 | 1.82 | - |
| Jiangshandao | 119°804′ E–25°443′ N | 7 | 14 | 56 | 70 | 0.47 | 68.1 | 10.01 | 1.94 | 10.71 | 19.94 | 1258 | 17.33 | 1.12 | - |
| Jinpaidao | 119°716′ E–26°357′ N | 9 | 4 | 16 | 20 | 0.05 | 18.82 | 17.27 | 1.12 | 4.53 | 18.54 | 1493 | 1.36 | 0.44 | - |
| Langqidao | 119°597′ E–26°094′ N | 11 | 60 | 240 | 300 | 55.5 | 275 | 0.59 | 1.23 | 3.09 | 19.35 | 1352 | 0.56 | 2.3 | 0.60 |
| LianjiangWuyu | 119°721′ E–26°270′ N | 5 | 6 | 24 | 30 | 0.11 | 41.68 | 16.45 | 1.51 | 5.36 | 18.81 | 1436 | 0.62 | 2.59 | - |
| Longmuyu | 119°692′ E–25°349′ N | 5 | 3 | 12 | 15 | 0.03 | 20.2 | 37.97 | 1.72 | 7.59 | 19.94 | 1258 | 3.95 | 0.57 | - |
| Muyudao | 119°721′ E–26°242′ N | 5 | 8 | 32 | 40 | 0.19 | 17.81 | 13.53 | 1.66 | 5.85 | 18.81 | 1436 | 3.64 | 2.52 | 0.01 |
| Nanchidao | 117°488′ E–23°706′ N | 16 | 2 | 8 | 10 | 0.02 | 49.52 | 28.19 | 1.25 | 5.73 | 21.28 | 1296 | 0.05 | 0.03 | 0.01 |
| Nanyu | 117°523′ E–23°721′ N | 15 | 3 | 12 | 15 | 0.04 | 22.7 | 20.09 | 1.17 | 6.39 | 21.22 | 1239 | 0.12 | 0.12 | 0.01 |
| Niguyu | 120°152′ E–26°558′ N | 9 | 32 | 128 | 160 | 10.48 | 231.83 | 0.57 | 0.52 | 11.67 | 18.14 | 1495 | 10.99 | 0.59 | - |
| Pingzhuodao | 119°821′ E–25°668′ N | 1 | 4 | 16 | 20 | 0.05 | 40 | 18.12 | 1.14 | 7.80 | 20.03 | 1259 | 21.09 | 3.38 | - |
| Qingyu | 119°385′ E–25°249′ N | 2 | 2 | 8 | 10 | 0.01 | 20 | 48.63 | 1.22 | 8.20 | 20.30 | 1190 | 1.95 | 5.32 | - |
| Shanbaidao | 119°853′ E–25°707′ N | 1 | 2 | 8 | 10 | 0.02 | 34.6 | 32.2 | 1.22 | 7.86 | 20.03 | 1259 | 22.75 | 8.21 | - |
| Shanzhuodao | 119°819′ E–25°673′ N | 4 | 3 | 12 | 15 | 0.04 | 52.2 | 21.98 | 1.24 | 7.74 | 20.03 | 1259 | 20.74 | 3.30 | - |
| Shichengdayu | 119°398′ E–25°256′ N | 2 | 3 | 12 | 15 | 0.04 | 31 | 19.09 | 1.09 | 9.01 | 20.3 | 1190 | 3.12 | 4.31 | - |
| Tayu | 117°551′ E–23°732′ N | 22 | 20 | 80 | 100 | 1.01 | 91.3 | 6.82 | 1.93 | 5.53 | 21.22 | 1239 | 2.44 | 1.16 | 0.26 |
| Toujinyu | 119°391′ E–25°251′ N | 0 | 2 | 8 | 10 | 0.01 | 21.1 | 52.37 | 1.35 | 8.22 | 20.3 | 1190 | 2.40 | 4.85 | - |
| Xiamendao | 118°132′ E–24°497′ N | 45 | 72 | 288 | 360 | 134.84 | 339.6 | 0.49 | 1.61 | 2.65 | 20.96 | 1093 | 0.71 | 5.42 | 0.80 |
| Xiaochiyu | 117°519′ E–23°749′ N | 7 | 2 | 8 | 10 | 0.01 | 19.2 | 16.85 | 0.54 | 5.83 | 21.22 | 1239 | 0.43 | 0.36 | - |
| Xiaosongdao | 119°792′ E–25°654′ N | 5 | 4 | 16 | 20 | 0.06 | 36.8 | 24.17 | 1.61 | 7.87 | 19.84 | 1257 | 19.43 | 0.28 | - |
| Xiaweidao | 120°124′ E–26°661′ N | 5 | 5 | 20 | 25 | 0.08 | 35.51 | 22.42 | 1.77 | 6.49 | 18.14 | 1495 | 0.27 | 2.63 | - |
| Xiluodao | 119°650′ E–25°755′ N | 2 | 6 | 24 | 30 | 0.10 | 39.85 | 15.96 | 1.42 | 7.46 | 20.03 | 1259 | 1.74 | 14.24 | - |
| Yuanyangyu | 120°379′ E–26°960′ N | 6 | 16 | 64 | 80 | 0.64 | 149.81 | 9.01 | 2.02 | 5.03 | 17.43 | 1406 | 12.85 | 0.25 | - |
| Zhangpuxiaosongdao | 117°942′ E–24°177′ N | 16 | 2 | 8 | 10 | 0.02 | 26.00 | 30.37 | 1.12 | 5.41 | 20.96 | 1142 | 0.35 | 1.39 | - |
| Zhujiangdao | 119°954′ E–26°776′ N | 14 | 6 | 24 | 30 | 0.15 | 47.30 | 12.82 | 1.42 | 3.47 | 18.32 | 1589 | 0.81 | 8.83 | 0.66 |
| Number | Family | Genus | Species |
|---|---|---|---|
| 1 | Acanthaceae | Andrographis | Andrographis paniculata (Burm. f.) Wall. ex Nees |
| 2 | Acanthaceae | Justicia | Justicia gendarussa Burm. f. |
| 3 | Aizoaceae | Tetragonia | Tetragonia tetragonioides (Pall.) Kuntze |
| 4 | Amaranthaceae | Alternanthera | Alternanthera pungens Kunth |
| 5 | Amaranthaceae | Amaranthus | Amaranthus spinosus L. |
| 6 | Amaranthaceae | Celosia | Celosia cristata L. |
| 7 | Amaranthaceae | Dysphania | Dysphania ambrosioides (L.) Mosyakin & Clemants |
| 8 | Amaranthaceae | Alternanthera | Alternanthera philoxeroides (Mart.) Griseb. |
| 9 | Amaranthaceae | Amaranthus | Amaranthus tricolor L. |
| 10 | Amaranthaceae | Amaranthus | Amaranthus viridis L. |
| 11 | Amaryllidaceae | Zephyranthes | Zephyranthes candida (Lindl.) Herb. |
| 12 | Amaryllidaceae | Hippeastrum | Hippeastrum vittatum (L’Hér.) Herb. |
| 13 | Apiaceae | Cyclospermum | Cyclospermum leptophyllum (Pers.) Sprague ex Britton & P. Wilson |
| 14 | Apocynaceae | Asclepias | Asclepias curassavica L. |
| 15 | Apocynaceae | Catharanthus | Catharanthus roseus (L.) G. Don |
| 16 | Asparagaceae | Agave | Agave sisalana Perr. ex Engelm. |
| 17 | Asparagaceae | Agave | Agave americana L. |
| 18 | Asteraceae | Chromolaena | Chromolaena odorata (L.) R. M. King & H. Rob. |
| 19 | Asteraceae | Bidens | Bidens pilosa L. |
| 20 | Asteraceae | Sonchus | Sonchus asper (L.) Hill |
| 21 | Asteraceae | Ageratum | Ageratum conyzoides L. |
| 22 | Asteraceae | Praxelis | Praxelis clematidea (Hieronymus ex Kuntze) R. M. King & H. Rob. |
| 23 | Asteraceae | Coreopsis | Coreopsis lanceolata L. |
| 24 | Asteraceae | Rudbeckia | Rudbeckia laciniata L. |
| 25 | Asteraceae | Coreopsis | Coreopsis basalis (A. Dietr.) S. F. Blake |
| 26 | Asteraceae | Sonchus | Sonchus oleraceus L. |
| 27 | Asteraceae | Bidens | Bidens bipinnata L. |
| 28 | Asteraceae | Cosmos | Cosmos bipinnatus Cav. |
| 29 | Asteraceae | Erigeron | Erigeron sumatrensis Retz. |
| 30 | Asteraceae | Glebionis | Glebionis coronaria (L.) Cass. ex Spach |
| 31 | Asteraceae | Ambrosia | Ambrosia artemisiifolia L. |
| 32 | Asteraceae | Tagetes | Tagetes erecta L. |
| 33 | Asteraceae | Erigeron | Erigeron bonariensis L. |
| 34 | Asteraceae | Erigeron | Erigeron canadensis L. |
| 35 | Asteraceae | Erigeron | Erigeron annuus (L.) Pers. |
| 36 | Asteraceae | Pluchea | Pluchea sagittalis (Lam.) Cabrera |
| 37 | Asteraceae | Parthenium | Parthenium hysterophorus L. |
| 38 | Asteraceae | Tridax | Tridax procumbens L. |
| 39 | Asteraceae | Tithonia | Tithonia diversifolia (Hemsl.) A. Gray |
| 40 | Asteraceae | Symphyotrichum | Symphyotrichum subulatum (Michx.) G. L. Nesom |
| 41 | Basellaceae | Basella | Basella alba L. |
| 42 | Bignoniaceae | Macfadyena | Macfadyena unguis-cati (L.) A.H.Gentry |
| 43 | Brassicaceae | Lepidium | Lepidium virginicum L. |
| 44 | Cactaceae | Selenicereus | Selenicereus undatus (Haw.) D.R.Hunt |
| 45 | Cactaceae | Opuntia | Opuntia dillenii (Ker Gawl.) Haw. |
| 46 | Caryophyllaceae | Stellaria | Stellaria aquatica (L.) Scop. |
| 47 | Caryophyllaceae | Cerastium | Cerastium glomeratum Thuill. |
| 48 | Casuarinaceae | Casuarina | Casuarina equisetifolia L. |
| 49 | Commelinaceae | Tradescantia | Tradescantia zebrina Bosse |
| 50 | Commelinaceae | Tradescantia | Tradescantia pallida (Rose) D. R. Hunt |
| 51 | Convolvulaceae | Ipomoea | Ipomoea nil (L.) Roth |
| 52 | Convolvulaceae | Ipomoea | Ipomoea cairica (L.) Sweet |
| 53 | Convolvulaceae | Ipomoea | Ipomoea purpurea (L.) Roth |
| 54 | Convolvulaceae | Ipomoea | Ipomoea alba L. |
| 55 | Crassulaceae | Bryophyllum | Bryophyllum pinnatum (L. f.) Oken |
| 56 | Cyperaceae | Cyperus | Cyperus involucratus Rottb. |
| 57 | Cyperaceae | Cyperus | Cyperus rotundus L. |
| 58 | Euphorbiaceae | Euphorbia | Euphorbia maculata L. |
| 59 | Euphorbiaceae | Ricinus | Ricinus communis L. |
| 60 | Euphorbiaceae | Euphorbia | Euphorbia hirta L. |
| 61 | Euphorbiaceae | Pedilanthus | Pedilanthus tithymaloides (L.) Poit. |
| 62 | Euphorbiaceae | Jatropha | Jatropha curcas L. |
| 63 | Euphorbiaceae | Euphorbia | Euphorbia prostrata Aiton |
| 64 | Euphorbiaceae | Euphorbia | Euphorbia hypericifolia L. |
| 65 | Malvaceae | Malvastrum | Malvastrum coromandelianum (L.) Garcke |
| 66 | Myrtaceae | Eucalyptus | Eucalyptus robusta Sm. |
| 67 | Euphorbiaceae | Euphorbia | Euphorbia pulcherrima Willd. ex Klotzsch |
| 68 | Fabaceae | Robinia | Robinia pseudoacacia L. |
| 69 | Fabaceae | Acacia | Acacia auriculiformis A. Cunn. ex Benth. |
| 70 | Fabaceae | Mimosa | Mimosa pudica L. |
| 71 | Fabaceae | Acacia | Acacia mearnsii De Wild. |
| 72 | Fabaceae | Senna | Senna surattensis (Burm. f.) H. S. Irwin & Barneby |
| 73 | Fabaceae | Vachellia | Vachellia farnesiana (L.) Wight & Arn. |
| 74 | Fabaceae | Albizia | Albizia lebbeck (L.) Benth. |
| 75 | Fabaceae | Erythrina | Erythrina corallodendron L. |
| 76 | Fabaceae | Arachis | Arachis duranensis Krapov. & W. C. Greg. |
| 77 | Fabaceae | Cajanus | Cajanus cajan (L.) Millsp. |
| 78 | Fabaceae | Acacia | Acacia confusa Merr. |
| 79 | Fabaceae | Senna | Senna occidentalis (L.) Link |
| 80 | Fabaceae | Leucaena | Leucaena leucocephala (Lam.) de Wit |
| 81 | Fabaceae | Melilotus | Melilotus indicus (L.) All. |
| 82 | Fabaceae | Crotalaria | Crotalaria pallida auct. non Aiton: T. L. Wu |
| 83 | Fabaceae | Medicago | Medicago sativa L. |
| 84 | Lamiaceae | Mentha | Mentha spicata L. |
| 85 | Lamiaceae | Salvia | Salvia splendens Ker Gawl. |
| 86 | Malvaceae | Sida | Sida szechuensis Matsuda |
| 87 | Malvaceae | Abelmoschus | Abelmoschus esculentus (L.) Moench |
| 88 | Poaceae | Paspalum | Paspalum conjugatum Bergius |
| 89 | Poaceae | Cymbopogon | Cymbopogon citratus (DC.) Stapf |
| 90 | Euphorbiaceae | Euphorbia | Euphorbia cyathophora Murray |
| 91 | Myrtaceae | Psidium | Psidium guajava L. |
| 92 | Nyctaginaceae | Bougainvillea | Bougainvillea glabra Choisy |
| 93 | Nyctaginaceae | Bougainvillea | Bougainvillea spectabilis Willd. |
| 94 | Nyctaginaceae | Mirabilis | Mirabilis jalapa L. |
| 95 | Onagraceae | Oenothera | Oenothera drummondii Hook. |
| 96 | Onagraceae | Oenothera | Oenothera parviflora L. |
| 97 | Onagraceae | Oenothera | Oenothera biennis L. |
| 98 | Orobanchaceae | Orobanche | Orobanche brassicae (Novopokr.) Novopokr. |
| 99 | Oxalidaceae | Oxalis | Oxalis corymbosa DC. |
| 100 | Papaveraceae | Argemone | Argemone mexicana L. |
| 101 | Papaveraceae | Papaver | Papaver rhoeas L. |
| 102 | Passifloraceae | Passiflora | Passiflora edulis Sims |
| 103 | Passifloraceae | Passiflora | Passiflora caerulea L. |
| 104 | Plantaginaceae | Scoparia | Scoparia dulcis L. |
| 105 | Plantaginaceae | Plantago | Plantago lanceolata L. |
| 106 | Poaceae | Bromus | Bromus catharticus Vahl |
| 107 | Poaceae | Lolium | Lolium perenne L. |
| 108 | Poaceae | Melinis | Melinis repens (Willd.) Zizka |
| 109 | Poaceae | Chloris | Chloris virgata Sw. |
| 110 | Poaceae | Spartina | Spartina alterniflora Loisel. |
| 111 | Poaceae | Paspalum | Paspalum urvillei Steud. |
| 112 | Poaceae | Pennisetum | Pennisetum purpureum Schumach. |
| 113 | Poaceae | Cymbopogon | Cymbopogon nardus (L.) Rendle |
| 114 | Poaceae | Arrhenatherum | Arrhenatherum elatius (L.) P. Beauv. ex J. Presl & C. Presl |
| 115 | Poaceae | Avena | Avena fatua L. |
| 116 | Polygonaceae | Antigonon | Antigonon leptopus Hook. & Arn. |
| 117 | Polygonaceae | Muehlenbeckia | Muehlenbeckia platyclada (F. Muell. ex Hook.) Meisn. |
| 118 | Pontederiaceae | Eichhornia | Eichhornia crassipes (Mart.) Solms |
| 119 | Portulacaceae | Portulaca | Portulaca grandiflora Hook. |
| 120 | Rubiaceae | Spermacoce | Spermacoce alata Aubl. |
| 121 | Solanaceae | Physalis | Physalis peruviana L. |
| 122 | Solanaceae | Atropa | Atropa belladonna L. |
| 123 | Solanaceae | Solanum | Solanum erianthum D. Don |
| 124 | Solanaceae | Physalis | Physalis angulata L. |
| 125 | Solanaceae | Datura | Datura stramonium L. |
| 126 | Solanaceae | Solanum | Solanum capsicoides All. |
| 127 | Solanaceae | Cestrum | Cestrum nocturnum L. |
| 128 | Urticaceae | Pilea | Pilea microphylla (L.) Liebm. |
| 129 | Verbenaceae | Duranta | Duranta erecta L. |
| 130 | Verbenaceae | Lantana | Lantana camara L. |
References
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and Rising Economic Costs of Biological Invasions Worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Roy, H.E.; Pauchard, A.; Stoett, P.; Renard Truong, T.; Bacher, S.; Galil, B.S.; Hulme, P.E.; Ikeda, T.; Sankaran, K.; McGeoch, M.A.; et al. IPBES Invasive Alien Species Assessment: Summary for Policymakers; IPBES: Bonn, Germany, 2024. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Q.; Shou, H.; Zeng, X.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.; Qi, S.; Ma, J. Classification and Geographical Distribution Patterns of Invasive Alien Plants in China. Biodiversity 2014, 22, 667–676. [Google Scholar]
- Zhang, A.; Hu, X.; Yao, S.; Yu, M.; Ying, Z. Alien, Naturalized and Invasive Plants in China. Plants 2021, 10, 2241. [Google Scholar] [CrossRef]
- Patiño, J.; Whittaker, R.J.; Borges, P.A.V.; Fernández-Palacios, J.M.; Ah-Peng, C.; Araújo, M.B.; Ávila, S.P.; Cardoso, P.; Cornuault, J.; de Boer, E.J.; et al. A Roadmap for Island Biology: 50 Fundamental Questions after 50 Years of The Theory of Island Biogeography. J. Biogeogr. 2017, 44, 963–983. [Google Scholar] [CrossRef]
- Simberloff, D. Why Do Introduced Species Appear to Devastate Islands More Than Mainland Areas? Pac. Sci. 1995, 49, 87–97. [Google Scholar]
- Nogué, S.; Santos, A.M.C.; Birks, H.J.B.; Björck, S.; Castilla-Beltrán, A.; Connor, S.; Boer, E.J.; Nascimento, L.; Felde, V.A.; Fernández-Palacios, J.M.; et al. The human dimension of biodiversity changes on islands. Science 2021, 372, 488–491. [Google Scholar] [CrossRef]
- Scientists’ Warning—The Outstanding Biodiversity of Islands Is in Peril. Glob. Ecol. Conserv. 2021, 31, e01847. [CrossRef]
- MacArthur, R.H.; Wilson, E.O. An Equilibrium Theory of Insular Zoogeography. Evolution 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford Biology; Oxford University Press: New York, NY, USA, 2007; ISBN 978-0-19-856612-0. [Google Scholar]
- Kreft, H.; Jetz, W.; Mutke, J.; Kier, G.; Barthlott, W. Global Diversity of Island Floras from a Macroecological Perspective. Ecol. Lett. 2008, 11, 116–127. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Community Diversity: Relative Roles of Local and Regional Processes. Science 1987, 235, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Rethinking Patch Size and Isolation Effects: The Habitat Amount Hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Honnay, O.; Piessens, K.; Van Landuyt, W.; Hermy, M.; Gulinck, H. Satellite Based Land Use and Landscape Complexity Indices as Predictors for Regional Plant Species Diversity. Landsc. Urban. Plan. 2003, 63, 241–250. [Google Scholar] [CrossRef]
- Sfenthourakis, S.; Triantis, K.A. Habitat Diversity, Ecological Requirements of Species and the Small Island Effect. Divers. Distrib. 2009, 15, 131–140. [Google Scholar] [CrossRef]
- Triantis, K.A.; Mylonas, M.; Lika, K.; Vardinoyannis, K. A Model for the Species–Area–Habitat Relationship. J. Biogeogr. 2003, 30, 19–27. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Ackerman, J.D. Tremblay Island Biogeography of Native and Alien Plant Species: Contrasting Drivers of Diversity across the Lesser Antilles. Divers. Distrib. 2020, 26, 1539–1550. [Google Scholar] [CrossRef]
- Kalmar, A.; Currie, D.J. A Global Model of Island Biogeography. Glob. Ecol. Biogeogr. 2006, 15, 72–81. [Google Scholar] [CrossRef]
- Kubota, Y.; Shiono, T.; Kusumoto, B. Role of Climate and Geohistorical Factors in Driving Plant Richness Patterns and Endemicity on the East Asian Continental Islands. Ecography 2015, 38, 639–648. [Google Scholar] [CrossRef]
- Yu, J.; Shen, L.; Li, D.D.; Guo, S.L. Determinants of Bryophyte Species Richness on the Zhoushan Archipelago, China. Basic. Appl. Ecol. 2019, 37, 38–50. [Google Scholar] [CrossRef]
- Valli, A.-T.; Kougioumoutzis, K.; Iliadou, E.; Panitsa, M.; Trigas, P. Determinants of Alpha and Beta Vascular Plant Diversity in Mediterranean Island Systems: The Ionian Islands, Greece. Nord. J. Bot. 2019, 37, e02156. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Schweiger, A.H.; Steinbauer, M.J.; Ah-Peng, C.; Arevalo, J.R.; Beierkuhnlein, C.; Chiarucci, A.; Daehler, C.C.; Fernandez-Palacios, J.M.; Flores, O.; et al. Human Impact, Climate and Dispersal Strategies Determine Plant Invasion on Islands. J. Biogeogr. 2021, 48, 1889–1903. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Hortal, J. Time, Area and Isolation: Factors Driving the Diversification of Azorean Arthropods. J. Biogeogr. 2009, 36, 178–191. [Google Scholar] [CrossRef]
- Jesse, W.A.M.; Behm, J.E.; Helmus, M.R.; Ellers, J. Human Land Use Promotes the Abundance and Diversity of Exotic Species on Caribbean Islands. Glob. Change Biol. 2018, 24, 4784–4796. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, X.; Zhang, Y.; Chen, S. Accelerated Expansion of Built-up Area after Bridge Connection with Mainland: A Case Study of Zhujiajian Island. Ocean. Coast. Manag. 2018, 152, 62–69. [Google Scholar] [CrossRef]
- Barajas-Barbosa, M.P.; Weigelt, P.; Borregaard, M.K.; Keppel, G.; Kreft, H. Environmental Heterogeneity Dynamics Drive Plant Diversity on Oceanic Islands. J. Biogeogr. 2020, 47, 2248–2260. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding Factors in the Detection of Species Responses to Habitat Fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef]
- Schrader, J.; König, C.; Triantis, K.A.; Trigas, P.; Kreft, H.; Weigelt, P. Species–Area Relationships on Small Islands Differ among Plant Growth Forms. Glob. Ecol. Biogeogr. 2020, 29, 814–829. [Google Scholar] [CrossRef]
- Jiang, M.; Pang, X.; Wang, J.; Cao, C. Islands Ecological Integrity Evaluation Using Multi Sources Data. Ocean. Coast. Manag. 2018, 158, 134–143. [Google Scholar] [CrossRef]
- Liu, M.; Chen, P. Significance of Phylogenetic Diversity and Phylogenetic Structure in Conservation of Island Plant Communities with Cases of 19 Islands along the Coast of Fujian Province. J. Appl. Oceanogr. 2022, 41, 275–285. [Google Scholar]
- Xie, Y.; Xie, X.; Weng, F.; Nong, L.; Lin, M.; Ou, J.; Wang, Y.; Mao, Y.; Chen, Y.; Qian, Z.; et al. Distribution Patterns and Environmental Determinants of Invasive Alien Plants on Subtropical Islands (Fujian, China). Forests 2024, 15, 1273. [Google Scholar] [CrossRef]
- Lin, P.; Qiu, X.; Zhang, R. Studies on the Vegetation of Three Islands of Pingtan, Nanri, and Meizhou on the Middle Coast of Fujian Province. Chin. J. Plant Ecol. 1984, 8, 74. [Google Scholar]
- Chen, H.; Xian, X.; Chen, Y.; Lin, N.; Wang, M.; Li, Z.; Zhao, J. Spatial Pattern and Driving Factors on the Prevalence of Red Imported Fire Ant (Solenopsis Invicta) in Island Cities: A Case Study of Haitan Island, Fujian. Biodivers. Sci. 2023, 31, 22501. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, W.; Zhang, W.; Ge, F.; Chen, B.; Hu, W.; Zhang, D.; Liao, J.; Huang, H.; Sun, Y.; et al. Conditions of Mainland China’s Island Ecosystems and Associated Influencing Factors: Integrated Assessment of 42 Typical Island Ecosystems. Front. Mar. Sci. 2022, 9, 920069. [Google Scholar] [CrossRef]
- Fujian Island Resources Comprehensive Survey Committee. Fujian Island Resources Comprehensive Survey Research Report; Ocean Press: Beijing, China, 1996. [Google Scholar]
- Yu, M.J.; Hu, G.; Feeley, K.J.; Wu, J.G.; Ding, P. Richness and Composition of Plants and Birds on Land-Bridge Islands: Effects of Island Attributes and Differential Responses of Species Groups. J. Biogeogr. 2012, 39, 1124–1133. [Google Scholar] [CrossRef]
- Hu, G.; Feeley, K.J.; Wu, J.G.; Xu, G.; Yu, M.J. Determinants of Plant Species Richness and Patterns of Nestedness in Fragmented Landscapes: Evidence from Land-Bridge Islands. Landsc. Ecol. 2011, 26, 1405–1417. [Google Scholar] [CrossRef]
- Yao, Z.; Wen, H.; Deng, Y.; Cao, M.; Lin, L. Drivers of Beta Diversity Patterns of Tree Species in Subtropical Mid-Mountain Humid Evergreen Broadleaf Forests of Ailao Mountain. Biodiversity 2020, 28, 445–454. [Google Scholar]
- Gao, K.; Li, F.; Liu, X.; Xiong, Y.; Li, J.; Zhao, C. Vertical Distribution Patterns of Diversity of Invasive Alien and Native Herbaceous Plants in Jiuwanshan National Nature Reserve, Guangxi. Biodiversity 2019, 27, 1047–1055. [Google Scholar]
- Lajiong; Zashi, C.; Zhu, W.; Xu, M.; Zhong, Y. Distribution Patterns and Environmental Interpretations of Plant Species Richness along the Yarlung Zangbo River. Biodiversity 2014, 22, 337–347. [Google Scholar]
- Fang, J.; Wang, X.; Tang, Z. Local and Regional Processes Jointly Control Community Species Diversity: The Species Pool Hypothesis. Biodiversity 2009, 17, 605–612. [Google Scholar]
- Ma, X.; Chi, Y.; Wang, J.; Qu, Y.; Zhang, Z.; Liu, Z.; Wang, Z.; Liu, D. How Plant Diversity Varies across an Archipelago with Highly Heterogeneous Human Activities. Glob. Ecol. Conserv. 2023, 43, e02446. [Google Scholar] [CrossRef]
- Weigelt, P.; Kreft, H. Quantifying Island Isolation—Insights from Global Patterns of Insular Plant Species Richness. Ecography 2013, 36, 417–429. [Google Scholar]
- Wu, J.G. Effects of Changing Scale on Landscape Pattern Analysis: Scaling Relations. Landsc. Ecol. 2004, 19, 125–138. [Google Scholar] [CrossRef]
- Hoffmeister, T.S.; Vet, L.E.M.; Biere, A.; Holsinger, K.; Filser, J. Ecological and Evolutionary Consequences of Biological Invasion and Habitat Fragmentation. Ecosystems 2005, 8, 657–667. [Google Scholar] [CrossRef]
- Neter, J.; Kutner, M.; Wasserman, W.; Nachtsheim, C. Applied Linear Statistical Models; Irwin Professional Publishing: Chicago, IL, USA, 1996; ISBN 978-0-256-11736-3. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Rojas-Sandoval, J.; Ackerman, J.D.; Duenas, M.-A.; Velez, J.; Diaz-Soltero, H. Habitat Affiliation of Non-Native Plant Species across Their Introduced Ranges on Caribbean Islands. Biol. Invasions 2024, 26, 2237–2249. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Millien, V. A Global Synthesis of the Small-Island Effect in Habitat Islands. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181868. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Perry, G. Detecting the small island effect and nestedness of herpetofauna of the West Indies. Ecol. Evol. 2016, 6, 5390–5403. [Google Scholar] [CrossRef] [PubMed]
- Panitsa, M.; Tzanoudakis, D.; Triantis, K.A.; Sfenthourakis, S. Patterns of Species Richness on Very Small Islands: The Plants of the Aegean Archipelago. J. Biogeogr. 2006, 33, 1223–1234. [Google Scholar] [CrossRef]
- Santos, A.M.C.; Whittaker, R.J.; Triantis, K.A.; Borges, P.A.V.; Jones, O.R.; Quicke, D.L.J.; Hortal, J. Are Species–Area Relationships from Entire Archipelagos Congruent with Those of Their Constituent Islands? Glob. Ecol. Biogeogr. 2010, 19, 527–540. [Google Scholar] [CrossRef]
- Lomolino, M. Ecology’s Most General, yet Protean Pattern: The Species-Area Relationship. J. Biogeogr. 2000, 27, 17–26. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Skinner, R.H.; Barker, D.J.; Edwards, G.R.; Tracy, B.F.; Wedin, D.A. Plant Species Diversity and Management of Temperate Forage and Grazing Land Ecosystems. Crop Sci. 2004, 44, 1132–1144. [Google Scholar] [CrossRef]
- Chiarucci, A.; Bacaro, G.; Triantis, K.A.; Fernandez-Palacios, J.M. Biogeographical Determinants of Pteridophytes and Spermatophytes on Oceanic Archipelagos. Syst. Biodivers. 2011, 9, 191–201. [Google Scholar] [CrossRef]
- Cabral, J.S.; Weigelt, P.; Kissling, W.D.; Kreft, H. Biogeographic, Climatic and Spatial Drivers Differentially Affect Alpha-, Beta- and Gamma-Diversities on Oceanic Archipelagos. P. R. Soc. B-Biol. Sci. 2014, 281, 20133246. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating Resources in Plant Communities: A General Theory of Invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Haeuser, E.; Dawson, W.; van Kleunen, M. The Effects of Climate Warming and Disturbance on the Colonization Potential of Ornamental Alien Plant Species. J. Ecol. 2017, 105, 1698–1708. [Google Scholar] [CrossRef]
- Gassó, N.; Pino, J.; Font, X.; Vilà, M. Regional Context Affects Native and Alien Plant Species Richness across Habitat Types. Appl. Veg. Sci. 2012, 15, 4–13. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate Warming Enhances Microbial Network Complexity and Stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather. Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting Plant Invasions in an Era of Global Change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate Change Threats to Plant Diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel Competitors Shape Species’ Responses to Climate Change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Pyšek, P. Mechanisms of Plant Invasions of North American and European Grasslands. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 133–153. [Google Scholar] [CrossRef]
- Parepa, M.; Fischer, M.; Bossdorf, O. Environmental Variability Promotes Plant Invasion. Nat. Commun. 2013, 4, 1604. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Huang, H.; Xie, X.; Ou, J.; Chen, Z.; Lu, X.; Kong, D.; Nong, L.; Lin, M.; Qian, Z.; et al. Landscape, Human Disturbance, and Climate Factors Drive the Species Richness of Alien Invasive Plants on Subtropical Islands. Plants 2024, 13, 2437. https://doi.org/10.3390/plants13172437
Xie Y, Huang H, Xie X, Ou J, Chen Z, Lu X, Kong D, Nong L, Lin M, Qian Z, et al. Landscape, Human Disturbance, and Climate Factors Drive the Species Richness of Alien Invasive Plants on Subtropical Islands. Plants. 2024; 13(17):2437. https://doi.org/10.3390/plants13172437
Chicago/Turabian StyleXie, Yanqiu, Hui Huang, Xinran Xie, Jingyao Ou, Zhen Chen, Xiaoxue Lu, Deyi Kong, Liebo Nong, Manni Lin, Zhijun Qian, and et al. 2024. "Landscape, Human Disturbance, and Climate Factors Drive the Species Richness of Alien Invasive Plants on Subtropical Islands" Plants 13, no. 17: 2437. https://doi.org/10.3390/plants13172437
APA StyleXie, Y., Huang, H., Xie, X., Ou, J., Chen, Z., Lu, X., Kong, D., Nong, L., Lin, M., Qian, Z., Mao, Y., Chen, Y., Wang, Y., Chen, Z., & Deng, C. (2024). Landscape, Human Disturbance, and Climate Factors Drive the Species Richness of Alien Invasive Plants on Subtropical Islands. Plants, 13(17), 2437. https://doi.org/10.3390/plants13172437






