Potentially Toxic Elements Uptake and Distribution in Betula middendorffii T. and Duschekia fruticosa R. Growing on Diamond Mining Area (Yakutia, Russia)
Abstract
1. Introduction
2. Materials and Methods
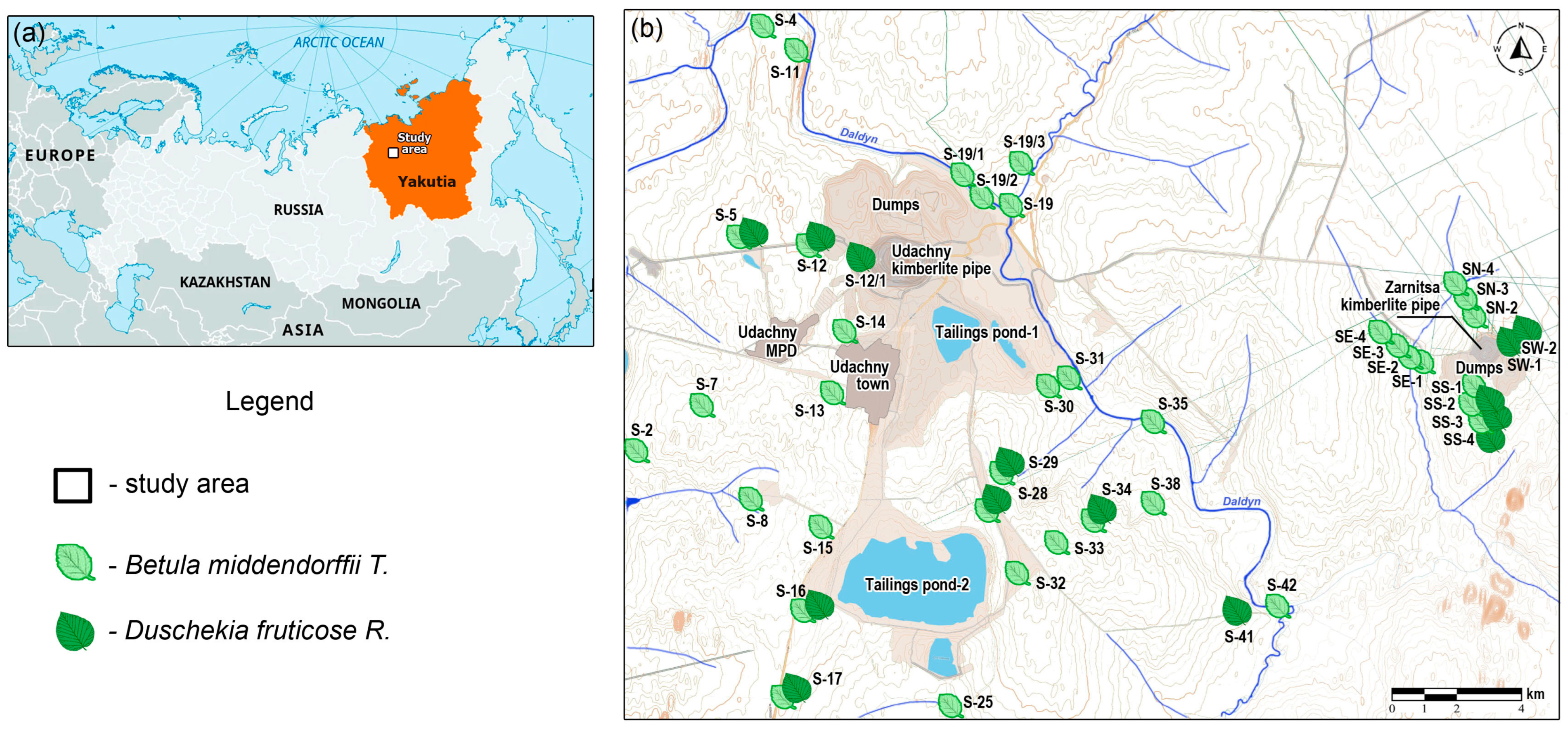
2.1. Research Area
2.2. Sample Collection and Preparation
2.3. Determination of Bioaccumulation Factor (BAF)
2.4. Statistical Analysis
3. Results and Discussion
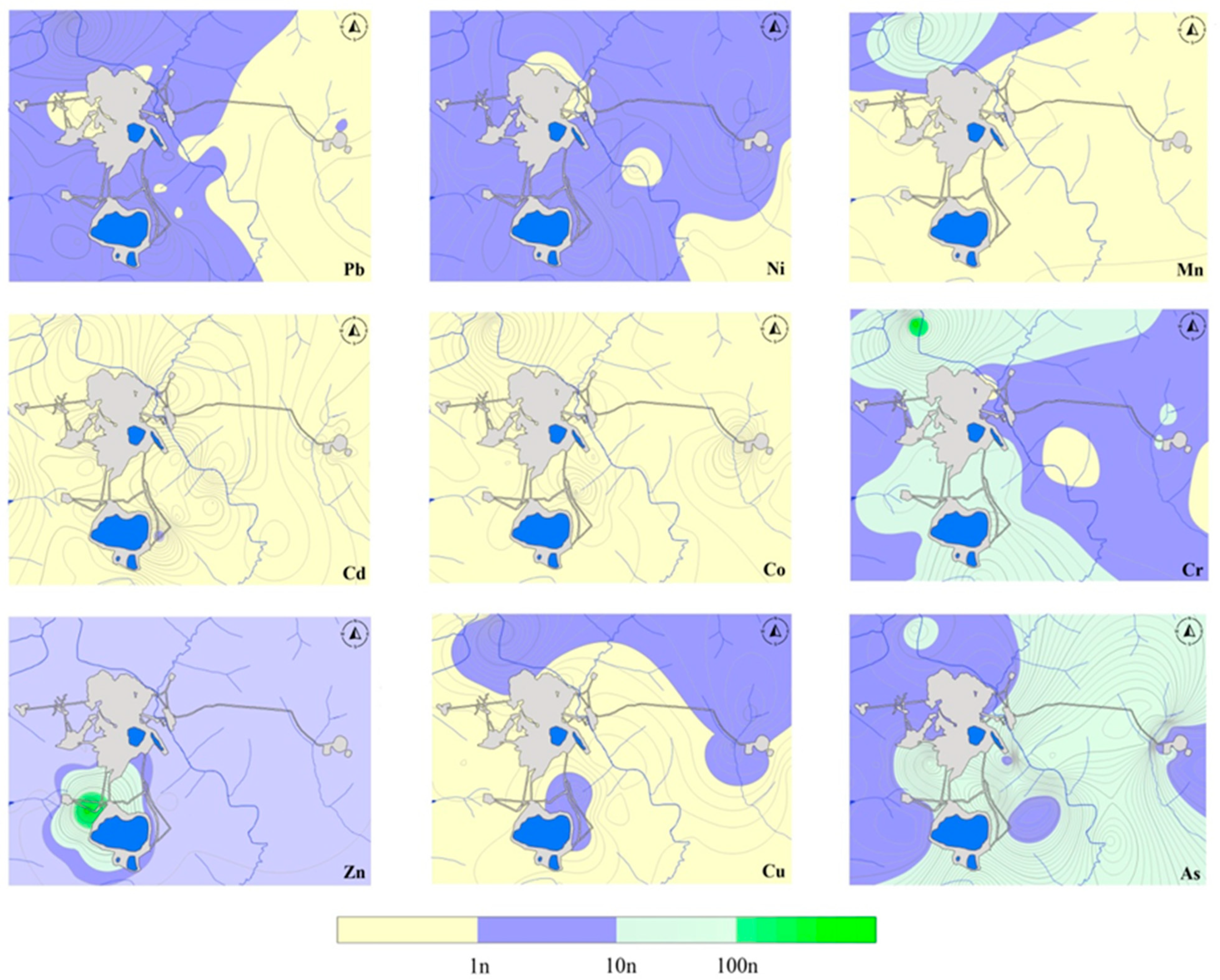
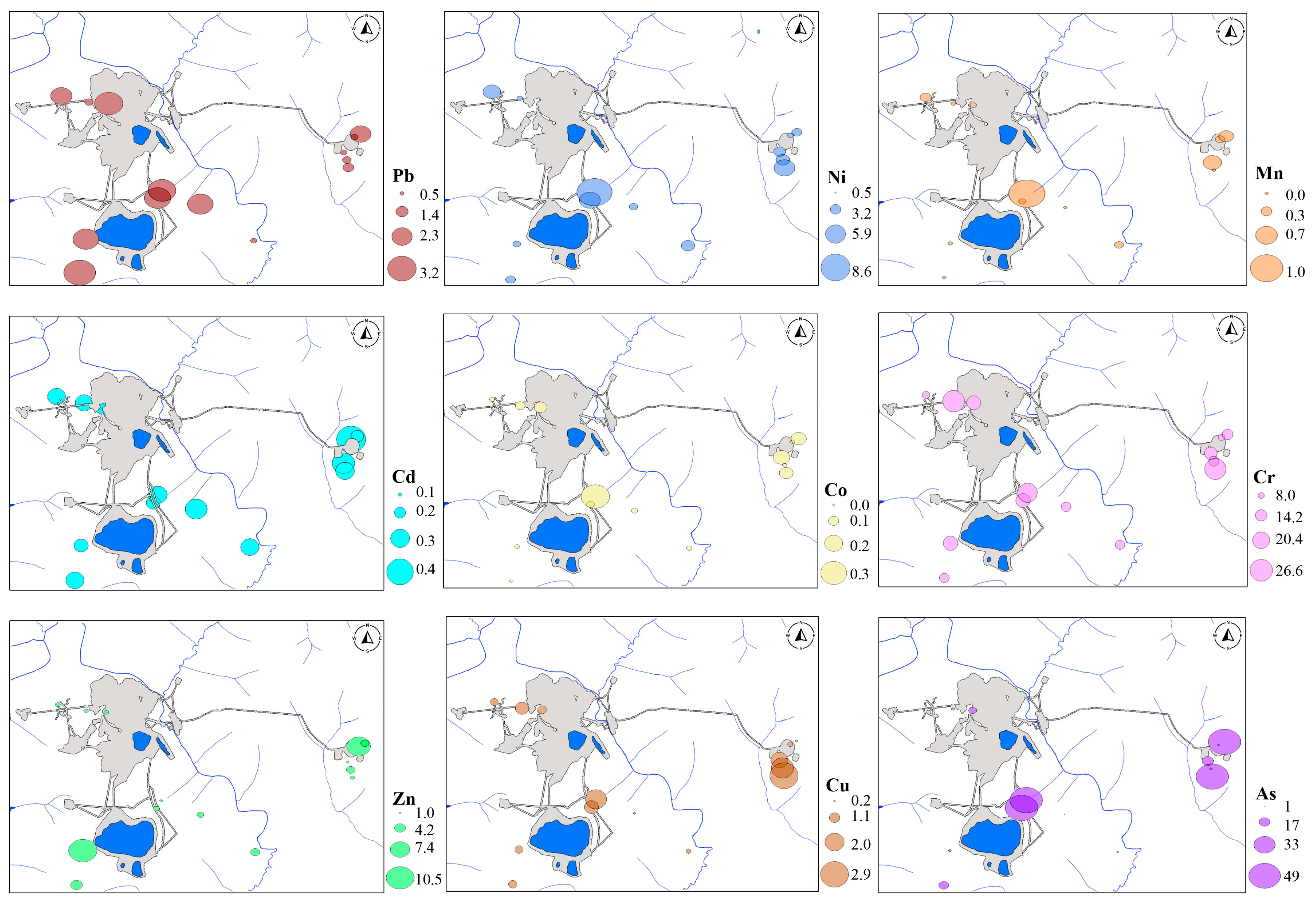
3.1. Concentrations of PTEs in Soils and Plants
3.2. Bioaccumulation Factor (BAF)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martinez-Carlos, J.; Martinez-Martinez, S.; Faz, A.; Zornoza, R.; Gabarron, M.; Soriano-Disla, M.; Gomez-Lopez, M.D.; Acosta, J.A. Are the soils and vegetation of a forest close to tailings ponds affected by metals and arsenic? Environ. Geochem. Health 2021, 44, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Roca-Perez, L.; Boluda, R.; Rodríguez-Martín, J.A.; Ramos-Miras, J.; Tume, P.; Roca, N.; Bech, J. Potentially harmful elements pollute soil and vegetation around the Atrevida mine (Tarragona, NE Spain). Environ. Geochem. Health 2023, 45, 9215–9230. [Google Scholar] [CrossRef] [PubMed]
- Radosteva, E.R.; Kulagin, A.Y. Bioaccumulation of metals in the organs of woody plants in the conditions of polymetallic dumps of the Uchalinsky mining and processing plant (Republic of Bashkortostan). Izv. Samara Sci. Cent. Russ. Acad. Sci. 2011, 13, 200–202. [Google Scholar]
- Lindahl, J. Environmental impacts of mining in Zambia: Towards better environmental management and sustainable exploitation of mineral resources. Geol. Surv. Swed. 2014, 22, 26. [Google Scholar]
- Torbati, S.; Kermani, S.; Abedini, A. Remediation of heavy metals by native plant species grown in Iran’s richest gold mine and study of plants’ pollution tolerance strategies. Front. Earth Sci. 2024, 12, 1304497. [Google Scholar] [CrossRef]
- Olobatoke, R.Y.; Mathuthu, M. Heavy metal concentration in soil in the tailing dam vicinity of an old gold mine in Johannesburg, South Africa. Can. J. Soil Sci. 2016, 96, 299–304. [Google Scholar] [CrossRef]
- Lotfi, S.; Chakit, M.; Belghyti, D. Groundwater quality and pollution index for heavy metals in Sais plain, Morocco. J. Health Pollut. 2020, 10, 200603. [Google Scholar] [CrossRef] [PubMed]
- Mulenga, C.; Phiri, D.; Ortega-Rodriguez, D.R.; Meincken, M. Bioaccumulation of potentially toxic elements by indigenous and exotic trees growing around a copper leaching plant in Mufulira, Zambia. Environ. Syst. Res. 2023, 12, 26. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Petukhov, A.S.; Kremleva, T.A. Bioacumulation of heavy metals oat from technogenically contaminated soils of Tyumen. Agrochem. Bull. 2021, 1, 73–80. [Google Scholar]
- Zhakypbek, Y.; Kossalbayev, B.D.; Belkozhayev, A.M.; Murat, T.; Tursbekov, S.; Abdalimov, E.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation. Plants 2024, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- Proshad, R.; Kormoker, T.; Islam, M.S.; Chandra, K. Potential health risk of heavy metals via consumption of rice and vegetables grown in the industrial areas of Bangladesh. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 921–943. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yuan, X.Z.; Xiong, T.; Wang, H.; Jiang, L.B. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Ryzhenko, N.; El Amrani, A.; Giltrap, M.; Furong, T.; Laptiev, V. Bioaccumulation of As, Cd, Cr, Cu, Pb, Zn in Ambrosia artemisiifolia L. in the polluted area by enterprise for the production and processing of batteries. Ann. Civ. Environ. Eng. 2022, 6, 26–30. [Google Scholar] [CrossRef]
- Legostaeva, Y.; Kozlova, I.; Popov, V.; Noev, D. Geoecological Situation in the Area of Aikhal MPD. In Proceedings of the Geology and Mineral Resources of the North-East of Russia, Yakutsk, Russia, 8 April 2020; pp. 482–485. [Google Scholar]
- Gololobova, A.G.; Legostaeva, Y.B. An assessment of the impact of the mining industry on soil and plant contamination by potentially toxic elements in boreal forests. Forests 2023, 14, 1641. [Google Scholar] [CrossRef]
- Gorelova, S.V.; Frontasyeva, M.V.; Gorbunov, A.V.; Lyapunov, S.M.; Okina, O.I. Bioindication and monitoring of atmospheric deposition using trees and shrubs. In Proceedings of the 27th Task Force Meeting of the UNECE ICP Vegetation, Paris, France, 28 January 2014; p. 63.2. [Google Scholar]
- Petrunina, N.S.; Ermakov, V.V. Modern aspects of plant geochemical ecology. Probl. Biogeochem. Geochem. Ecol. 2012, 1, 147–155. [Google Scholar]
- Neverova, O.A.; Kolmogorova, E.Y. Woody Plants and the Urbanized Environment: Ecological and Biotechnological Aspects; Nauka: Novosibirsk, Russia, 2003. [Google Scholar]
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. Version 2020-3. In UNECE ICP Forests Programme Co-Ordinating Centre (Editor): Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 16. Available online: http://www.icp-forests.org/Manual.htm (accessed on 25 July 2024).
- ISO 11464-2015; Soil Quality. Pretreatment of Samples for Physico-Chemical Analysis. Inter-Governmental Council on Standardization, Metrology and Certification 77-П: Moscow, Russia, 2015.
- Aziz, R.A.; Yiwen, M.; Saleh, M.; Salleh, M.N.; Gopinath, S.C.B.; Giap, S.G.E.; Chinni, S.V.; Gobinath, R. Bioaccumulation and Translocation of Heavy Metals in Paddy (Oryza sativa L.) and Soil in Different Land Use Practices. Sustainability 2023, 15, 13426. [Google Scholar] [CrossRef]
- Ilyin, V.B. Heavy Metals in the Soil-Plant System; Nauka: Novosibirsk, Russia, 1991. [Google Scholar]
- Ladonin, D.V. Compounds of heavy metals in soils—Problems and methods of study. Soil Sci. 2002, 6, 682–692. [Google Scholar]
- Method M 03-07-2014; Measurement of the mass fraction of elements (As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, V, Zn) in samples of soil, subsoil, bottom sediments and sewage sludge, FER 16.1:2:2.2:2.3.63-09. Lumex: Moscow, Russia, 2014.
- ISO 10390; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005.
- GOST-26483-85; State Standard of the Union of, S.S.R. Soils. Preparation of Salt Extract and Determination of Its pH by CINAO Method. GOST: Moscow, Russia, 1985.
- ISO 14235; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. ISO: Geneva, Switzerland, 1998.
- GOST-26213-91; State Standard of the Union of, S.S.R. Soils. Methods for Determination of Organic Matter. GOST: Moscow, Russia, 1991.
- GOST R 58596-2019; Soils. Methods for Determining Total Nitrogen. National Standard of the Russian Federation; GOST: Moscow, Russia, 2019.
- NF ISO 11261; Dosage de L’azote Total—Methode de Kjeldahl Modifiee; Qualite des Sols. Association Française de Normalization: Paris, France, 1995.
- Shakeel, T.; Shah, G.M.; Zeb, B.S.; Gul, I.; Bibi, S.; Hussain, Z.; Irshad, M. Phytoremediation potential and vegetation assessment of plant species growing on multi-metals contaminated coal mining site. Environ. Res. Commun. 2024, 6, 055006. [Google Scholar] [CrossRef]
- Dessalew, G.; Beyene, A.; Nebiyu, A.; Astatkie, T. Effect of brewery spent diatomite sludge on trace metal availability in soil and uptake by wheat crop, and trace metal risk on human health through the consumption of wheat grain. Heliyon 2018, 4, e00783. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; García, G.; Werner, O.; Navarro-Pedreño, J.; Ros, R.M. Implications of the phytoremediationof heavy metal contamination of soils and wild plants inthe industrial area of Haina, Dominican Republic. Sustainability 2021, 13, 1403. [Google Scholar] [CrossRef]
- Liu, W.X.; Liu, J.W.; Wu, M.Z.; Li, Y.; Zhao, Y.; Li, S.R. Accumulation and translocation of toxic heavy metals in winter wheat (Triticum aestivum L.) growing in agricultural soil of Zhengzhou, China. Bull. Environ. Contam. Toxicol. 2009, 82, 343–347. [Google Scholar] [CrossRef]
- Perelman, A.I.; Kasimov, N.S. Geochemistry of Landscape; Astrea: Moscow, Russia, 1999. [Google Scholar]
- Degtyarev, A.P. Biological accumulation coefficients as a basis for biochemical classification of chemical elements. Biosphere 2024, 1–16, 5–19. [Google Scholar]
- Aitchison, J. The Statistical Analysis of Compositional Data; Chapman and Hall: London, UK, 1986; p. 416. [Google Scholar]
- Aitchison, J. The Statistical Analysis of Compositional Data; Blackburn Press: Caldwell, NJ, USA, 2003; p. 460. [Google Scholar]
- Pawlowsky-Glahn, V.; Buccianti, A. (Eds.) Compositional Data Analysis: Theory and Applications; Wiley: Chichester, UK; West Sussex, UK, 2011. [Google Scholar]
- Fedorov-Davydov, D.G.; Davydov, S.P.; Davydova, A.I.; Shmelev, D.G.; Ostroumov, V.E.; Kholodov, A.L.; Sorokovikov, V.A. Thermal state of soils in Northern Yakutia. Cryosphere Earth 2018, 22, 52–66. [Google Scholar]
- Wang, M.S.; Han, Q.; Gui, C.L.; Cao, J.L.; Liu, Y.P.; He, X.D.; He, Y.C. Differences in the risk assessment of soil heavy metals between newly built and original parks in Jiaozuo, Henan Province, China. Sci. Total Environ. 2019, 676, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abliz, A.; Shi, Q.; Abulizi, A. Contamination status and health risk assessment of soil heavy metals in the northern slope of eastern Tianshan mountains industrial belt in Xinjiang, Northwest China. Forests 2022, 13, 1914. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRS Press: Boca Raton, FL, USA, 2011; p. 548. [Google Scholar]
- Dobrovolsky, V.V. Fundamentals of Biogeochemistry; Academy: Moscow, Russia, 2003; p. 400. [Google Scholar]
- Syso, A.I.; Siromlya, T.I.; Myadelets, M.A.; Cherevko, A.S. Ecological and biogeochemical assessment of the elemental and biochemical composition of vegetation in anthropogenically disturbed ecosystems (using Achillea millefolium L. as an example). Sib. Ecol. J. 2016, 5, 782–792. [Google Scholar]
- Brooks, R.R. Biological Methods of Mineral Exploration: Trans; Nedra: Moscow, Russia, 1986; p. 311. [Google Scholar]
- Yagnyshev, B.S.; Yagnysheva, T.A.; Zinchuk, M.N.; Legostaeva, Y.B. Ecology of Western Yakutia (Geochemistry of Geoecosystems: State and Problems); YNTS SB RAS: Yakutsk, Russia, 2005. [Google Scholar]
- Legostaeva, Y.B.; Gololobova, A.G. Features of the distribution of trace elements in the soils of the background and impact zones at diamond mining sites in the northwest of the Siberian Platform. Bull. Tomsk Polytech. University. Geo Assets Eng. 2021, 332, 142–153. [Google Scholar]
- Zinchuk, N.N. The role of petrological-mineralogical and geochemical studies in assessing the potential diamond content of kimberlites. Domest. Geol. 2022, 1, 59–70. [Google Scholar] [CrossRef]
- Zinchuk, N.N. On the geochemical features of formations of different ages in diamond-promising territories. Domest. Geol. 2023, 1, 46–69. [Google Scholar] [CrossRef]
- López-Bucio, J.S.; Ravelo-Ortega, G.; López-Bucio, J. Chromium in plant growth and development: Toxicity, tolerance and hormesis. Environ. Pollut. 2022, 312, 120084. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Microbial Life in the Presence of Heavy Metals, Arsenic and Antimony; Mir: Moscow, Russia, 1981; p. 469. [Google Scholar]
- Elbekyan, K.S. Ecological and Experimental Characteristics of the Toxicity of Heavy Metals and Assessment of a Possible Antitoxic Mechanism: Abstract of the Dissertation for the Degree of Doctor of Biological Sciences; STGMU: Saratov, Russia, 2008; p. 35. [Google Scholar]
- Liao, H.T.; Hedly, X.; Wooley, D.J.; Brooks, R.R.; Nockols, M.A. Copper uptake and translocation in chicory (Cichorium in tybus L., cv. Grassland Punas) and tomato (Lycopersicon esulentum Mill., cv. Rondy) plants grown in NFT system II. The role of nicotinamide and histidine in xylem sap copper transport. Plant Soil 2000, 223, 243–252. [Google Scholar] [CrossRef]
- Ortiz, D.F.; Kreppler, L.; Speiser, D.M. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette type vacuolar membrane. J. EMBO 1992, 11, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Vatamamuk, O.K.; Mari, S.S.; Lu, Y.-P. Mechanisms of heavy metal ion activation of phytochelatin synthease (PS). Blocked thiols are sufficient for PS-synthease-catalyzed transpeptidation of glutetione and related thiol peptides. J. Biol. Chem. 2000, 275, 31451–31459. [Google Scholar]
- Reed, R.; Darring, S. Physiological responce of ship-fouling and non-fouling isolates of Entermorpha compressa to copper. In Heavy Metals Environment Internetional Conference; CEP Consultants, Edinburgh: Heidelberg, Germany, 1983; Volume 69, pp. 322–325. [Google Scholar]
- Choi, Y.-E.; Harada, E.; Wada, M. Detoxification of cadmium in tobacco plants: Formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 2001, 213, 45–50. [Google Scholar] [CrossRef] [PubMed]

| Index | Mean | Geometric Mean | Median | Minimum | Maximum | Variance | Std. Dev. | |
|---|---|---|---|---|---|---|---|---|
| Physicochemical properties | pH | 7.62 | 7.56 | 7.80 | 4.32 | 9.30 | 0.72 | 0.85 |
| Humus, % | 9.88 | 7.01 | 6.20 | 1.10 | 47.00 | 72.52 | 8.52 | |
| SOC, % | 5.73 | 4.07 | 3.60 | 0.64 | 27.26 | 24.40 | 4.94 | |
| TN, % | 0.81 | 0.62 | 0.76 | 0.03 | 1.98 | 0.23 | 0.48 | |
| SOC/TN | 7.06 | 6.56 | 4.72 | 25.52 | 13.80 | 106.55 | 10.32 | |
| Granulometric fractions | 1–0.25 mm | 1.04 | 0.44 | 0.34 | 0.05 | 2.81 | 1.47 | 1.21 |
| 0.25–0.05 mm | 7.65 | 6.66 | 6.26 | 3.00 | 12.19 | 18.06 | 4.25 | |
| 0.05–0.01 mm | 23.45 | 22.57 | 26.62 | 15.61 | 30.86 | 47.76 | 6.91 | |
| 0.01–0.005 mm | 10.34 | 10.31 | 10.19 | 9.55 | 11.55 | 0.68 | 0.83 | |
| 0.005–0.001 mm | 15.83 | 15.28 | 15.72 | 11.67 | 23.66 | 24.01 | 4.90 | |
| <0.001 mm | 25.21 | 24.12 | 27.92 | 12.70 | 29.74 | 49.90 | 7.06 | |
| <0.01 mm | 51.38 | 51.24 | 50.02 | 46.55 | 57.71 | 18.09 | 4.25 | |
| >0.01 mm | 32.14 | 31.43 | 33.08 | 22.14 | 42.59 | 55.80 | 7.47 | |
| Index | Pb | Ni | Mn | Cd | Co | Cr | Zn | Cu | As | |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | Mean | 2.21 | 15.39 | 311.63 | 0.14 | 4.53 | 1.94 | 12.10 | 8.21 | 0.22 |
| Geom. mean | 1.80 | 3.43 | 199.9 | 0.106 | 2.73 | 0.967 | 9.36 | 6.14 | 0.12 | |
| Minimum | 0.33 | 1.00 | 34.76 | 0.03 | 0.53 | 0.11 | 0.05 | 0.82 | 0.03 | |
| Maximum | 6.60 | 163.8 | 1856 | 1.12 | 34.65 | 21.45 | 36.46 | 28.44 | 0.84 | |
| Standard deviation | 1.41 | 40.97 | 415.5 | 0.18 | 6.92 | 4.08 | 6.99 | 6.40 | 0.21 | |
| Variance | 1.99 | 1678 | 172,608 | 0.03 | 47.94 | 16.62 | 48.91 | 40.95 | 0.04 | |
| Background value | 1.79 | 3.12 | 189 | 0.11 | 2.64 | 0.93 | 9.47 | 5.81 | 0.13 | |
| Betula middendorffii | Mean | 3.03 | 8.08 | 144.8 | 0.04 | 0.39 | 12.30 | 42.94 | 4.73 | ND |
| Geom. mean | 2.85 | 7.70 | 55.64 | 0.03 | 0.27 | 12.19 | 37.48 | 4.59 | ND | |
| Minimum | 1.51 | 4.00 | 14.67 | 0.03 | 0.13 | 9.92 | 25.00 | 2.92 | ND | |
| Maximum | 7.33 | 15.9 | 2237 | 0.26 | 2.71 | 15.62 | 97.54 | 8.83 | ND | |
| Standard deviation | 1.15 | 2.61 | 408.5 | 0.04 | 0.46 | 1.67 | 24.18 | 1.27 | ND | |
| Variance | 1.33 | 6.81 | 166,887 | 0.002 | 0.22 | 2.79 | 584.6 | 1.62 | ND | |
| Duschekia fruticosa | Mean | 3.09 | 6.81 | 31.5 | 0.03 | 0.15 | 12.08 | ND | 5.16 | ND |
| Geom.mean | 2.98 | 6.63 | 25.71 | 0.03 | 0.14 | 11.98 | ND | 5.08 | ND | |
| Minimum | 2.14 | 3.45 | 9.65 | 0.03 | 0.13 | 9.60 | ND | 4.13 | ND | |
| Maximum | 4.58 | 9.02 | 98.9 | 0.05 | 0.44 | 14.49 | ND | 7.04 | ND | |
| Standard deviation | 0.86 | 1.47 | 24.2 | 0.01 | 0.08 | 1.59 | ND | 0.97 | ND | |
| Variance | 0.74 | 2.153 | 586.1 | 0.0001 | 0.007 | 2.54 | ND | 0.936 | ND | |
| Background values | 2.88 | 7.39 | 44.82 | 0.03 | 0.22 | 12.13 | 33.46 | 4.72 | ND | |
| Parameters | Pb | Ni | Mn | Cd | Co | Cr | Zn | Cu | As | |
|---|---|---|---|---|---|---|---|---|---|---|
| Soils | ||||||||||
| * F | 100.0–400.0 | 100.0 | 1500–3000 | 3.0–8.0 | 25.0–50.0 | 75.0–100.0 | 70.0–250.0 | 60.0–100.0 | 15.0–50.0 | |
| General for soils of the study area | Mean Max | 2.21 6.60 | 15.39 163.8 | 311.63 1856.0 | 0.14 1.12 | 4.53 34.65 | 1.94 21.45 | 12.10 36.46 | 8.21 28.44 | 0.22 0.84 |
| ** KF | 0.07 | 1.64 | 1.24 | 0.37 | 1.41 | 0.29 | 0.52 | 0.47 | 0.06 | |
| Soils in the impact zone of the Udachny quarry | 1.09 | 2.89 | 167.7 | 0.123 | 2.79 | 0.48 | 14.59 | 10.54 | 0.03 | |
| KF1 | 0.01 | 0.02 | 0.11 | 0.04 | 0.11 | 0.01 | 0.21 | 0.17 | 0.002 | |
| Soils in the impact zone of waste rock dumps | Udachny quarry | 2.39 | 148.5 | 1770.6 | 0.059 | 34.65 | 16.44 | 13.83 | 18.01 | 0.41 |
| KF2 | 0.02 | 1.48 | 1.18 | 0.02 | 1.41 | 0.22 | 0.19 | 0.3 | 0.03 | |
| Zarnitsa quarry | 4.45 | 2.64 | 1856.0 | 0.440 | 3.13 | 0.74 | 23.85 | 4.02 | 0.07 | |
| KF3 | 0.04 | 0.03 | 1.23 | 0.14 | 0.12 | 0.04 | 0.34 | 0.07 | 0.004 | |
| Tailings pond | 1.69 | 3.07 | 1463.0 | 0.134 | 9.40 | 0.48 | 11.59 | 16.25 | 0.38 | |
| KF4 | 0.02 | 0.03 | 0.97 | 0.04 | 0.38 | 0.06 | 0.16 | 0.27 | 0.02 | |
| Plants | ||||||||||
| Plants of terrestrial land *** | 1.25–1.50 | 1.54 | 6.86 | 0.035–4.40 | 1.37 | 1.03 | 11.76–30.0 | 2.27–8.0 | 0.2 **** | |
| Betula middendorffii | 3.03 | 8.08 | 144.8 | 0.04 | 0.39 | 12.30 | 42.94 | 4.73 | n/d | |
| Duschekia fruticosa | 3.09 | 6.81 | 31.5 | 0.03 | 0.15 | 12.08 | n/d | 5.16 | n/d | |
| Concentration of elements in mature leaf tissues based on generalized data from many species [44] | Deficiency or less than the established required quantities of elements | - | - | 15.0–25.0 | - | - | - | 10.0–20.0 | 2.0–5.0 | - |
| Sufficient or normal | 5.0–10.0 | 0.1–5.0 | 20.0–300.0 | 0.05–0.2 | 0.02–1.0 | 0.1–0.5 | 27.0–150.0 | 5.0–30.0 | 1.0–1.7 | |
| Excess or toxic | 30.0–300.0 | 10.0–100.0 | 300.0–500.0 | 5.0–30.0 | 15.0–50.0 | 5.0–30.0 | 100.0–400.0 | 20.0–100.0 | 5.0–20.0 | |
| Vegetation Species | Parameters | Pb | Ni | Mn | Cd | Co | Cr | Zn | Cu | As |
|---|---|---|---|---|---|---|---|---|---|---|
| Betula middendorffii (n = 36) | Mean | 2.07 | 3.26 | 2.32 | 0.36 | 0.16 | 18.85 | 10.15 | 1.02 | - |
| Median | 1.39 | 3.00 | 0.22 | 0.28 | 0.08 | 13.70 | 3.22 | 0.76 | - | |
| Geom. mean | 1.62 | 2.25 | 0.27 | 0.29 | 0.09 | 12.30 | 3.77 | 0.76 | - | |
| Minimum | 0.39 | 0.07 | 0.02 | 0.06 | 0.01 | 0.52 | 0.99 | 0.18 | - | |
| Maximum | 9.64 | 8.40 | 64.36 | 1.07 | 0.78 | 135.6 | 210.0 | 4.27 | - | |
| Std. Dev. | 1.76 | 1.91 | 10.75 | 0.25 | 0.19 | 22.89 | 34.60 | 0.84 | - | |
| Duschekia fruticosa (n = 14) | Mean | 1.86 | 3.24 | 0.26 | 0.25 | 0.08 | 15.97 | 3.43 | 1.21 | - |
| Median | 2.15 | 2.73 | 0.19 | 0.26 | 0.06 | 14.46 | 2.39 | 0.88 | - | |
| Geom. mean | 1.58 | 2.68 | 0.18 | 0.23 | 0.07 | 15.01 | 2.71 | 0.95 | - | |
| Minimum | 0.65 | 0.55 | 0.02 | 0.06 | 0.03 | 8.05 | 1.05 | 0.24 | - | |
| Maximum | 3.23 | 8.59 | 0.97 | 0.41 | 0.24 | 26.66 | 10.55 | 2.90 | - | |
| Std. Dev. | 0.98 | 2.05 | 0.24 | 0.08 | 0.06 | 5.92 | 2.83 | 0.81 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gololobova, A.; Legostaeva, Y. Potentially Toxic Elements Uptake and Distribution in Betula middendorffii T. and Duschekia fruticosa R. Growing on Diamond Mining Area (Yakutia, Russia). Plants 2024, 13, 2440. https://doi.org/10.3390/plants13172440
Gololobova A, Legostaeva Y. Potentially Toxic Elements Uptake and Distribution in Betula middendorffii T. and Duschekia fruticosa R. Growing on Diamond Mining Area (Yakutia, Russia). Plants. 2024; 13(17):2440. https://doi.org/10.3390/plants13172440
Chicago/Turabian StyleGololobova, Anna, and Yana Legostaeva. 2024. "Potentially Toxic Elements Uptake and Distribution in Betula middendorffii T. and Duschekia fruticosa R. Growing on Diamond Mining Area (Yakutia, Russia)" Plants 13, no. 17: 2440. https://doi.org/10.3390/plants13172440
APA StyleGololobova, A., & Legostaeva, Y. (2024). Potentially Toxic Elements Uptake and Distribution in Betula middendorffii T. and Duschekia fruticosa R. Growing on Diamond Mining Area (Yakutia, Russia). Plants, 13(17), 2440. https://doi.org/10.3390/plants13172440






