Bioassessment of Cd and Pb at Multiple Growth Stages of Wheat Grown in Texturally Different Soils Using Diffusive Gradients in Thin Films and Traditional Extractants: A Comparative Study
Abstract
:1. Introduction
2. Results
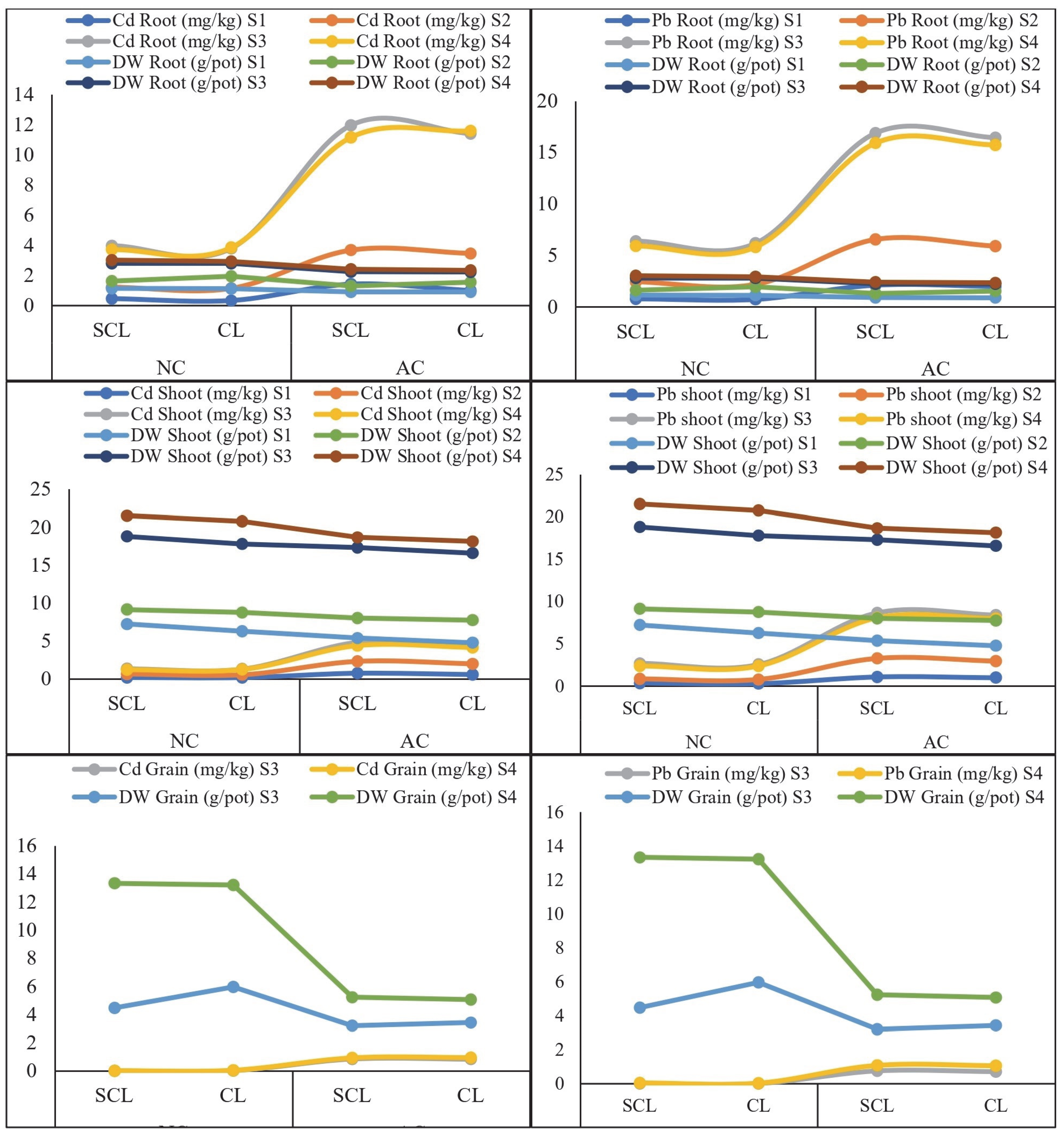
2.1. Response of Wheat Plant Growth and Concentration of Cd and Pb in Plant Tissues
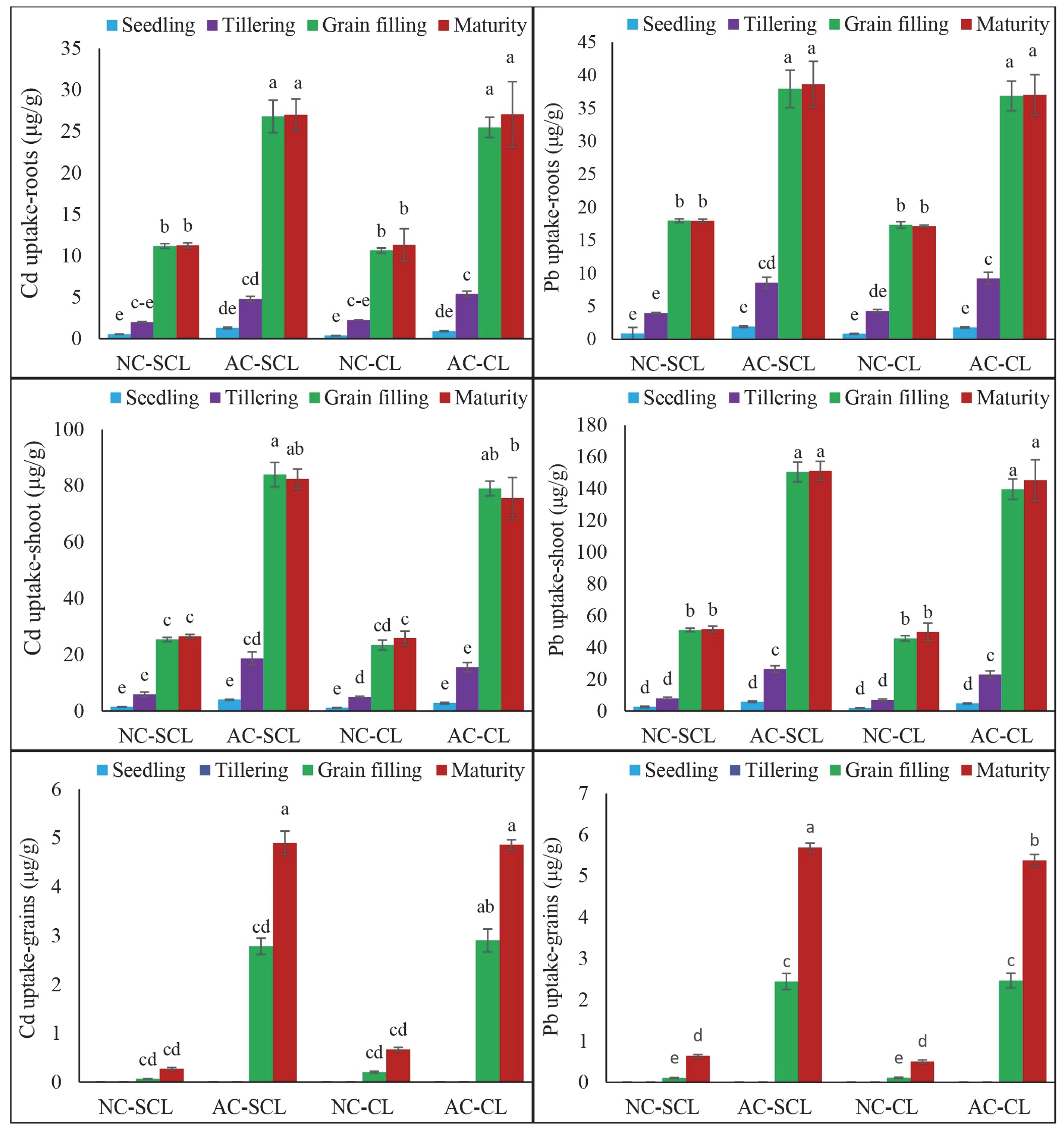
2.2. Uptake of Cd and Pb in Wheat Plant Tissues at Different Growth Stages in Different Types of Soils
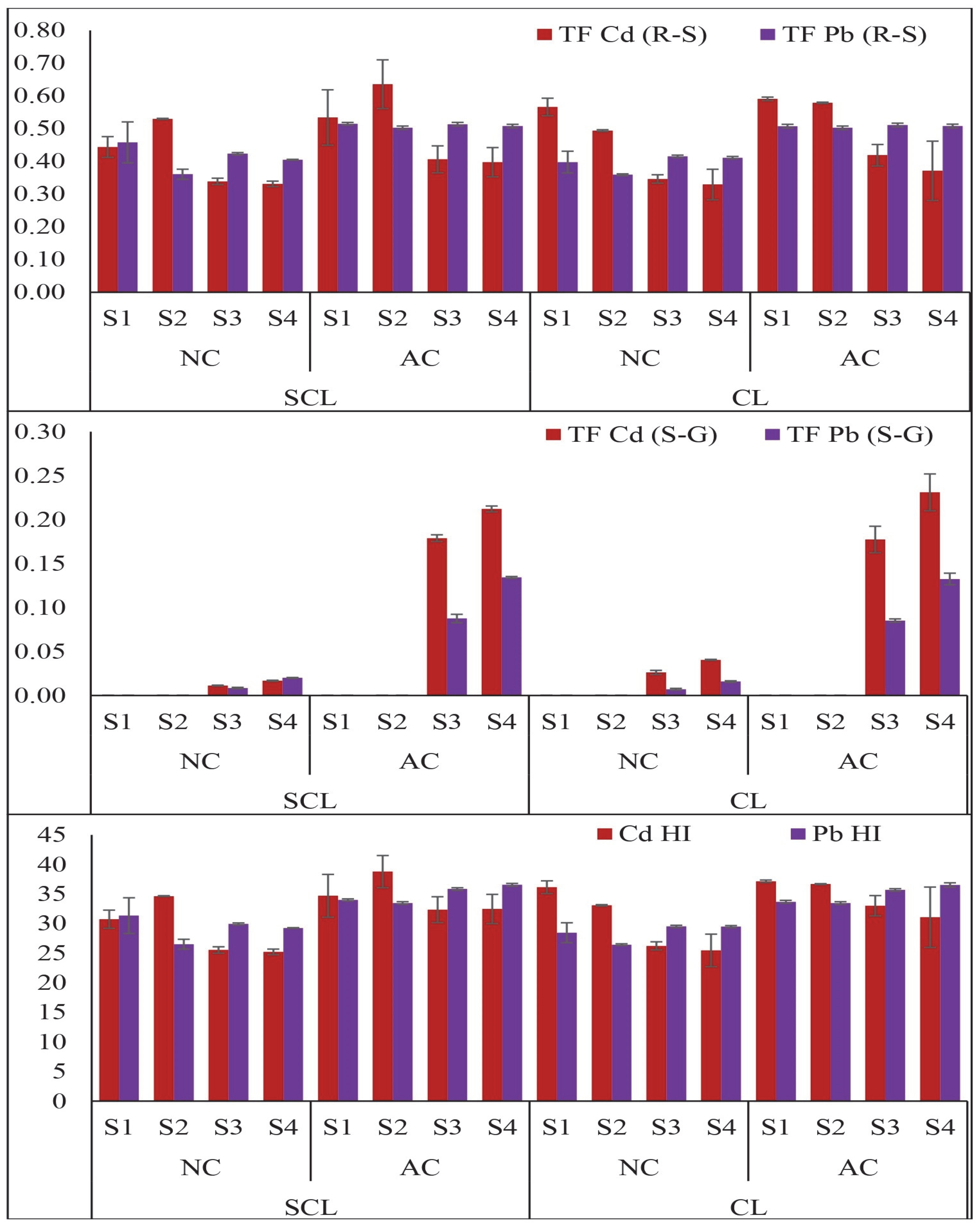
2.3. Translocation and Harvest Indices of Cd and Pb in Wheat Plants
2.4. Determination of Cd and Pb Concentration in Soil Using DGT and Single-Step Traditional Extraction Methods
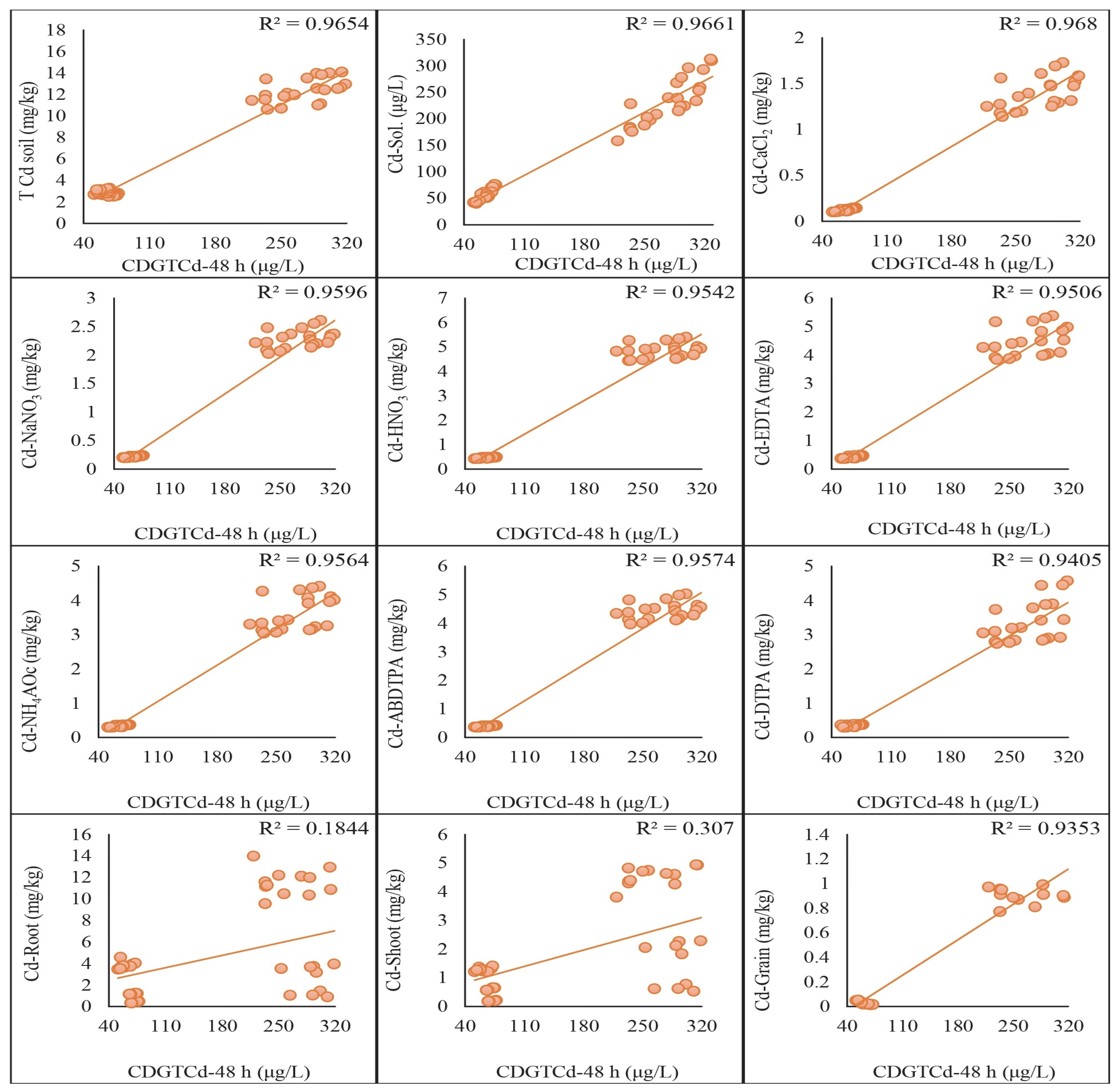
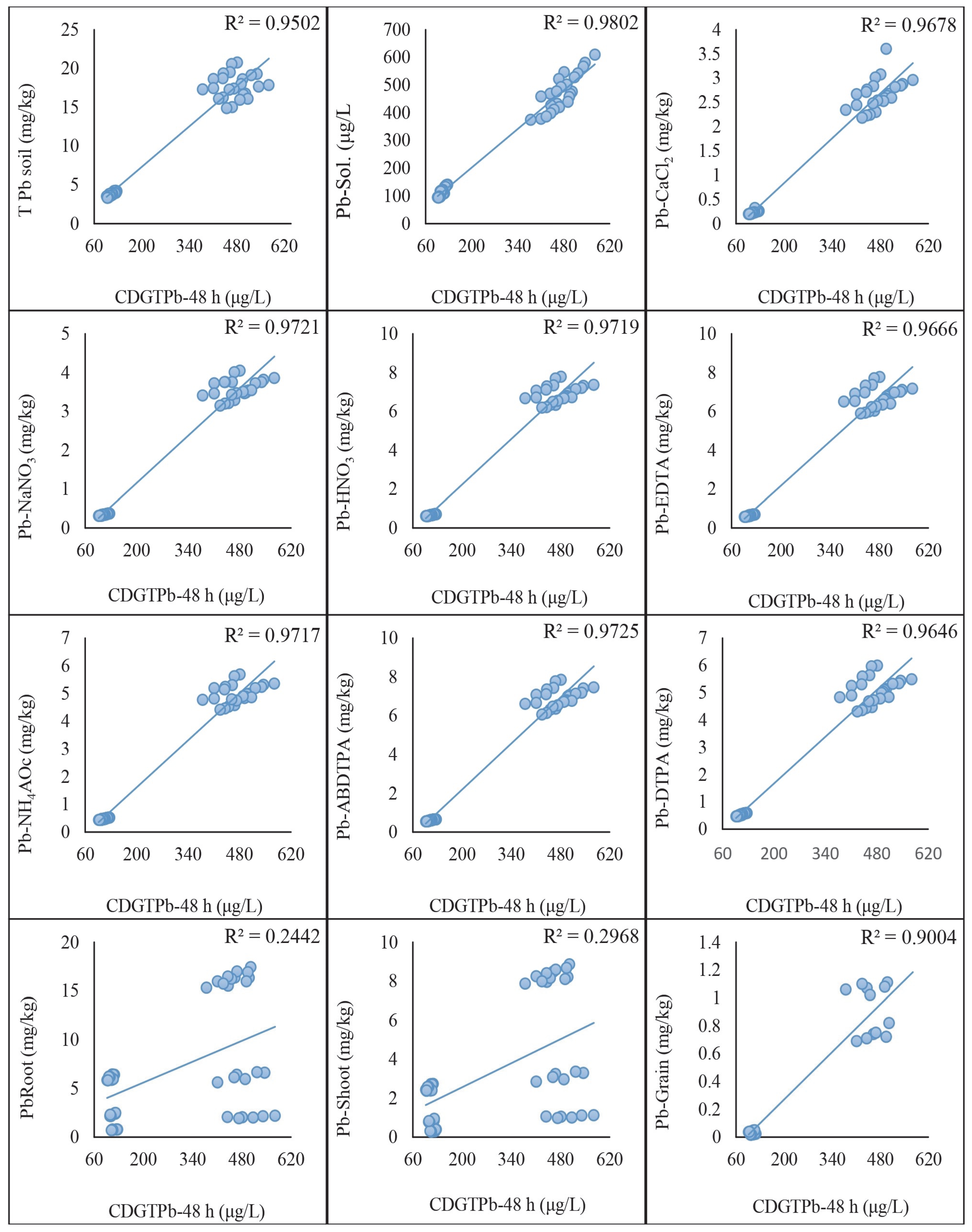
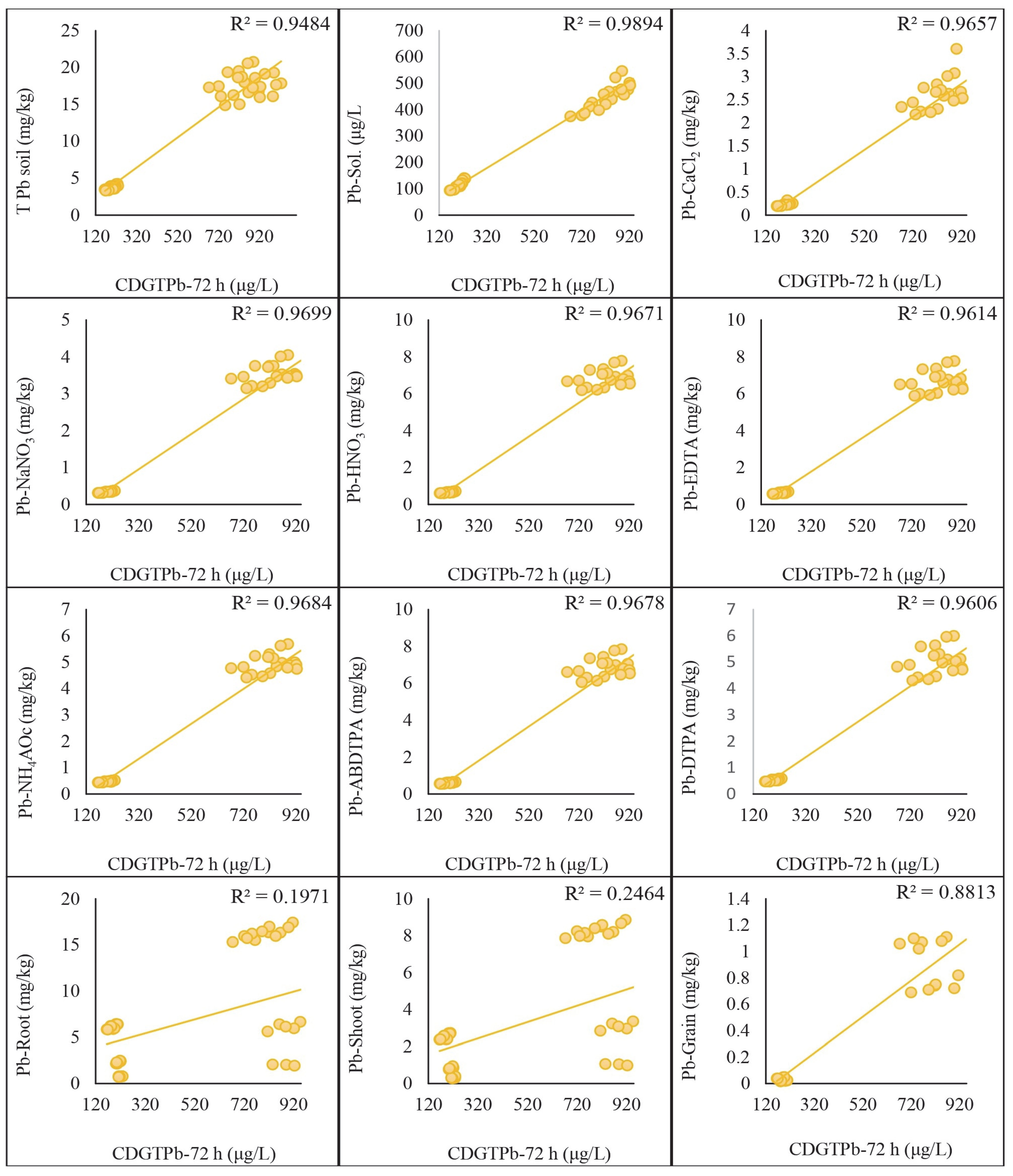
2.5. Regression Analysis of DGT-Assessed Cd and Pb with Traditional Extraction Methods
2.6. Regression Analysis of DGT-Assessed Cd and Pb with Wheat Plant Tissue Concentrations
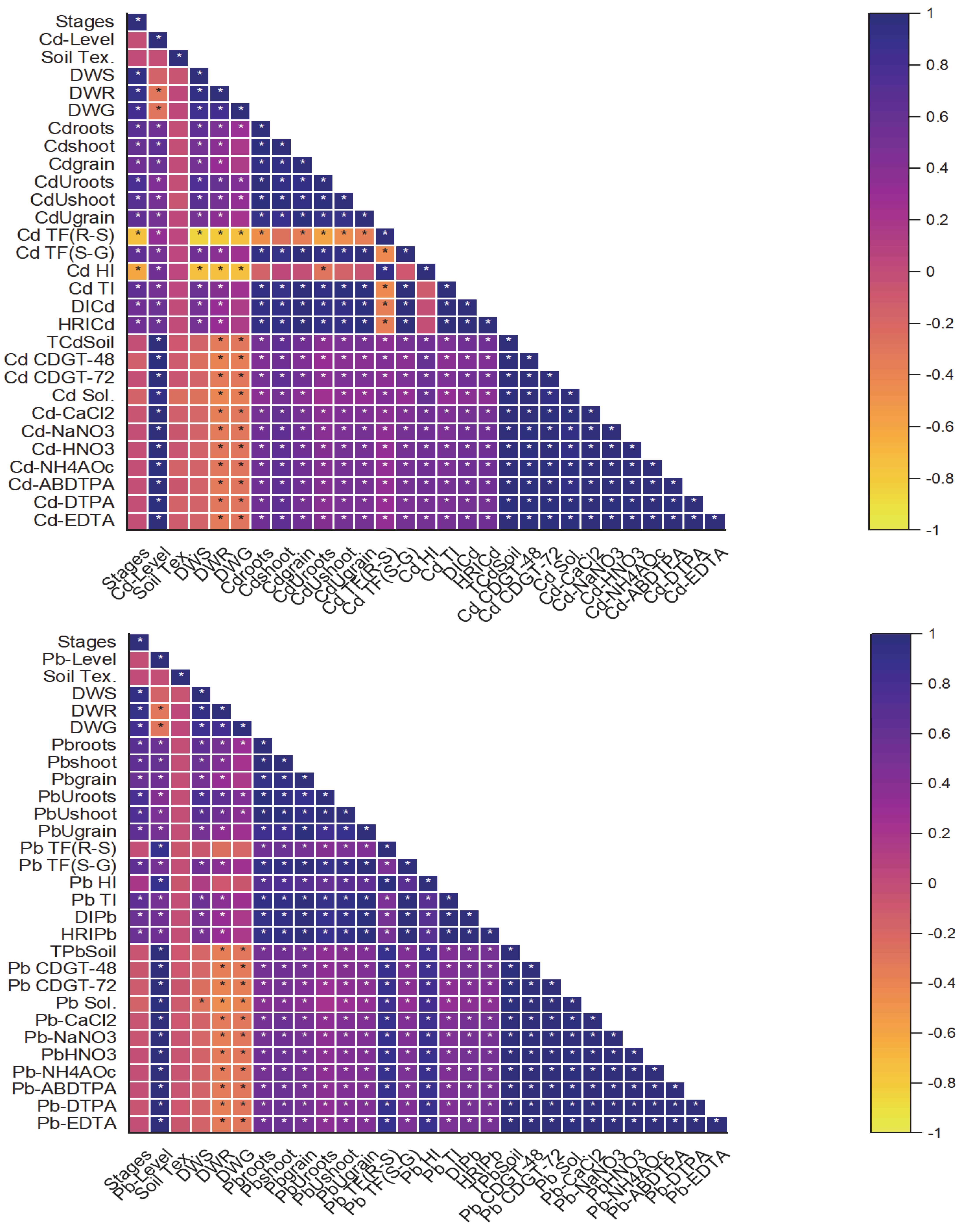
2.7. Correlation Analysis of Studied Parameters
3. Discussion
3.1. Wheat Plant Growth Response and Concentration of Cd and Pb in Plant Tissues
3.2. Uptake of Cd and Pb in Wheat Plant Tissues
3.3. Translocation and Harvest Indices of Cd and Pb in Wheat Plants at Different Growth Stages in Various Contaminated Soils
3.4. Assessment of Cd and Pb Concentration in Soil Using DGT and Single-Step Traditional Extraction Methods
3.5. Comparative Effectiveness of DGT and Traditional Extraction Methods through Regression Analysis
3.6. Regression Analysis of DGT-Assessed Cd and Pb with Wheat Plant Tissue Concentrations
4. Materials and Methods
4.1. Soil Collection and Characterization
4.2. Spiking of Soil and Pot Filling
4.3. Cultivation of Wheat and Experimentation
4.4. Arrangement and Deployment of DGT Devices
4.5. Assessment of Cd and Pb Adsorption on DGT Devices
4.6. Soil Solution and Single-Step Traditional Extraction Methods
4.7. Harvesting and Determination of Cd and Pb
4.8. Secondary Parameter Calculations
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Sharma, M.; Kant, R.; Sharma, A.K.; Sharma, A.K. Exploring the Impact of Heavy Metals Toxicity in the Aquatic Ecosystem. Int. J. Energy Water Resour. 2024. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, J.; Katnoria, J.K. Assessment of Spatial Variations in Pollution Load of Agricultural Soil Samples of Ludhiana District, Punjab. Environ. Monit. Assess. 2022, 195, 222. [Google Scholar] [CrossRef] [PubMed]
- Proshad, R.; Islam, M.S.; Kormoker, T. Assessment of Heavy Metals with Ecological Risk of Soils in the Industrial Vicinity of Tangail District, Bangladesh. Int. J. Adv. Geosci. 2018, 6, 108. [Google Scholar] [CrossRef]
- Mushtaq, N.; Singh, D.V.; Bhat, R.A.; Dervash, M.A.; Hameed, O. Freshwater Contamination: Sources and Hazards to Aquatic Biota. In Fresh Water Pollution Dynamics and Remediation; Qadri, H., Bhat, R., Mehmood, M., Dar, G., Eds.; Springer: Singapore, 2019; pp. 27–50. [Google Scholar]
- Dong, W.; Zhang, Y.; Quan, X. Health Risk Assessment of Heavy Metals and Pesticides: A Case Study in the Main Drinking Water Source in Dalian, China. Chemosphere 2020, 242, 125113. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, L.; Rao, S.; Zhang, M.; Zhao, K.; Fu, W. Spatial Variation of Heavy Metals and Their Ecological Risk and Health Risks to Local Residents in a Typical E-Waste Dismantling Area of Southeastern China. Environ. Monit. Assess. 2022, 194, 604. [Google Scholar] [CrossRef]
- Guan, D.-X.; He, S.-X.; Li, G.; Teng, H.H.; Ma, L.Q. Application of Diffusive Gradients in Thin-Films Technique for Speciation, Bioavailability, Modeling and Mapping of Nutrients and Contaminants in Soils. Crit. Rev. Environ. Sci. Technol. 2021, 52, 3035–3079. [Google Scholar] [CrossRef]
- Dellavia, A.; Marković, M.; Habuda-Stanić, M.; Kovačić, Đ.; Bubalo, A.; Popović, B. Wastewater-Based Crop Irrigation: An Issue or a Solution? Poljoprivreda 2023, 29, 43–52. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Ji, Y.; Fu, Y.; Lang, T.; Shi, T.; Liu, S. Risk Assessment of Cr, As, and Sb to Aquatic Communities at the Water-Sediment Interface in the Lower Yellow River, China, Integrated with the Diffusive Gradients in Thin-films Technique. J. Environ. Chem. Eng. 2024, 12, 111942. [Google Scholar] [CrossRef]
- Antoniadis, V.; Golia, E.E.; Liu, Y.-T.; Wang, S.-L.; Shaheen, S.M.; Rinklebe, J. Soil and Maize Contamination by Trace Elements and Associated Health Risk Assessment in the Industrial Area of Volos, Greece. Environ. Int. 2019, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Rosado Rodríguez, G.; Otero Morales, E. Assessment of Heavy Metal Contamination at Tallaboa Bay (Puerto Rico) by Marine Sponges’ Bioaccumulation and Fungal Community Composition. Mar. Pollut. Bull. 2020, 161, 111803. [Google Scholar] [CrossRef] [PubMed]
- Xin, J. Enhancing Soil Health to Minimize Cadmium Accumulation in Agro-Products: The Role of Microorganisms, Organic Matter, and Nutrients. Environ. Pollut. 2024, 348, 123890. [Google Scholar] [CrossRef]
- Davidson, C.M. Methods for the Determination of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils. Environmental Pollution; Alloway, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 22, pp. 97–140. [Google Scholar]
- van Hullebusch, E.D.; Guibaud, G.; Simon, S.; Lenz, M.; Yekta, S.S.; Fermoso, F.G.; Jain, R.; Duester, L.; Roussel, J.; Guillon, E.; et al. Methodological Approaches for Fractionation and Speciation to Estimate Trace Element Bioavailability in Engineered Anaerobic Digestion Ecosystems: An Overview. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1324–1366. [Google Scholar] [CrossRef]
- Kelepertzis, E.; Paraskevopoulou, V.; Argyraki, A.; Fligos, G.; Chalkiadaki, O. Evaluation of Single Extraction Procedures for the Assessment of Heavy Metal Extractability in Citrus Agricultural Soil of a Typical Mediterranean Environment (Argolida, Greece). J. Soils Sediments 2015, 15, 2265–2275. [Google Scholar] [CrossRef]
- Senila, M.; Kovacs, E. Use of Diffusive Gradients in Thin-Film Technique to Predict the Mobility and Transfer of Nutrients and Toxic Elements from Agricultural Soil to Crops-an Overview of Recent Studies. Environ. Sci. Pollut. Res. Int. 2024, 31, 34817–34838. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.A.; Frentiu, T.; Mihali, C.; Angyus, S.B. Assessment of Mercury Bioavailability in Garden Soils around a Former Nonferrous Metal Mining Area Using DGT, Accumulation in Vegetables, and Implications for Health Risk. Environ. Monit. Assess. 2023, 195, 1554. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Lamb, D.T.; Wang, L.; Megharaj, M.; Naidu, R. Copper Interactions on Arsenic Bioavailability and Phytotoxicity in Soil. Ecotoxicol. Environ. Saf. 2018, 148, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Rong, Q.; Zhang, H.; Jones, K.C.; Li, Y.; Luo, J. Evaluation and Application of a Novel Diffusive Gradients in Thin-Films Technique for In Situ Monitoring of Glucocorticoids in Natural Waters. Environ. Sci. Technol. 2022, 56, 15499–15507. [Google Scholar] [CrossRef]
- Martins de Barros, R.; Rougerie, J.; Guibal, R.; Lissalde, S.; Buzier, R.; Simon, S.; Guibaud, G. Interest of a New Large Diffusive Gradients in Thin Films (L-DGT) for Organic Compounds Monitoring: On-Field Comparison with Conventional Passive Samplers. Environ. Pollut. 2023, 323, 121257. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, C.; Wei, J.; Li, Y.; Chen, C.; Xu, L.; Mao, M.; Luo, Y. Extended Application of Diffusive Gradients in Thin Films (DGT) in Assessing Arsenic Bioavailability and Human Health Risks in Brownfield Soils. Environ. Technol. Innov. 2023, 32, 103346. [Google Scholar] [CrossRef]
- Wei, T.-J.; Guan, D.-X.; Li, X.-Y.; Hao, Y.-L.; Teng, H.H.; Yang, J.-F.; Xu, Y.-Y.; Li, G. Analysis of Studies on Environmental Measurements Using Diffusive Gradients in Thin-Films (DGT) from 1994 to 2020. J. Soils Sediments 2022, 22, 1069–1079. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview Assessment of Risk Evaluation and Treatment Technologies for Heavy Metal Pollution of Water and Soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Alhaj Hamoud, Y.; Shaghaleh, H.; Zia-ur-Rehman, M.; Rizwan, M.; Umair, M.; Usman, M.; Ayub, M.A.; Riaz, U.; Alnusairi, G.S.H.; Alghanem, S.M.S. Cadmium and Lead Accumulation in Important Food Crops Due to Wastewater Irrigation: Pollution Index and Health Risks Assessment. Heliyon 2024, 10, e24712. [Google Scholar] [CrossRef]
- Ali, M. Comparative Assessment of Phytoavailable Cadmium by Using Various Extractants in Texturally Different Contaminated Soils during the Growth of Maize Crop. Pak. J. Agric. Sci. 2023, 60, 389–400. [Google Scholar] [CrossRef]
- Usman, M.; Zia-ur-Rehman, M.; Rizwan, M.; Abbas, T.; Ayub, M.A.; Naeem, A.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Qamar, M.J.; et al. Effect of Soil Texture and Zinc Oxide Nanoparticles on Growth and Accumulation of Cadmium by Wheat: A Life Cycle Study. Environ. Res. 2023, 216, 114397. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Anayatullah, S.; Irfan, E.; Hussain, S.M.; Rizwan, M.; Sohail, M.I.; Jafir, M.; Ahmad, T.; Usman, M.; Alharby, H.F. Nanoparticles Assisted Regulation of Oxidative Stress and Antioxidant Enzyme System in Plants under Salt Stress: A Review. Chemosphere 2023, 314, 137649. [Google Scholar] [CrossRef]
- Doležal, J.; Chondol, T.; Chlumská, Z.; Altman, J.; Čapková, K.; Dvorský, M.; Fibich, P.; Korznikov, K.A.; Ruka, A.T.; Kopecký, M.; et al. Contrasting Biomass Allocations Explain Adaptations to Cold and Drought in the World’s Highest-Growing Angiosperms. Ann. Bot. 2024, 134, 401–414. [Google Scholar] [CrossRef]
- Yu, L.; Luo, X.; Croft, H.; Rogers, C.A.; Chen, J.M. Seasonal Variation in the Relationship between Leaf Chlorophyll Content and Photosynthetic Capacity. Plant Cell Environ. 2024, 1–13. [Google Scholar] [CrossRef]
- Saltelli, A.; Ratto, M.; Tarantola, S.; Campolongo, F. Sensitivity Analysis Practices: Strategies for Model-Based Inference. Reliab. Eng. Syst. Saf. 2006, 91, 1109–1125. [Google Scholar] [CrossRef]
- Ullah, S.; Zia, M.; Meers, E.; Ghafoor, A.; Murtaza, G.; Sabir, M.; Zia-ur-Rehman, M.; Tack, F. Chemically Enhanced Phytoextraction of Pb by Wheat in Texturally Different Soils. Chemosphere 2010, 79, 652–658. [Google Scholar]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.W.; Zia-ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A Critical Review on Effects, Tolerance Mechanisms and Management of Cadmium in Vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef]
- Sohail, M.I.; Zia ur Rehman, M.; Rizwan, M.; Yousaf, B.; Ali, S.; Anwar ul Haq, M.; Anayat, A.; Waris, A.A. Efficiency of Various Silicon Rich Amendments on Growth and Cadmium Accumulation in Field Grown Cereals and Health Risk Assessment. Chemosphere 2020, 244, 125481. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Zia ur Rehman, M.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon Nanoparticles Enhanced the Growth and Reduced the Cadmium Accumulation in Grains of Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.I.; Zia ur Rehman, M.; Aziz, T.; Akmal, F.; Azhar, M.; Nadeem, F.; Aslam, M.; Siddiqui, A.; Khalid, M.A. Iron Bio-Fortification and Heavy Metal/(Loid)s Contamination in Cereals: Successes, Issues, and Challenges. Crop Pasture Sci. 2022, 73, 877–895. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Zia-ur-Rehman, M.; Hussain, S.M.; Qayyum, M.F.; Rizwan, M.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Ali, S. Combined Effects of Zinc Oxide Nanoparticles and Melatonin on Wheat Growth, Chlorophyll Contents, Cadmium (Cd) and Zinc Uptake under Cd Stress. Sci. Total Environ. 2023, 864, 161061. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Rauf, A.; Ayub, M.A.; Ali, S.; Qayyum, M.F.; Waris, A.A.; Naeem, A.; Sanaullah, M. Split Application of Silicon in Cadmium (Cd) Spiked Alkaline Soil Plays a Vital Role in Decreasing Cd Accumulation in Rice (Oryza sativa L.) Grains. Chemosphere 2019, 226, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.Z.; Batool, Z.; Ayub, M.A.; Hussaini, K.M.; Murtaza, G.; Usman, M.; Naeem, A.; Khalid, H.; Rizwan, M.; Ali, S. Effect of Acidified Biochar on Bioaccumulation of Cadmium (Cd) and Rice Growth in Contaminated Soil. Environ. Technol. Innov. 2020, 19, 101015. [Google Scholar] [CrossRef]
- Ayub, M.A.; Zia ur Rehman, M.; Ahmad, H.R.; Fox, J.-P.; Clubb, P.; Wright, A.L.; Anwar-ul-Haq, M.; Nadeem, M.; Rico, C.M.; Rossi, L. Influence of Ionic Cerium and Cerium Oxide Nanoparticles on Zea mays Seedlings Grown with and without Cadmium. Environ. Pollut. 2023, 322, 121137. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Akram, M.A.; Zia-ur-Rehman, M.; Wang, X.; Zhang, X.; Huang, L. Physiological and Transcriptome Analyses Demonstrate the Silver Nanoparticles Mediated Alleviation of Salt Stress in Pearl Millet (Pennisetum glaucum L). Environ. Pollut. 2023, 318, 120863. [Google Scholar] [CrossRef]
- Rizwan, A.; Zia-ur-Rehman, M.; Rizwan, M.; Usman, M.; Anayatullah, S.; Alharby, H.F.; Bamagoos, A.A.; Alharbi, B.M.; Ali, S. Effects of Silicon Nanoparticles and Conventional Si Amendments on Growth and Nutrient Accumulation by Maize (Zea mays L.) Grown in Saline-Sodic Soil. Environ. Res. 2023, 227, 115740. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Haider, G.; Rizwan, M.; Schofield, H.K.; Qayyum, M.F.; Zia-ur-Rehman, M.; Ali, S. Different Feedstocks of Biochar Affected the Bioavailability and Uptake of Heavy Metals by Wheat (Triticum aestivum L.) Plants Grown in Metal Contaminated Soil. Environ. Res. 2023, 217, 114845. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium Stress in Rice: Toxic Effects, Tolerance Mechanisms, and Management: A Critical Review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Liu, G.-Z.; Zhao, K.; Zhang, S.-Q.; Liang, Y.-M.; Yue, Y.-J.; Liu, G.-H.; Qin, F.-C. Biomass Allocation and Allometric Relationship of Salix Gordejevii Branches in Sandy Habitats Heterogeneity in Northen China. Sustainability 2024, 16, 5483. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Mubsher, A.; Rizwan, M.; Usman, M.; Jafir, M.; Umair, M.; Alharby, H.F.; Bamagoos, A.A.; Alshamrani, R.; Ali, S. Effect of Farmyard Manure, Elemental Sulphur and EDTA on Growth and Phytoextraction of Cadmium by Spider Plants (Chlorophytum comosum L.) under Cd Stress. Chemosphere 2023, 313, 137385. [Google Scholar] [CrossRef]
- Dai, Y.; Nasir, M.; Zhang, Y.; Gao, J.; Lv, Y.; Lv, J. Comparison of DGT with Traditional Extraction Methods for Assessing Arsenic Bioavailability to Brassica Chinensis in Different Soils. Chemosphere 2018, 191, 183–189. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Xu, F.; Chen, X.; Du, L.; Wang, X.; Li, Y. Assessing the Remobilization and Fraction of Cadmium and Lead in Sediment of the Jialing River by Sequential Extraction and Diffusive Gradients in Films (DGT) Technique. Chemosphere 2020, 257, 127181. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, L.; Deng, H.; Hu, S.; Xie, X.; Luo, D.; Xiao, T. Hydrogeochemistry of Groundwater Surrounding a Pyrite Mine in Southern China: Assessment of the Diffusive Gradients in Thin Films Technique for in Situ Monitoring. J. Hydrol. 2023, 622, 129685. [Google Scholar] [CrossRef]
- Zhang, T.; Zeng, X.; Zhang, H.; Lin, Q.; Su, S.; Wang, Y.; Bai, L. Investigation of Synthetic Ferrihydrite Transformation in Soils Using Two-Step Sequential Extraction and the Diffusive Gradients in Thin Films (DGT) Technique. Geoderma 2018, 321, 90–99. [Google Scholar] [CrossRef]
- Luo, J.; Cheng, H.; Ren, J.; Davison, W.; Zhang, H. Mechanistic Insights from DGT and Soil Solution Measurements on the Uptake of Ni and Cd by Radish. Environ. Sci. Technol. 2014, 48, 7305–7313. [Google Scholar] [CrossRef]
- Senila, M. Real and Simulated Bioavailability of Lead in Contaminated and Uncontaminated Soils. J. Environ. Health Sci. Eng. 2014, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, O.; Pierzynski, G.; Kaya, C.; Aydemir, S. The Effects of Phosphorus Addition on Phytoavailability of Zinc by Diffusive Gradientsin Thin Films (DGT). Turk. J. Agric. For. 2016, 40, 379–385. [Google Scholar] [CrossRef]
- Herníková, V.; Dočekalová, H.; Diviš, P. The Diffusive Gradient in Thin Films Technique (DGT) for the Mercury Determination in Aquatic Systems. Chem. Listy 2005, 99. [Google Scholar]
- Duquène, L.; Vandenhove, H.; Tack, F.; Van Hees, M.; Wannijn, J. Diffusive Gradient in Thin FILMS (DGT) Compared with Soil Solution and Labile Uranium Fraction for Predicting Uranium Bioavailability to Ryegrass. J. Environ. Radioact. 2010, 101, 140–147. [Google Scholar] [CrossRef]
- Nowack, B.; Koehler, S.; Schulin, R. Use of Diffusive Gradients in Thin Films (DGT) in Undisturbed Field Soils. Environ. Sci. Technol. 2004, 38, 1133–1138. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, S.; Xu, D.; Tang, Y.; Wong, M.H. Bioavailability Assessment of Phosphorus and Metals in Soils and Sediments: A Review of Diffusive Gradients in Thin Films (DGT). Environ. Monit. Assess. 2014, 186, 7367–7378. [Google Scholar] [CrossRef]
- Yobouet, Y.A.; Adouby, K.; Trokourey, A.; Yao, B. Cadmium, Copper, Lead and Zinc Speciation in Contaminated Soils. Int. J. Eng. Sci. Technol. 2010, 2, 802–812. [Google Scholar]
- Wu, Q.; Hendershot, W.H.; Marshall, W.D.; Ge, Y. Speciation of Cadmium, Copper, Lead, and Zinc in Contaminated Soils. Commun. Soil Sci. Plant Anal. 2000, 31, 1129–1144. [Google Scholar] [CrossRef]
- Williams, P.N.; Zhang, H.; Davison, W.; Zhao, S.; Lu, Y.; Dong, F.; Zhang, L.; Pan, Q. Evaluation of in Situ DGT Measurements for Predicting the Concentration of Cd in Chinese Field-Cultivated Rice: Impact of Soil Cd:Zn Ratios. Environ. Sci. Technol. 2012, 46, 8009–8016. [Google Scholar] [CrossRef]
- Agbenin, J.O.; Welp, G. Bioavailability of Copper, Cadmium, Zinc, and Lead in Tropical Savanna Soils Assessed by Diffusive Gradient in Thin Films (DGT) and Ion Exchange Resin Membranes. Environ. Monit. Assess. 2012, 184, 2275–2284. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance Characteristics of Diffusion Gradients in Thin Films for the In Situ Measurement of Trace Metals in Aqueous Solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Soltanpour, P.N. Use of Ammonium Bicarbonate DTPA Soil Test to Evaluate Elemental Availability and Toxicity. Commun. Soil Sci. Plant Anal. 1985, 16, 323–338. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Wear, J.I.; Evans, C.E. Relationship of Zinc Uptake by Corn and Sorghum to Soil Zinc Measured by Three Extractants. Soil Sci. Soc. Am. J. 1968, 32, 543–546. [Google Scholar] [CrossRef]
- Novozamsky, I.; Lexmond, T.M.; Houba, V.J.G. A Single Extraction Procedure of Soil for Evaluation of Uptake of Some Heavy Metals by Plants. Int. J. Environ. Anal. Chem. 1993, 51, 47–58. [Google Scholar] [CrossRef]
- Gupta, S.K.; Aten, C. Comparison and Evaluation of Extraction Media and Their Suitability in a Simple Model to Predict the Biological Relevance of Heavy Metal Concentrations in Contaminated Soils. Int. J. Environ. Anal. Chem. 1993, 51, 25–46. [Google Scholar] [CrossRef]
- Groenenberg, J.E.; Römkens, P.F.A.M.; Van Zomeren, A.; Rodrigues, S.M.; Comans, R.N.J. Evaluation of the Single Dilute (0.43 M) Nitric Acid Extraction to Determine Geochemically Reactive Elements in Soil. Environ. Sci. Technol. 2017, 51, 2246–2253. [Google Scholar] [CrossRef]
- Symeonides, C.; McRae, S.G. The Assessment of Plant-Available Cadmium in Soils. J. Environ. Qual. 1977, 6, 120–123. [Google Scholar] [CrossRef]

| Parameters | Units | Values | |
|---|---|---|---|
| Sand | % | 54.44 | 41.89 |
| Silt | % | 22.01 | 26.56 |
| Clay | % | 23.55 | 31.55 |
| Soil Texture | Sandy clay loam | Clay loam | |
| Saturation percentage | % | 33.00 | 35.00 |
| Soil-saturated paste pH | 7.78 | 7.69 | |
| Electrical conductivity | dS/m | 4.78 | 4.99 |
| Cation exchange capacity | cmolc/kg | 12.00 | 15.00 |
| Sodium adsorption ratio | (mmol/L)1/2 | 10.45 | 11.55 |
| Total Cd | mg/kg | 2.75 | 3.10 |
| Total Pb | mg/kg | 4.18 | 3.78 |
| ABDTPA Cd | mg/kg | 0.45 | 0.42 |
| ABDTPA Pb | mg/kg | 0.71 | 0.63 |
| Organic matter | % | 0.78 | 0.83 |
| Extraction Method | Soil Weight (g) | Solution Composition | Solution Volume (mL) | Shaking Time | References |
|---|---|---|---|---|---|
| AB-DTPA | 10 | 1 N NH4HCO3 + 0.005 M DTPA | 20 | 15 min | [64] |
| 0.005 M DTPA | 10 | 0.005 M DTPA + 0.01 M TEA + 0.01 M CaCl2 | 20 | 2 h | [65] |
| 0.05 M EDTA | 2 | 0.05 M EDTA, pH 7.0 | 20 | 1 h | [66] |
| 0.01 M CaCl2 | 2 | 0.01 M CaCl2 | 20 | 3 h | [67] |
| 0.1 M NaNO3 | 8 | 0.1 M NaNO3 | 20 | 2 h | [68] |
| 0.43 M HNO3 | 2 | 0.43 M HNO3 | 20 | 2 h | [69] |
| 1 N NH4.AOc | 2 | 1 N NH4.AOc | 20 | 2 h | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaghaleh, H.; Rana, S.; Zia-ur-Rehman, M.; Usman, M.; Ali, M.; Alharby, H.F.; Majrashi, A.; Alamri, A.M.; Abu Zeid, I.M.; Alhaj Hamoud, Y. Bioassessment of Cd and Pb at Multiple Growth Stages of Wheat Grown in Texturally Different Soils Using Diffusive Gradients in Thin Films and Traditional Extractants: A Comparative Study. Plants 2024, 13, 2445. https://doi.org/10.3390/plants13172445
Shaghaleh H, Rana S, Zia-ur-Rehman M, Usman M, Ali M, Alharby HF, Majrashi A, Alamri AM, Abu Zeid IM, Alhaj Hamoud Y. Bioassessment of Cd and Pb at Multiple Growth Stages of Wheat Grown in Texturally Different Soils Using Diffusive Gradients in Thin Films and Traditional Extractants: A Comparative Study. Plants. 2024; 13(17):2445. https://doi.org/10.3390/plants13172445
Chicago/Turabian StyleShaghaleh, Hiba, Sana Rana, Muhammad Zia-ur-Rehman, Muhammad Usman, Mujahid Ali, Hesham F. Alharby, Ali Majrashi, Amnah M. Alamri, Isam M. Abu Zeid, and Yousef Alhaj Hamoud. 2024. "Bioassessment of Cd and Pb at Multiple Growth Stages of Wheat Grown in Texturally Different Soils Using Diffusive Gradients in Thin Films and Traditional Extractants: A Comparative Study" Plants 13, no. 17: 2445. https://doi.org/10.3390/plants13172445
APA StyleShaghaleh, H., Rana, S., Zia-ur-Rehman, M., Usman, M., Ali, M., Alharby, H. F., Majrashi, A., Alamri, A. M., Abu Zeid, I. M., & Alhaj Hamoud, Y. (2024). Bioassessment of Cd and Pb at Multiple Growth Stages of Wheat Grown in Texturally Different Soils Using Diffusive Gradients in Thin Films and Traditional Extractants: A Comparative Study. Plants, 13(17), 2445. https://doi.org/10.3390/plants13172445









