A Comprehensive Review of Climate Change and Plant Diseases in Brazil
Abstract
:1. Introduction
2. Plant Disease
3. Impacts of Climate Change on Food Security
4. The Impacts of Climate Change on Plant Disease
5. Diseases Transmitted by Vectors
6. Research Gap
7. What to Do after Risk Assessment
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Eskander, S.M.; Fankhauser, S. Reduction in greenhouse gas emissions from national climate legislation. Nat. Clim. Change 2020, 10, 750–756. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2023: Synthesis Report; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- IPPC Secretariat. Summary for Policymakers of the Scientific Review of the Impact of Climate Change on Plant Pests: A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Gregory, P.J.; Johnson, S.N.; Newton, A.C.; Ingram, J.S. Integrating pests and pathogens into the climate change/food security debate. J. Exp. Bot. 2009, 60, 2827–2838. [Google Scholar] [CrossRef] [PubMed]
- Mahmuti, M.; West, J.S.; Watts, J.; Gladders, P.; Fitt, B.D. Controlling crop disease contributes to both food security and climate change mitigation. Int. J. Agric. Sustain. 2009, 7, 189–202. [Google Scholar] [CrossRef]
- Manning, W.J.; Tiedemann, A.V. Climate change: Potential effects of increased atmospheric carbon dioxide (CO2), ozone (O3), and ultraviolet-B (UV-B) radiation on plant diseases. Environ. Pollut. 1995, 88, 219–245. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tebeest, D.O.; Teng, P.S.; Fabellar, N.G. Simulation studies on risk analysis of rice leaf blast epidemics associated with global climate change in several Asian countries. J. Biogeogr. 1995, 22, 673–678. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Luck, J.; Spackman, M.; Freeman, A.; Trebicki, P.; Griffiths, W.; Finlay, K.; Chakraborty, S. Climate change and diseases of food crops. Plant Pathol. 2011, 60, 113–121. [Google Scholar] [CrossRef]
- Stack, J.; Fletcher, J.; Gullino, M.L. Climate change and plant biosecurity: A new world disorder? In Global Environmental Change: New Drivers for Resistance, Crime and Terrorism; Bodo, B., Burnley, C., Comardicea, I., Maas, A., Roffey, R., Eds.; Nomos: Baden Baden, Germany, 2013; pp. 161–181. [Google Scholar]
- Heeb, L.; Jenner, E.; Cock, M.J.W. Climate-smart pest management: Building resilience of farms and landscapes to changing pest threats. J. Pest Sci. 2019, 92, 951–969. [Google Scholar] [CrossRef]
- Trebicki, P.; Finlay, K. Pests and diseases under climate change; its threat to food security. In Food Security and Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Ebert, A.W., Hunter, D., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2019; pp. 229–249. [Google Scholar]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Ingram, J.S.I.; Gregory, P.J.; Izac, A.-M. The role of agronomic research in climate change and food security policy. Agric. Ecosyst. Environ. 2008, 126, 4–12. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Managan, N. Climate change, food security and mycotoxins: Do we know enough? Fung Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Leonard, L. Climate change impacts and challenges of combating food insecurity in rural Somkhele, KwaZulu-Natal, South Africa. Sustainability 2022, 14, 16023. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019. International Year of Plant Health, 2020; FAO: Rome, Italy, 2019. [Google Scholar]
- Assad, E.D.; Pinto, H.S.; Zullo Júnior, J.; Ávila, A.M.H. Impacto das mudanças climáticas no zoneamento agroclimático do café no Brasil. Pesq. Agropecu. Bras. 2004, 39, 1057–1064. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Coates, K.D.; Hamann, A. Is an unprecedented dothistroma needle blight epidemic related to climate change? BioScience 2005, 55, 761–769. [Google Scholar] [CrossRef]
- Woods, A. Is the health of British Columbia’s forests being influenced by climate change? If so, was this predictable? Can. J. Plant Pathol. 2011, 33, 117–126. [Google Scholar] [CrossRef]
- Zhao, D.L.; Reddy, K.R.; Kakani, V.G.; Mohammed, A.R.; Read, J.J.; Gao, W. Leaf and canopy photosynthetic characteristics of cotton (Gossypium hirsutum) under elevated CO2 concentration and UV-B radiation. J. Plant Physiol. 2004, 161, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Tubby, K.V.; Webber, J.F. Pests and diseases threatening urban trees under a changing climate. Forestry 2010, 83, 451–459. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Juroszek, P.; Tiedemann, A.V. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Amthor, J.S. Crops and Environmental Change; Food Products Press: Binghamton, NY, USA, 2005; p. 421. [Google Scholar]
- Melloy, P.; Hollaway, G.; Luck, J.; Norton, R.; Aitken, E.; Chakraborty, S. Production and fitness of Fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE. Glob. Change Biol. 2010, 16, 3363–3373. [Google Scholar] [CrossRef]
- Chakraborty, S.; Luck, J.; Hollaway, G.; Fitzgerald, G.; White, N. Rust-proofing wheat for a changing climate. Euphytica 2011, 179, 19–32. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Skelsey, P.; Cooke, D.E.L.; Lynott, J.S.; Lees, A.K. Crop connectivity under climate change: Future environmental and geographic risks of potato late blight in Scotland. Glob. Change Biol. 2016, 22, 3724–3738. [Google Scholar] [CrossRef] [PubMed]
- Sparks, A.H.; Forbes, G.A.; Hijmans, R.J.; Garrett, K.A. Climate change may have limited effect on global risk of potato late blight. Glob. Change Biol. 2014, 20, 3621–3631. [Google Scholar] [CrossRef]
- Choudhary, J.S.; Kumari, M.; Fand, B.B. Linking insect pest models with climate change scenarios to project against future risks of agricultural insect pests. CAB Rev. 2019, 14, 055. [Google Scholar] [CrossRef]
- Tavares, P.D.S.; Giarolla, A.; Chou, S.C.; Silva, A.J.D.P.; Lyra, A.D.A. Climate change impact on the potential yield of Arabica coffee in Southeast Brazil. Reg. Environ. Change 2018, 18, 873–883. [Google Scholar] [CrossRef]
- Gäumann, E. Principles of Plant Infection; Crosby Lockwood & Sons: London, UK, 1950. [Google Scholar]
- Stevens, R.B. Cultural practices in disease control. In Plant Pathology: An Advanced Treatise; Horsfall, J.G., Dimond, A.E., Eds.; Academic Press: New York, UY, USA, 1960; pp. 357–429. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, NY, USA, 2005; p. 922. [Google Scholar]
- Ghini, R. Mudanças Climáticas Globais e Doenças de Plantas; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2005. [Google Scholar]
- Francl, L.J. The disease triangle: A plant pathological paradigm revisited. Plant Health Instruct 2001. Available online: https://www.apsnet.org/edcenter/foreducators/TeachingNotes/Pages/DiseaseTriangle.aspx (accessed on 10 May 2022). [CrossRef]
- Karnosky, D.F.; Percy, K.E.; Xiang, B.; Callan, B.; Noormets, A.; Mankovska, B.; Hopkin, A.; Sober, J.; Jones, W.; Dickson, R.E.; et al. Interacting elevated CO2 and tropospheric O3 predisposes aspen (Populus tremuloides Michx.) to infection by rust (Melampsora medusae f. sp. tremuloidae). Glob. Change Biol. 2002, 8, 329–338. [Google Scholar] [CrossRef]
- Tiedemann, A.; Firsching, K.H. Interactive effects of elevated ozone and carbon dioxide on growth and yield of leaf rust-infected versus non-infected wheat. Environ. Pollut. 2000, 108, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.E.; Reich, P.B.; Tilman, D.; Groth, J.V. Effects of elevated CO2, nitrogen deposition, and decreased species diversity on foliar fungal plant disease. Glob. Change Biol. 2003, 9, 438–451. [Google Scholar] [CrossRef]
- Plessl, M.; Elstner, E.F.; Rennenberg, H.; Habermeyer, J.; Heiser, I. Influence of elevated CO2 and ozone concentrations on late blight resistance and growth of potato plants. Environ. Exp. Bot. 2007, 60, 447–457. [Google Scholar] [CrossRef]
- Percy, K.E.; Awmack, C.S.; Lindroth, R.L.; Kubiske, M.E.; Kopper, B.J.; Isebrands, J.G.; Pregitzerk, K.S.; Hendrey, G.R.; Dickson, R.E.; Zak, D.R.; et al. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 2002, 420, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Flint, S.D. Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystems. Clim. Change 1994, 28, 375–394. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bjorn, L.O.; Bornman, J.F.; Flint, S.D.; Kulandaivelu, G.; Teramura, A.H.; Tevini, M. Effects of increased solar ultraviolet radiation on terrestrial ecosystems. J. Photochem. Photobiol. B Biol. 1998, 46, 40–52. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.F.; Ballare, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with bother climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [CrossRef]
- Evans, N.; Baierl, A.; Semenov, M.A.; Gladders, P.; Fitt, B.D.L. Range and severity of plant disease increased by global warming. J. R. Soc. Interface 2008, 5, 525–531. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Garrett, K.A.; Nita, M.; Wolf, E.D.D.; Gomez, L.; Sparks, A.H. Plant pathogens as indicators of climate change. In Climate Change: Observed Impacts on Planet Earth; Letcher, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 425–437. [Google Scholar]
- Garrett, K.A.; Forbes, G.A.; Savary, S.; Skelsey, P.; Sparks, A.H.; Valdivia, C.; van Bruggen, A.H.C.; Willocquet, L.; Djurle, A.; Duveiller, E.; et al. Complexity in climate-change impacts: An analytical framework for effects mediated by plant disease. Plant Pathol. 2011, 60, 15–30. [Google Scholar] [CrossRef]
- Gunasekera, T.S.; Paul, N.D.; Ayres, P.G. The effects of ultraviolet-B (UV-B: 290–320 nm) radiation on blister blight disease of tea (Camellia sinensis). Plant Pathol. 1997, 46, 179–185. [Google Scholar] [CrossRef]
- Johnson, D. Response of terrestrial microorganisms to ultraviolet-B radiation in ecosystems. Res. Microbiol. 2003, 154, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.P.; Wreford, A.; Butterworth, M.H.; Semenov, M.A.; Moran, D.; Evans, N.; Fitt, B.D.L. Adaptation to increasing severity of phoma stem canker on winter oilseed rape in the UK under climate change. J. Agric. Sci. 2010, 148, 683–694. [Google Scholar] [CrossRef]
- Wu, J.; Guan, D.; Yuan, F.; Zhang, X. Research advances on the biological effects of elevated ultraviolet-B radiation on terrestrial plants. J. For. Res. 2009, 20, 383–390. [Google Scholar] [CrossRef]
- Paul, N.D. Stratospheric ozone depletion, UV-B radiation and crop disease. Environ. Pollut. 2000, 108, 343–355. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishiguro, K.; Nakajima, T.; Kim, H.Y.; Okada, M.; Kobayashi, K. Effects of elevated atmospheric CO2 concentration on the infection of rice blast and sheath blight. Phytopathology 2006, 96, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ghini, R.; Hamada, E.; Bettiol, W. Climate change and plant diseases. Sci. Agric. 2008, 65, 98–107. [Google Scholar] [CrossRef]
- Eastburn, D.M.; Degennaro, M.M.; Delucia, E.H.; Dermody, O.; McElrone, A.J. Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Glob. Change Biol. 2010, 16, 320–330. [Google Scholar] [CrossRef]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant–pathogen interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Robin, C.; Reynaud, G.; Deque, M.; Badeau, V.; Piou, D.; Husson, C.; Marcais, B. Simulating the effects of a climate-change scenario on the geographical range and activity of forest-pathogenic fungi. Can. J. Plant Pathol. 2007, 29, 101–120. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Fernandes, J.M.C.; Pavan, W.; Baethgen, W.E. A model-based assessment of the impacts of climate variability on fusarium head blight seasonal risk in Southern Brazil. J. Phytopathol. 2009, 157, 675–681. [Google Scholar] [CrossRef]
- Hannukkala, A.O.; Kaukoranta, T.; Lehtinen, A.; Rahkonen, A. Late-blight epidemics on potato in Finland, 1933–2002; increased and earlier occurrence of epidemics associated with climate change and lack of rotation. Plant Pathol. 2007, 56, 167–176. [Google Scholar] [CrossRef]
- Carter, T.R.; Saarikko, R.A.; Niemi, K.J. Assessing the risks and uncertainties of regional crop potential under a changing climate in Finland. Agric. Food Sci. 1996, 5, 329–350. [Google Scholar] [CrossRef]
- Salinari, F.; Giosue, S.; Tubiello, F.N.; Rettori, A.; Rossi, V.; Spanna, F.; Rosenzweig, C.; Gullino, M.L. Downy mildew (Plasmopara viticola) epidemics on grapevine under climate change. Glob. Change Biol. 2006, 12, 1299–1307. [Google Scholar]
- Shaw, M.W.; Osborne, T.M. Geographic distribution of plant pathogens in response to climate change. Plant Pathol. 2011, 60, 31–43. [Google Scholar] [CrossRef]
- Roos, J.; Hopkins, R.; Kvarnheden, A.; Dixelius, C. The impact of global warming on plant diseases and insect vectors in Sweden. Eur. J. Plant Pathol. 2011, 129, 9–19. [Google Scholar] [CrossRef]
- Silva Dias, M.A.F.; Silva Dias, P.L. As incertezas regionais nos cenários de mudanças climáticas globais. Bol. Soc. Bras. Meteorol. 2007, 31, 12–16. [Google Scholar]
- Souza, A.P.; Gaspar, M.; Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y., Jr.; Santos, R.V.; Teixeira, M.M.; Souza, G.M.; Buckeridge, M.S. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef]
- Butterworth, M.H.; Semenov, M.A.; Barnes, A.; Moran, D.; West, J.S.; Fitt, B.D.L. North–South divide: Contrasting impacts of climate change on crop yields in Scotland and England. J. R. Soc. Interface 2010, 7, 123–130. [Google Scholar] [CrossRef]
- Boag, B.; Crawford, J.W.; Neilson, R. The effect of potential climatic changes on the geographical distribution of the plant-parasitic nematodes Xiphinema and Longidorus in Europe. Nematologica 1991, 37, 312–323. [Google Scholar]
- Ghini, R.; Mac Leod, R.E.O.; Torre Neto, A.; Cardoso, D.C.; Bettiol, W.; Morais, L.A.S.; Vique, B. Increased atmospheric carbon dioxide concentration: Effects on eucalypt rust (Puccinia psidii), C:N ratio and essential oils in eucalypt clonal plantlets. For. Pathol. 2014, 44, 409–416. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pangga, I.B. Plant disease and climate change. In Plant Microbiology; Gillings, M., Holmes, A., Eds.; BIOS Scientific: London, UK, 2004; pp. 163–180. [Google Scholar]
- Ghini, R.; Hamada, E.; Bettiol, W. Diseases in tropical and plantation crops as affected by climate changes: Current knowledge and perspectives. Plant Pathol. 2011, 60, 122–132. [Google Scholar] [CrossRef]
- Ghini, R.; Torre-Neto, A.; Dentzien, A.F.; Guerreiro-Filho, O.; Iost, R.; Patrício, F.R.; Prado, J.S.; Thomaziello, R.A.; Bettiol, W.; DaMatta, F.M. Coffee growth, pest and yield response to free-air CO2 enrichment. Clim. Change 2015, 132, 307–320. [Google Scholar] [CrossRef]
- Raja, M.U.; Mukhtar, T.; Shaheen, F.A.; Bodlah, I.; Jamal, A.; Fatima, B.; Ismail, M.; Shah, I. Climate change and its impact on plant health: A Pakistan’s prospective. Plant Protect. 2018, 2, 51–56. [Google Scholar]
- van der Putten, W.H.; Bradford, M.A.; Pernilla Brinkman, E.; van de Voorde, T.F.; Veen, G.F. Where, when and how plant-soil feedback matters in a changing world. Funct. Ecol. 2016, 30, 1109–1121. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.; Sims, J.; Martins, V.F.; Swerbillow, J.; Romero, M.; Alborn, H.T.; et al. Effects of elevated [CO2] on maize defense against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 2014, 37, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.A.; Gomez-Gallego, M.; Jones, E.; Smaill, S.; Lear, G.; Lambie, S. Climate change induced impacts on plant diseases in New Zealand. Australas. Plant Path. 2018, 47, 101–114. [Google Scholar] [CrossRef]
- McElrone, A.J.; Reid, C.D.; Hoye, K.A.; Hart, E.; Jackson, R.B. Elevated CO2 reduces disease incidence and severity of a red maple fungal pathogen via changes in host physiology and leaf chemistry. Glob. Change Biol. 2005, 11, 1828–1836. [Google Scholar] [CrossRef]
- Pangga, I.B.; Hanan, J.; Chakraborty, S. Pathogen dynamics in a crop canopy and their evolution under changing climate. Plant Pathol. 2011, 60, 70–81. [Google Scholar] [CrossRef]
- Gullino, M.L.; Pugliese, M.; Gilardi, G.; Garibaldi, A. Effect of increased CO2 and temperature on plant diseases: A critical appraisal of results obtained in studies carried out under controlled environment facilities. J. Plant Pathol. 2018, 100, 371–389. [Google Scholar] [CrossRef]
- Mikkelsen, B.L.; Jørgensen, R.B.; Lyngkjær, M.F. Complex interplay of future climate levels of CO2, ozone and temperature on susceptibility to fungal diseases in barley. Plant Pathol. 2014, 64, 319–327. [Google Scholar] [CrossRef]
- Koo, T.H.; Hong, S.J.; Yun, S.C. Changes in the aggressiveness and fecundity of hot pepper anthracnose pathogen (Colletotrichum acutatum) under elevated CO2 and temperature over 100 infection cycles. Plant Pathol. J. 2016, 32, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Coakley, S.M.; Scherm, H.; Chakraborty, S. Climate change and plant disease management. Annu. Rev. Phytopathol. 1999, 37, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; McCarl, B.A. An investigation of the relationship between pesticide usage and climate change. Clim. Change 2001, 50, 475–487. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Amthor, J.S. Crops and Environmental Change; Food Products Press: New York, NY, USA, 2005. [Google Scholar]
- Coakley, S.M.; Scherm, H. Plant disease in a changing global environment. Asp. Appl. Biol. 1996, 45, 227–238. [Google Scholar]
- Chakraborty, S.; Luck, J.; Hollaway, G.; Freeman, A.; Norton, R.; Garrett, K.A.; Percy, K.; Hopkins, A.; Davis, C.; Karnosky, D.F. Impacts of global change on diseases of agricultural crops and forest trees. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–15. [Google Scholar] [CrossRef]
- Chakraborty, S.; Murray, G.M.; Magarey, P.A.; Yonow, T.; O’Brien, R.G.; Croft, B.J.; Barbetti, M.J.; Sivasithamparam, K.; Old, K.M.; Dudzinski, M.J.; et al. Potential impact of climate change on plant diseases of economic significance to Australia. Australas. Plant Pathol. 1998, 27, 15–35. [Google Scholar] [CrossRef]
- Chakraborty, S.; Datta, S. How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Pathol. 2003, 159, 733–742. [Google Scholar] [CrossRef]
- Ghini, R.; Hamada, E.; Angelotti, F.; Costa, L.B.; Bettiol, W. Research approaches, adaptation strategies, and knowledge gaps concerning the impacts of climate change on plant diseases. Trop. Plant Pathol. 2012, 37, 5–24. [Google Scholar]
- Garrett, K.A.; Bebber, D.P.; Etherton, B.A.; Gold, K.M.; Sula, A.P.; Selvaraj, M.G. Climate change effects on pathogen emergence: Artificial intelligence to translate big data for mitigation. Annu. Rev. Phytopathol. 2022, 60, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, J.M.; Whitbread, R.; Farrar, J.F. Effect of elevated concentrations of CO2 on infection of barley by Erysiphe graminis. Physiol. Mol. Plant Pathol. 1996, 48, 37–53. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Huang, Y.J.; Huang, Y.J.; Evans, N.; Li, Z.Q.; Eckert, M.; Chèvre, A.M.; Renard, M.; Fitt, B.D.L. Temperature and leaf wetness duration affect phenotypic expression of Rlm6-mediated resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2006, 170, 129–141. [Google Scholar] [CrossRef]
- Osswald, W.F.; Fleischmann, F.; Heiser, I. Investigations on the effect of ozone, elevated CO2 and nitrogen fertilization on host-parasite interactions. Summa Phytopathol. 2006, 32, 111–113. [Google Scholar]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; American Phytopathological Society: St. Paul, MN, USA, 1983. [Google Scholar]
- Cook, R.J. Biological control of the pathogens: Theory to application. Phytopathology 1985, 75, 25–29. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate change impact on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Gilardi, G.; Gisi, U.; Garibaldi, A.; Gullino, M.L. Effect of elevated atmospheric CO2 and temperature on the chemical and biological control of powdery mildew of zucchini and the Phoma leaf spot of leaf beet. Eur. J. Plant Pathol. 2017, 148, 229–236. [Google Scholar] [CrossRef]
- Waage, J.K.; Greathead, D.J. Biological control: Challenges and opportunities. Philos. Trans. R. Soc. 1988, 318, 111–128. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; World Resources Institute: Washington, NY, USA, 2005. [Google Scholar]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, E.; Mendes, R.; Bakker, P.A.H.; Raaijmakers, J.M. Fungal invasion of the rhizosphere microbiome. ISME J. 2016, 10, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Rossmann, M.; Pérez-Jaramillo, J.E.; Kavamura, V.N.; Chiaramonte, J.B.; Dumack, K.; Fiore-Donno, A.M.; Mendes, L.W.; Ferreira, M.M.C.; Bonkowski, M.; Raaijmakers, J.M.; et al. Multitrophic interactions in the rhizosphere microbiome of wheat: From bacteria and fungi to protists. FEMS Microbiol. Ecol. 2020, 96, fiaa032. [Google Scholar] [CrossRef]
- Silva, J.C.P.; Medeiros, F.H.V.; Campos, V.P. Building soil suppressiveness against plant-parasitic nematodes. Biocontrol Sci. Technol. 2018, 28, 423–445. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Sutton, J.C.; Li, D.W.; Peng, G.; Yu, H.; Zhang, P.; Valdebenito-Sanhueza, R.M. Gliocladium roseum, a versatile adversary of Botrytis cinerea in crops. Plant Dis. 1997, 81, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Morandi, M.A.B.; Maffia, L.A.; Sutton, J.C. Development of Clonostachys rosea and interations with Botytis cinerea in rose leaves and residues. Phytoparasitica 2001, 29, 103–113. [Google Scholar] [CrossRef]
- Sivagnanapazham, K.; Karthikeyan, M.; Raguchander, T.; Swarnapriya, R.; Kamalakannan, A. Effect of media, pH, temperature and light on the growth of Coniothyrium minitans (Campbell 1947)—A novel biocontrol agent for cabbage head rot caused by Sclerotinia sclerotiorum (Lib.) De Bary. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1885–1894. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pangga, I.B.; Lupton, J.; Hart, L.; Room, P.M.; Yates, D. Production and dispersal of Colletotrichum gloeosporioides spores on Stylosanthes scabra under elevated CO2. Environ. Pollut. 2000, 108, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ghini, R.; Hamada, E.; Gonçalves, R.R.V.; Gasparotto, L.; Pereira, J.C.R. Análise de risco das mudanças climáticas globais sobre a sigatoka-negra da bananeira no Brasil. Fitopatol. Bras. 2007, 32, 197–204. [Google Scholar] [CrossRef]
- Ghini, R.; Hamada, E.; Pedro Junior, M.J.; Gonçalves, R.R.V. Incubation period of Hemileia vastatrix in coffee plants in Brazil simulated under climate change. Summa Phytopathol. 2011, 37, 85–93. [Google Scholar] [CrossRef]
- Das, T.; Majumdart, H.D.; Devi, R.K.T.; Rajesh, T. Climate change impacts on plant diseases. SAARC J. Agric. 2016, 14, 200–209. [Google Scholar] [CrossRef]
- Zayan, S.A. Impact of climate change on plant diseases and IPM strategies. In Plant Diseases: Current Threats and Management Trends; Topolovec-Pintarić, S., Ed.; IntechOpen: London, UK, 2019; pp. 1–12. [Google Scholar]
- Juroszek, P.; Racca, P.; Link, S.; Farhumand, J.; Kleinhez, B. Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risk. Plant Pathol. 2020, 69, 179–193. [Google Scholar] [CrossRef]
- Amari, K.; Huang, C.; Heinlein, M. Potential impact of global warming on virus propagation in infected plants and agricultural productivity. Front. Plant Sci. 2021, 12, 649768. [Google Scholar] [CrossRef]
- Iost, R.; Ghini, R.; Nechet, K.L.; Bettiol, W. Effect of elevated atmospheric CO2 concentration on the incidence of rust and leaf miners, and growth of coffee. Australas. Plant Pathol. 2022, 51, 507–517. [Google Scholar] [CrossRef]
- Jeger, M.J. The impact of climate change on disease in wild plant populations and communities. Plant Pathol. 2022, 71, 111–130. [Google Scholar] [CrossRef]
- Juroszek, P.; Bartsch, L.; Fontaine, J.F.; Racca, P.; Kleinhenz, B. Summary of the worldwide available crop disease risk simulation studies that were driven by climate change scenarios and published during the past 20 years. Plant Pathol. 2022, 71, 1815–1838. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Evans, J.R.; McCaffery, S.; Cavagnaro, T.R. Growth and nutritive value of cassava (Manihot esculenta Cranz.) are reduced when grown in elevated CO2. Plant Biol. 2009, 11, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Taoussi, M.; Laasli, S.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; Jarroudi, M.E.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Jeger, M.J.; Pautasso, M. Plant disease and global change the importance of long term data sets. New Phytol. 2008, 177, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, T.; Palojarvi, A.; Ramo, K.; Ojanpera, K.; Esala, M.; Manninen, S. A 3-year exposure to CO2 and O3 induced minor changes in soil N cycling in a meadow ecosystem. Plant Soil 2006, 286, 61–73. [Google Scholar] [CrossRef]
- Gay, C.; Estrada, C.G.; Conde, C.; Eakin, H.; Villers, L. Potential impacts of climate change on agriculture: A case of study of coffee production in Veracruz, Mexico. Clim. Change 2006, 79, 259–288. [Google Scholar] [CrossRef]
- Riikonen, J.; Syrjälä, L.; Tulva, I.; Mänd, P.; Oksanen, E.; Poteri, M.; Vapaavuor, E. Stomatal characteristics and infection biology of Pyrenopeziza betulicola in Betula pendula trees grown under elevated CO2 and O3. Environ. Pollut. 2008, 156, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Goins, E.W. Elevated atmospheric carbon dioxide and weed populations in glyphosate treated soybean. Crop Sci. 2006, 46, 1354–1359. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Global Biopesticides Market by Type (Biofungicides, Bioinsecticides, Bionematicides), Source (Beneficial Insects, Microbials, Plant Extracts), Form, Crop, Application—Forecast 2024–2030. Available online: https://www.researchandmarkets.com/report/biopesticide (accessed on 29 July 2024).
- van Lenteren, J.C.; Bueno, V.H.P.; Luna, M.G. Biological Control in Latin America and the Caribbean: Its Rich History and Bright Future; Colmenarez, Y.C., Ed.; CABI: Boston, MA, USA, 2020. [Google Scholar]
- Agrianual. Anuário Da Agricultura Brasileira; IHS Markit: São Paulo, Brazil, 2022. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations, FAOSTAT Statistics Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 May 2022).
- Torres, S.M.; Moran, E.F.; Silva, R.F.B. Property rights and the soybean revolution: Shaping how China and Brazil are telecoupled. Sustainability 2017, 9, 954. [Google Scholar] [CrossRef]
- Silva, R.F.B.; Batistella, M.; Moran, E.; Celidonio, O.L.M.; Millington, J.D.A. The soybean trap: Challenges and risks for brazilian producers. Front. Sustain. Food. Syst. 2020, 4, 12. [Google Scholar]
- Canabarro, N.I.; Silva-Ortiz, P.; Nogueira, L.A.H.; Cantarella, H.; Maciel-Filho, R. Sustainability assessment of etanol and biodiesel production in Argentina, Brazil, Colombia, and Guatemala. Ren. Sustain. Energ. Rev. 2023, 171, 113019. [Google Scholar] [CrossRef]
- Chum, H.L.; Warner, E.; Seabra, J.E.A.; Macedo, I.C. A comparison of commercial ethanol production systems from Brazilian sugarcane and US corn. Biofuels Bioprod. Biorefining 2014, 8, 205–223. [Google Scholar] [CrossRef]
- Junqueira, T.L.; Chagas, M.F.; Gouveia, V.L.R.; Rezende, M.C.A.F.; Watanabe, M.D.B.; Jesus, C.D.F.; Cavalett, O.; Milanez, A.Y.; Bonomi, A. Techno-economic analysis and climate change impacts of sugarcane biorefineries considering different time horizons. Biotechnol. Biofuels 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.C.; Chagas, M.F.; Watanabe, M.D.B.; Bonomi, A.; Maciel Filho, R. Low carbon biofuels and the New Brazilian National Biofuel Policy (RenovaBio): A case study for sugarcane mills and integrated sugarcane-microalgae biorefineries. Renew. Sustain. Energy Rev. 2019, 115, 109365. [Google Scholar] [CrossRef]
- Chagas, M.F.; Bordonal, R.O.; Cavalett, O.; Carvalho, J.L.N.; Bonomi, A.; La Scala, N. Environmental and economic impacts of different sugarcane production systems in the ethanol biorefinery: Impacts of ethanol from sugarcane using different agricultural technologies are evaluated with focus on harvesting system, reduced tillage, controlled. Biofuels Bioprod. Biorefining 2016, 10, 89–106. [Google Scholar] [CrossRef]
- Alves, M.C.; Carvalho, L.G.; Pozza, E.A.; Sanches, L.; Maia, J.C.S. Ecological zoning of soybean rust, coffee rust and banana black sigatoka based on Brazilian climate changes. Proc. Environ. Sci. 2011, 6, 35–49. [Google Scholar] [CrossRef]
- Alves, M.C.; Sanches, L. Potential effects of spatio-temporal temperature variation for monitoring coffee leaf rust progress under CMIP6 climate change scenarios. Earth Syst. Environ. 2022, 6, 421–436. [Google Scholar] [CrossRef]
- Sanches, R.F.E.; Centeno, D.C.; Braga, M.R.; Silva, E.A. Impact of high atmospheric CO2 concentrations on the seasonality of water-related processes, gas exchange, and carbohydrate metabolism in coffee trees under field conditions. Clim. Change 2020, 162, 1231–1248. [Google Scholar] [CrossRef]
- Catarino, I.C.A.; Monteiro, G.B.; Ferreira, M.J.P.; Torres, L.M.B.; Domingues, D.S.; Centeno, D.C.; Lobo, A.K.M.; Silva, E.A. Elevated [CO2] mitigates drought effects and increases leaf 5-O-caffeoylquinic acid and caffeine concentrations during the early growth of Coffea arabica plants. Front. Sustain. Food Syst. 2021, 5, 676207. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and agronomic performance of the coffee crop in the context of climate change and global warming: A review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- Sanches, R.F.E.; Catarino, I.C.A.; Braga, M.R.; Silva, E.A. Influência da alta concentração atmosférica de CO2 (↑[CO2]atm) × disponibilidade hídrica nas relações hídricas, trocas gasosas e acúmulo de carboidratos em Coffea arabica L. Hoehnea 2017, 44, 635–643. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Campo, C.B.; Moscardi, F.; Correa-Ferreira, B.S.; Oliveira, L.J.; Sosa-Gomez, D.R.; Panizzi, A.R.; Corso, I.C.; Gazzoni, D.L.; Oliveira, E.B. Pragas da Soja No Brasil e Seu Manejo Integrado; Embrapa Soja: Londrina, Brazil, 2000. [Google Scholar]
- Tokeshi, H.; Rago, A.M. Doenças da cana-de-açúcar. In Manual de Fitopatologia: Doenças das Plantas Cultivadas; Kimati, H., Amorim, L., Rezende, J.A.M., Bergamin Filho, A., Camargo, L.E.A., Eds.; Ceres: São Paulo, Brazil, 2005; pp. 185–196. [Google Scholar]
- Gonçalves, M.C.; Pinto, L.; Creste, S.; Landell, M.G.A. Virus diseases of sugarcane. A constant challenge to sugarcane breeding in Brazil. Funct. Plant Sci. Biotechnol. 2012, 6, 108–116. [Google Scholar]
- Henning, A.A.; Almeida, A.M.R.; Godoy, C.V.; Seixas, C.D.S.; Yorinori, J.T.; Costamilan, L.M.; Ferreira, L.P.; Meyer, M.C.; Soares, R.M.; Dias, W.P. Manual de Identificação de Doenças da Soja; Embrapa Soja: Londrina, Brazil, 2014. [Google Scholar]
- Gaitán, A.L.; Cristancho, M.A.; Caicedo, B.L.C.; Rivillas, C.A.; Gómez, G.C. Compendium of Coffee Diseases and Pests; American Phytopathological Society: St. Paul, MN, USA, 2016. [Google Scholar]
- Godoy, C.V.; Seixas, C.D.S.; Soares, R.M.; Marcelino-Guimarães, F.C.; Meyer, M.C.; Costamilan, L.M. Asian soybean rust in Brazil: Past, present, and future. Pesqui. Agropecu. Bras. 2016, 51, 407–421. [Google Scholar] [CrossRef]
- Hartman, G.L.; Rupe, J.C.; Sikora, E.J.; Domier, L.L.; Davis, J.A.; Steffey, K.L. Compendium of Soybean Diseases and Pests; American Phytopathological Society: St. Paul, MN, USA, 2016. [Google Scholar]
- Mesquita, C.M.; Rezende, J.E.; Carvalho, J.C.; Fabri Junior, M.A.; Moraes, N.C.; Dias, P.T.; Carvalho, R.M.; Araújo, W.G. Manual do Café: Distúrbios Fisiológicos, Pragas e Doenças do Cafeeiro (Coffea arabica L.); EMATER: Belo Horizonte, Brazil, 2016. [Google Scholar]
- Zambolim, L. Current status and management of coffee leaf rust in Brazil. Trop. Plant Pathol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Marins, E.F.C.; Silva, M.J.S.; Silva, J.L.; Silva-Cabral, J.R.; Costa, J.F.O.; Feijó, F.M.; Assunção, I.P.; Lima, G.S.A. Colletotrichum species associated with sugarcane red rot in Brazil. Fungal Biol. 2022, 126, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Avelino, J.; Allinne, C.; Cerda, R.; Willocquet, L.; Savary, S. Multiple-disease system in coffee: From crop loss assessment to sustainable management. Annu. Rev. Phytopathol. 2018, 56, 611–635. [Google Scholar] [CrossRef]
- Chaloner, T.M.; Gurr, S.J.; Bebber, D.P. Plant pathogen infection risk tracks global crop yields under climate change. Nat. Clim. Change 2021, 11, 710–715. [Google Scholar] [CrossRef]
- Koutouleas, A.; Collinge, D.B.; Raebild, A. Alternative plant protection strategies for tomorrow’s coffee. Plant Pathol. 2022, 72, 409–429. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Mehdi, F.; Cao, Z.; Zhang, S.; Gan, Y.; Cai, W.; Peng, L.; Wu, Y.; Wang, W.; Yang, B. Factors affecting the production of sugarcane yield and sucrose accumulation: Suggested potential biological solutions. Front. Plant Sci. 2024, 15, 1374228. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Iglesius, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events—Implications for food production, plant diseases, and pests. Glob. Change Hum. Health 2001, 2, 90–104. [Google Scholar] [CrossRef]
- Reddy, P.P. Impact of climate change on insect pests, pathogens and nematodes. Pest Manag. Hortic. Ecosyst. 2013, 19, 225–233. [Google Scholar]
- Souza, W.R.; Oliveira, N.G.; Vinecky, F.; Ribeiro, A.P.; Basso, M.F.; Casari, R.A.D.C.N.; da Cunha, B.A.D.B.; Duarte, K.E.; Santiago, T.R.; Martins, P.K.; et al. Field evaluation of At DREB 2A CA overexpressing sugarcane for drought tolerance. J. Agron. Crop Sci. 2019, 205, 545–553. [Google Scholar] [CrossRef]
- Ortega, G.; Arias, P.A.; Villegas, J.C.; Marquet, P.A.; Nobre, P. Present-day and future climate over central and South America according to CMIP5/CMIP6 models. Int. J. Climatol. 2021, 41, 6713–6735. [Google Scholar] [CrossRef]
- Carvalho, D.; Rafael, S.; Monteiro, A.; Rodrigues, V.; Lopes, M.; Rocha, A. How well have CMIP3, CMIP5 and CMIP6 future climate projections portrayed the recently observed warming. Sci. Rep. 2022, 12, 11983. [Google Scholar] [CrossRef]
- Hamada, E.; Ghini, R.; Marengo, J.A.; Thomaz, M.C. Projeções de mudanças climáticas para o Brasil no final do século XXI. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 41–74. [Google Scholar]
- Hamada, E.; Gonçalves, R.R.V.; Marengo, J.A.; Ghini, R. Future climate scenarios for Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 2. [Google Scholar]
- IPCC. Summary for policymakers. In Special Report on Emissions Scenarios. A Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Nakicenovic, N., Swart, R., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 1–20. ISBN 0-521-80081-1. [Google Scholar]
- Ghini, R.; Hamada, E. Mudanças Climáticas: Impactos Sobre Doenças de Plantas No Brasil; Embrapa Informação Tecnológica: Brasília, Brazil, 2008. [Google Scholar]
- Silva, H.S.A.; Andrade, E.C. Impacto potencial das mudanças climáticas sobre as doenças da mandioca no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 263–272. [Google Scholar]
- Pozza, E.A.; Alves, M.C. Potential climate change impact on coffee fungal diseases. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 13. [Google Scholar]
- Pinto, N.F.J.A.; Oliveira, E.; Fernandes, F.T. Potential climate change impact on maize diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 9. [Google Scholar]
- Prabhu, A.S.; Silva, S.C.; Filippi, M.C. Potential climate change impact on rice diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 7. [Google Scholar]
- Oliveira, E.; Fernandes, F.T.; Rodrigues, J.A.S.; Tardin, F.D.; Landau, E.C. Impacto potencial de mudanças climáticas sobre as doenças do sorgo no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 319–330. [Google Scholar]
- Centurion, M.A.P.C.; Ghini, R. Potential climate change impact on soybean plant growth and diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 10. [Google Scholar]
- Sanguino, A. Potential climate change impact on sugarcane diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 12. [Google Scholar]
- Reis, E.M.; Casa, R.T.; Zoldan, S. Potential climate change impact on diseases of small grains in southern Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 8. [Google Scholar]
- Santos, A.F.; Auer, C.G.; Wrege, M.S.; Luz, E.D.M.N. Impacto potencial das mudanças climáticas sobre a gomose da acácia-negra no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 119–128. [Google Scholar]
- Furtado, E.L.; Santos, C.A.G.; Masson, M.V. Potential climate change impact on Eucalyptus rust in São Paulo state. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 16. [Google Scholar]
- Mafia, R.G.; Alfenas, A.C.; Loos, R.A. Impacto potencial das mudanças climáticas sobre doenças na eucaliptocultura no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 211–225. [Google Scholar]
- Auer, C.G.; Santos, A.F.; Wrege, M.S. Impacto potencial das mudanças climáticas sobre as doenças do pínus no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 305–317. [Google Scholar]
- Furtado, E.L. Potential climate change impact on rubber diseases in São Paulo state. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 15. [Google Scholar]
- Gasparotto, L.; Pereira, J.C.R. Potential climate change impact on banana diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 11. [Google Scholar]
- Cardoso, J.E.; Viana, F.M.P. Impacto potencial das mudanças climáticas sobre as doenças do cajueiro no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 161–176. [Google Scholar]
- Jesus Junior, W.C.; Morandi, M.A.B.; Christiano, R.S.C.; Yamamoto, P.T. Potential climate change impact on major citrus diseases in São Paulo state. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 14. [Google Scholar]
- Warwick, D.R.N.; Talamini, V.; Carvalho, R.R.C.; Silva, A.M.F. Impacto potencial das mudanças climáticas sobre as doenças do coqueiro no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 199–210. [Google Scholar]
- Garrido, L.R.; Angelotti, F. Impacto potencial das mudanças climáticas sobre as doenças da videira no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 331–356. [Google Scholar]
- Angelotti, F.; Magalhães, E.E. Impacto potencial das mudanças climáticas sobre as doenças da mangueira no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 273–284. [Google Scholar]
- Brunelli, K.R.; Kobori, R.F.; Gioria, R. Potential climate change impact on melon diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 6. [Google Scholar]
- Oliveira, A.A.R.; Santos Filho, H.P.; Andrade, E.C.; Meissner Filho, P.E. Impacto potencial das mudanças climáticas sobre as doenças do mamoeiro no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 249–262. [Google Scholar]
- Matos, A.P.; Junghans, D.T.; Andrade, E.C.; Meissner Filho, P.E. Impacto potencial das mudanças climáticas sobre as doenças do abacaxi no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 105–114. [Google Scholar]
- Garrido, L.R.; Mio, L.L.M.; Ueno, B.; Campos, A.D. Impacto das mudanças climáticas sobre as doenças de fruteiras de caroço no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 227–247. [Google Scholar]
- Morandi, M.A.B.; Costa, H. Impacto potencial das mudanças climáticas sobre as doenças do morangueiro no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 285–304. [Google Scholar]
- Brunelli, K.R.; Gioria, R.; Kobori, R. Impacto potencial das mudanças climáticas sobre as doenças das brássicas no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 145–160. [Google Scholar]
- Kobori, R.F.; Brunelli, K.R.; Gioria, R. Impacto potencial das mudanças climáticas sobre as doenças da alface no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 129–144. [Google Scholar]
- Gioria, R.; Della Vecchia, P.T.; Brunelli, K.R.; Kobori, R.F. Impacto potencial das mudanças climáticas sobre as doenças da cebola no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 177–198. [Google Scholar]
- Lopes, C.A.; Reis, A.; Shimoyama, N.Y. Potential climate change impact on potato diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 3. [Google Scholar]
- Kobori, R.F.; Gioria, R.; Brunelli, K.R. Potential climate change impact on pepper diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 5. [Google Scholar]
- Gioria, R.; Brunelli, K.R.; Kobori, R.F. Potential climate change impact on tomato diseases in Brazil. In Climate Change: Impacts on Plant Diseases in Brazil; Ghini, R., Hamada, E., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014; Chapter 4. [Google Scholar]
- Ghini, R.; Hamada, E.; Bettiol, W. Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas No Brasil; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011. [Google Scholar]
- Ghini, R.; Hamada, E. Climate Change: Impacts on Plant Diseases in Brazil; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2014. [Google Scholar]
- Jesus Junior, W.C.; Valadares Junior, R.; Cecilio, R.A.; Moraes, W.B.; Vale, F.X.R.; Alves, F.R.; Paul, P.A. Worldwide geographical distribution of black sigatoka for banana: Predictions based on climate change models. Sci. Agric. 2008, 65, 40–53. [Google Scholar] [CrossRef]
- Andrade, S.O.; Páez, G.T.; Feria, T.P.; Muñoz, J. Climate change and the risk of spread of the fungus from the high mortality of Theobroma cocoa in Latin America. Neotrop. Biodivers. 2017, 3, 30–40. [Google Scholar] [CrossRef]
- Moraes, W.B.; Jesus Junior, W.C.; Peixoto, L.A.; Moraes, W.B.; Furtado, E.L.; Silva, L.G.; Cecílio, R.A.; Alves, F.R. An analysis of the risk of Cocoa moniliasis occurrence in Brazil as the result of climate change. Summa Phytopathol. 2012, 38, 30–35. [Google Scholar] [CrossRef]
- Alfonsi, W.M.V.; Coltri, P.P.; Zullo Junior, J.; Patrício, F.R.A.; Gonçalves, R.R.V.; Shinji, K.; Alfonsi, E.L.; Koga-Vicente, A. Geographical distribution of the incubation period of coffee leaf rust in climate change scenarios. Pesqui. Agropecu. Bras. 2019, 54, 00273. [Google Scholar] [CrossRef]
- Ghini, R.; Hamada, E.; Pedro Junior, M.; Marengo, J.A.; Gonçalves, R.R.V. Risk analysis of climate change on coffee nematodes and leaf miner in Brazil. Pesqui. Agropecu. Bras. 2008, 43, 187–194. [Google Scholar] [CrossRef]
- Moraes, W.B.; Jesus Junior, W.C.; Peixoto, L.A.; Cecílio, R.A. Análise de risco do estabelecimento da mancha americana do cafeeiro no Brasil face às mudanças climáticas globais. Encicl. Biosf. 2011, 7, 1–15. [Google Scholar]
- Moraes, W.B.; Jesus Junior, W.C.; Peixoto, L.A.; Moraes, W.B.; Coser, S.M.; Cecílio, R.A. Impact of climate change on the phoma leaf spot of coffee in Brazil. Interciencia 2012, 37, 272–278. [Google Scholar]
- Macedo, R.; Sales, L.P.; Yoshida, F.; Silva-Abud, L.L.; Lobo Junior, M. Potential worldwide distribution of Fusarium dry root rot in common beans based on the optimal environment for disease occurrence. PLoS ONE 2017, 12, e0187770. [Google Scholar] [CrossRef]
- Moraes, W.B.; Jesus Junior, W.C.; Cecílio, R.A.; Mafia, R.G.; Moraes, W.B.; Cosmi, F.C.; Valadares Junior, R. Potential impact of the global climate changes on the spatial distribution of areas of risk for the occurrence of eucalyptus rust in Brazil. Summa Phytopathol. 2014, 40, 114–122. [Google Scholar] [CrossRef]
- Hamada, E.; Angelotti, F.; Garrido, L.R.; Ghini, R.; Carvalho, M.C.; Palladino, R.P. Efeito das mudanças climáticas sobre a favorabilidade às podridões da uva madura e cinzenta da videira no Nordeste brasileiro. Rev. Bras. Geogr. Física 2011, 6, 1213–1221. [Google Scholar]
- Angelotti, F.; Hamada, E.; Magalhães, E.E.; Ghini, R.; Garrido, L.R.; Pedro Junior, M.J. Climate change and the occurrence of downy mildew in Brazilian grapevines. Pesqui. Agropecuária Bras. 2017, 52, 424–432. [Google Scholar] [CrossRef]
- Hamada, E.; Angelotti, F.; Garrido, L.R.; Ghini, R. Cenários futuros de epidemia do oídio da videira com as mudanças climáticas para o Brasil. Rev. Bras. Geogr. Física 2015, 8, 454–470. [Google Scholar]
- Moraes, W.B.; Moraes, W.B.; Cosmi, F.C.; Jesus Junior, W.C.; Cecílio, R.A.; Valadares Junior, R.; Souza, A.F. Análise de risco do impacto das mudanças climáticas globais na distribuição espacial da pinta preta do mamoeiro no Brasil. Nucleus 2011, 8, 7. [Google Scholar] [CrossRef]
- Bisonard, E.M.; Hamada, E.; Angelotti, F.; Gonçalves, R.R.V.; Rago, A.M. Evolução da mancha preta do amendoim nas principais regiões produtoras da Argentina e do Brasil frente às mudanças no clima. Rev. Bras. Geogr. Física 2020, 13, 1778–1791. [Google Scholar] [CrossRef]
- Santos, M.S.; Ghini, R.; Fernandes, B.V.; Silva, C.A. Increased carbon dioxide concentration in the air reduces the severity of Ceratocystis wilt in Eucalyptus clonal plantlets. Australas. Plant Pathol. 2013, 42, 595–599. [Google Scholar] [CrossRef]
- Silva, C.E.O.; Ghini, R. Plant growth and leaf-spot severity on eucalypt at different CO2 concentrations in the air. Pesqui. Agropecu. Bras. 2014, 49, 232–235. [Google Scholar] [CrossRef]
- Araújo, A.L.S.; Angelotti, F.; Ribeiro Junior, P.M. Severity of melon powdery mildew as a function of increasing temperature and carbon dioxide concentration. Rev. Bras. Cienc. Agrar. 2019, 14, e6916. [Google Scholar] [CrossRef]
- Braga, M.R.; Aidar, M.P.M.; Marabesi, M.A.; Godoy, J.R.L. Effects of elevated CO2 on the phytoalexin production of two soybean cultivars differing in the resistance to stem canker disease. Environ. Exp. Bot. 2006, 58, 85–92. [Google Scholar] [CrossRef]
- Lessin, R.C.; Ghini, R. Efeito do aumento da concentração de CO2 atmosférico sobre o oídio e o crescimento de plantas de soja. Trop. Plant Pathol. 2009, 34, 385–392. [Google Scholar] [CrossRef]
- Gória, M.M.; Ghini, R.; Bettiol, W. Elevated atmospheric CO2 concentration increases rice blast severity. Trop. Plant Pathol. 2013, 38, 253–257. [Google Scholar] [CrossRef]
- Tozzi, F.R.O.; Ghini, R. Impacto do aumento da concentração atmosférica de dióxido de carbono sobre a ferrugem e o crescimento do cafeeiro. Pesqui. Agropecu. Bras. 2016, 51, 933–941. [Google Scholar] [CrossRef]
- Dorneles, K.R.; Martins, A.C.; Fernando, J.A.; Amarante, L.; Avila, L.A.; Deuner, S.; Dallagnol, L.J. Increased atmospheric CO2 concentration causes modification of physiological, biochemical and histological characteristics that affects rice-Bipolaris oryzae interaction. Eur. J. Plant Pathol. 2020, 157, 29–38. [Google Scholar] [CrossRef]
- Dorneles, K.R.; Refatti, J.P.; Pazdiora, P.C.; Avila, L.A.; Deuner, S.; Dallagnol, L.J. Biochemical defenses of rice against Bipolaris oryzae increase with high atmospheric concentration of CO2. Physiol. Mol. Plant Pathol. 2020, 110, 101484. [Google Scholar] [CrossRef]
- Cerri, C.E.P.; Sparovek, G.; Bernoux, M.; Easterling, W.E.; Melillo, J.M.; Cerri, C.C. Tropical agriculture and global warming: Impacts and mitigation options. Sci. Agric. 2007, 64, 83–99. [Google Scholar] [CrossRef]
- Gigot, C.; Hamerning, D.; Deytieux, V.; Diallo, I.; Deudon, O.; Gourdain, E. Developing a method to simulate and evaluate effects of adaptation strategies to climate change on wheat crop production: A challenging multi-criteria analysis. Eng. Proc. 2021, 9, 21. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Carvajal-Yepes, M.; Kumar, P.L.; Kawarazuka, N.; Liu, Y.; Mulema, A.A.; McCutcheon, S.; Ibabao, X. Sustainable management of transboundary pests requires holistic and inclusive solutions. Food Secur. 2022, 14, 1449–1457. [Google Scholar] [CrossRef]
- Bartoli, C.; Frachon, L.; Barret, M.; Rigal, M.; Huard-Chauveau, C.; Mayjonade, B.; Zanchetta, C.; Bouchez, O.; Roby, D.; Carrère, S.; et al. In situ relationships between microbiota and potential pathobiota in Arabidopsis thaliana. Multidiscipl. J. Microb. Ecol. 2018, 12, 2024–2038. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host–multi-pathogen warfare: Pathogen interactions in co-infected plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef]
- O’Hara, N.B.; Rest, J.S.; Franks, S.J. Increased susceptibility to fungal disease accompanies adaptation to drought in Brassica rapa. Evolution 2016, 70, 241–248. [Google Scholar] [CrossRef]
- Brunelli, K.R.; Gioria, R.; Kobori, R.F. Influência do aquecimento global na quebra de resistência genética a doenças em hortaliças. In Aquecimento Global e Problemas Fitossanitários; Bettiol, W., Hamada, E., Angelotti, F., Auad, A.M., Ghini, R., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2017; pp. 159–176. [Google Scholar]
- Catullo, R.A.; Ferrier, S.; Hoffmann, A.A. Extending spatial modelling of climate-change responses beyond the realized niche: Estimating, and accommodating, physiological limits and adaptive evolution. Glob. Ecol. Biogeogr. 2015, 24, 1192–1202. [Google Scholar] [CrossRef]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Barzman, M.; Booij, K.; Boonekamp, P.; Desneux, N.; Huber, L.; Kudsk, P.; Langrell, S.R.H.; Ratnadass, A.; Ricci, P.; et al. Robust cropping systems to tackle pests under climate change: A review. Agron. Sustain. Dev. 2015, 35, 443–459. [Google Scholar] [CrossRef]
- Gilardi, G.; Garibaldi, A.; Gullino, M.L. Emerging pathogens as a consequence of globalization and climate change: Leafy vegetables as a case study. Phytopathol. Mediterr. 2018, 57, 146–152. [Google Scholar]
- Cheatham, M.R.; Rouse, M.N.; Esker, P.D.; Ignacio, S.; Pradel, W.; Raymundo, R.; Sparks, A.H.; Forbes, G.A.; Gordon, T.R.; Garrett, K.A. Beyond yield: Plant disease in the context of ecosystem services. Phytopathology 2009, 99, 1228–1236. [Google Scholar] [CrossRef]
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.Z.; Borgemeister, C. Future risks of pest species under changing climatic conditions. PLoS ONE 2016, 11, e0153237. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Constable, F.; Finlay, K.J.; Harrington, R.; Luck, J.; Zalucki, M.P. Adapting to crop pest and pathogen risks under a changing climate. WIREs Clim. Change 2011, 2, 220–237. [Google Scholar] [CrossRef]
- Faraz, M.; Mereu, V.; Spano, D.; Trabuco, A.; Marras, S.; Chami, D. A systematic review of analytical and modelling assess climate change impacts and adaptation on coffee agrosystems. Sustainability 2023, 15, 14582. [Google Scholar] [CrossRef]
- Gomes, L.C.; Bianchi, F.J.J.A.; Cardoso, I.M.; Fernandes, R.B.A.; Fernandes Filho, E.I.; Schulte, R.P.O. Agroforestry systems can mitigate the impacts of climate change on coffee production: A spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 2020, 294, 106858. [Google Scholar] [CrossRef]
- Pfordt, A.; Ramos Romero, L.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear- and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- van Capelle, C.; Meyer-Wolfarth, F.; Meiners, T.; Schrader, S. Lumbricus terrestris regulating the ecosystem service/disservice balance in maize (Zea mays) cultivation. Plant Soil 2021, 462, 459–475. [Google Scholar] [CrossRef]
- Angelotti, F.; Morales, C.C.; Hamada, E.; Bisonard, E.M.; Gonçalves, R.R.V.; Rago, A.M. Climate risk scenarios of orange rust for the sugarcane-producing regions of Argentina and Brazil. Res. Soc. Dev. 2022, 11, e428111536648. [Google Scholar] [CrossRef]
- Gao, D.; Sun, Q.; Hu, B.; Zhang, S. A framework for agricultural pest and disease monitoring based on internet-of-things and unmanned aerial vehicles. Sensors 2020, 20, 1487. [Google Scholar] [CrossRef]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; Bellis, L.; Luvisi, A.; Maruccio, G. Advances in plant disease detection and monitoring: From traditional assays to in-field diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; White, J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, J.M.; Chikte, R.G.; Paknikar, K.M. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biot. 2020, 104, 1437–1461. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.; Gally, M.E.; Romero, A.M.; Poggio, S.L. Functional groups of plant pathogens in agroecosystems: A review. Eur. J. Plant Pathol. 2019, 153, 695–713. [Google Scholar] [CrossRef]
- Rangel, L.E.P. A política fitossanitária brasileira. In Defesa Vegetal: Fundamentos, Ferramentas, Políticas e Perspectivas; Sugayama, R.L., Silva, M.L., da Silva, S.X., de, B., Ribeiro, L.C., Rangel, L.E.P., Eds.; SBDA: Belo Horizonte, Brazil, 2015; Chapter 2. [Google Scholar]

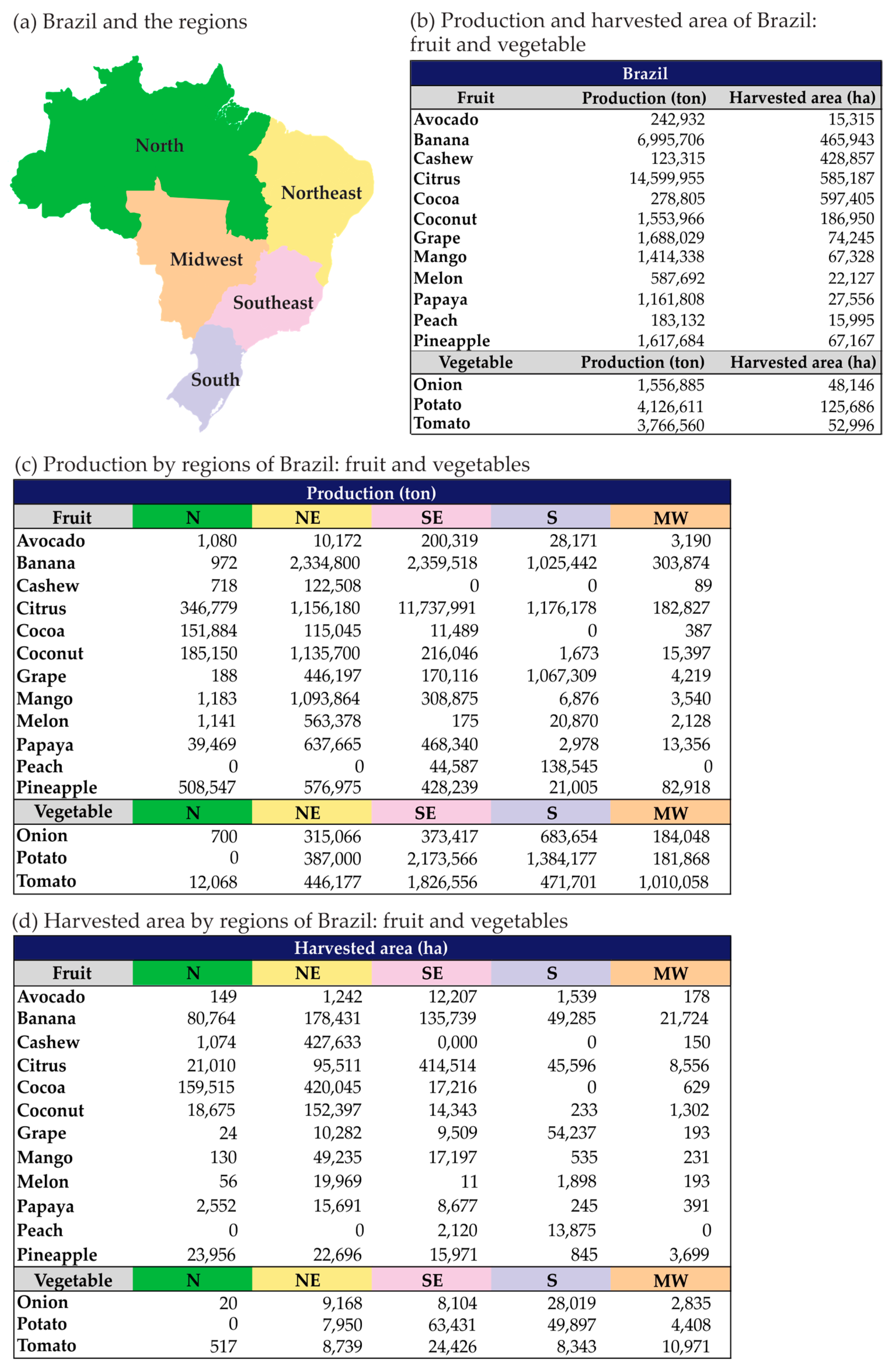
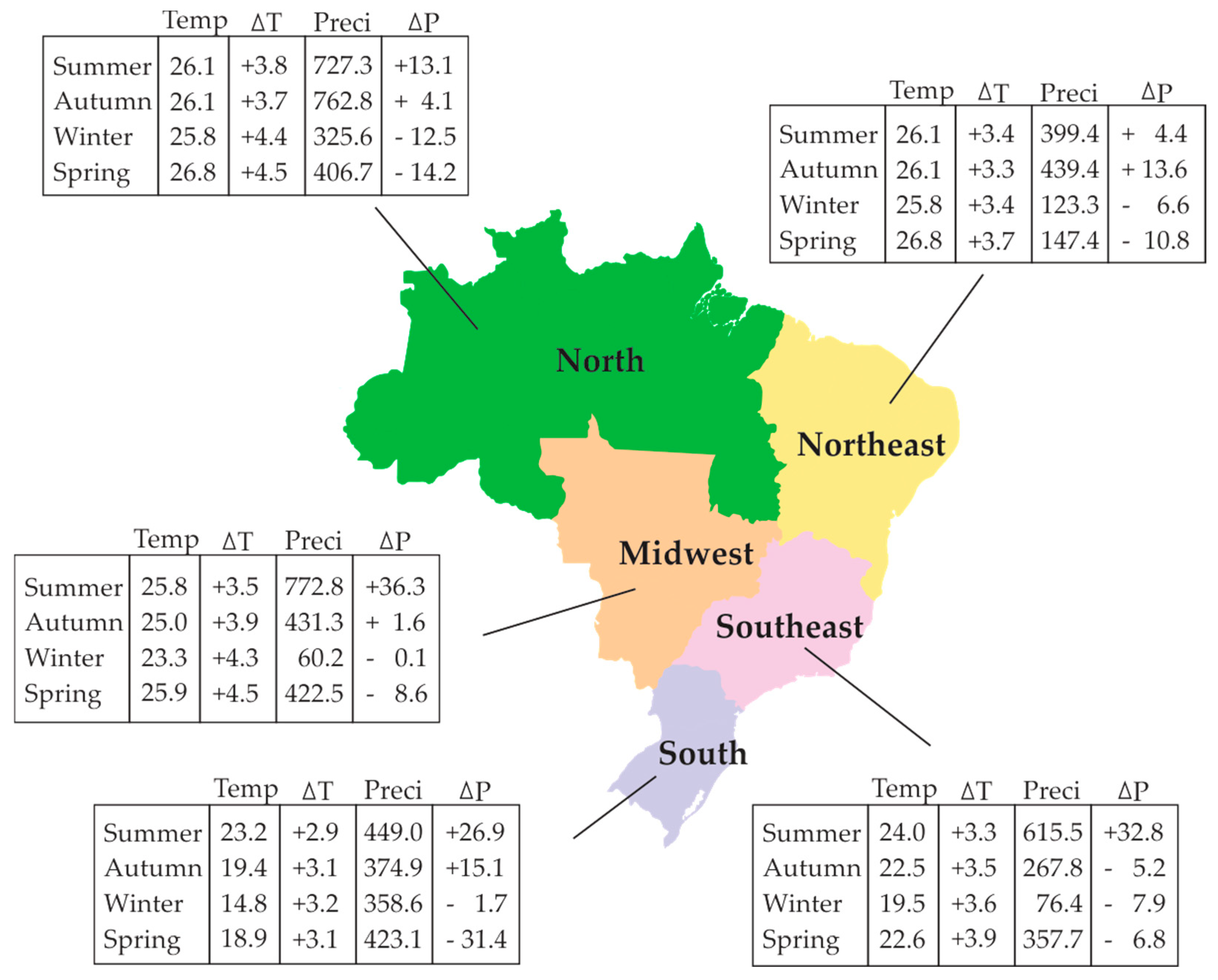


| Host—Reference | Pathogen (Disease) | Appropriate Environmental Conditions of Temperature, Relative Humidity (RH) and Precipitation for the Occurrence of Diseases | Effects of Climate Change on Future Importance of the Disease in Different Regions |
|---|---|---|---|
| Crops and plantation crops | |||
| Cassava—[179] | Cercosporidium henningsii and Cercospora vicosae (Brown leaf spot, Diffuse leaf spot) | Rainy season | Remain similar |
| Colletotrichum gloeosporiodes f. sp. manihotis (Anthracnose) | 18–28 °C and high RH | Reduce in North, Northeast and Midwest, and will be remain similar in South and Southeast regions | |
| Oidium manihotis (Powdery mildew) | 15–35 °C and RH between 85–95% | Increase in South | |
| Phaeoramularia manihotis (White leaf spot) | Mild weather | Remain similar | |
| Phytophthora drechsleri and Fusarium solani (Root rot) | Prolonged periods of rain and poorly drained soils | Reduction for Phytophthora, except in the Southern region. Remain similar importance for Fusarium | |
| Sphaceloma manihoticola (Superalongation) | 20–28 °C and high precipitation | Reduce | |
| Uromyces manihotis (Rust) | 18–23 °C and high RH | Reduce in North, Northeast, and Midwest | |
| Xanthomonas axonopodis pv. manihotis (Cassava bacterial blight) | 20–30 °C and RH > 90% | Increase in Midwest, South and Southeast | |
| Cassava Common Mosaic Virus (CsCMV) | Mild weather | Reduce | |
| Cassava Vein Mosaic Virus (CsVMV) | High temperature | Increase | |
| Coffee—[180] | Cercospora coffeicola (Brown eye spot) | 18–24 °C and precipitation greater than 3 mm/day | Reduce |
| Hemileia vastatrix (Coffee leaf rust) | 18–26 °C and precipitation greater than 3 mm/day | Increase | |
| Phoma spp. (Phoma leaf spot) | 16–20 °C and precipitation greater than 4 mm/day | Reduce | |
| Maize—[181] | Colletotrichum graminicola (Anthracnose) | Increase | |
| Fusarium graminearum (Red ear rot) | Increase | ||
| Peronosclerospora sorghi (Downy mildew), Puccinia sorghi (Common rust) Exserohilum turcicum (Northern corn leaf blight) | 15–23 °C and RH > 60% | Increase | |
| Puccinia polysora (Polysora rust), Physopella zeae (Tropical rust), Cercospora zeae-maydis (Cercospora leaf spot, leaf streak), Bipolaris maydis (Leaf blight, Southern maize leaf blight) | 24–32 °C and RH > 75% | Reduce | |
| Stenocarpella macrospora, Stenocarpella maydis (White ear rot), Fusarium verticillioides, Fusarium subglutinans (Pink ear rot), Pythium aphanidermatum (Stalk rot) | Remain similar | ||
| Ustilago maydis (Common smut), and Macrophomina phaseolina (Stalk rot) | 24–32 °C and water deficit | Increase | |
| Erwinia chrysanthemi (Soft rot), E. carotovora pv. zeae (Stalk rot) and Pseudomonas alboprecipitans (Bacterial leaf blight) | >32 °C and high humidity | Reduce | |
| Rice—[182] | Pyricularia grisea (P. oryzae) (Rice blast) | 20–30 °C | Reduce in Midwest of Brazil, and increase in Rio Grande do Sul |
| Monographella albescens (Syn. Metasphera albscens) (Leaf scald) | Wetting the leaves | Increase | |
| Bipolaris oryzae, Alternaria padwickii, P. grisea, Monographella albescens, Sarocladium oryzae, Phoma sorghina, Drechslera, Curvularia, Nigrospora, Fusarium, Coniothyrium, Epicoccum, Pithomyces, Chetomium, Pseudomonas, Erwinia (Sheath blight, grain blight) | High temperatures, high RH and low soil fertility | Increase | |
| Rhizoctonia solani (Sheath blight) | 28–32 °C and UR ± 95% | Increase | |
| Sorghum—[183] | Claviceps africana (Ergot) | 20–25 °C and UR > 80% | Reduce |
| Colletotrichum sublineolum (Anthracnose) | 22–30 °C and high RH | Increase | |
| Exserohilum turcicum (Northern leaf blight) | 18–27 °C and wetting of the leaves | Increase | |
| Fusarium moniliforme (Fusarium head blight, root and stalk rot) | 25–35 °C and high soil moisture | ||
| Gloeocercospora sorghi (Zonate leaf spot) | 28–30 °C and high RH | ||
| M. phaseolina (Charcoal rot) | 35–37 °C and low soil moisture | ||
| P. sorghi (Downy mildew) | 21–23 °C and wetting of the leaves | Reduce | |
| Puccinia purpurea (Rust) | 26–29 °C | Increase | |
| Ramulispora sorghi (Oval leaf spot) | 28 °C and high RH | ||
| Soybean—[184] | Phakopsora pachyrhizi (Asian soybean rust) | 20–25 °C and wetting of the leaves | Reduce |
| Sugarcane—[185] | Puccinia melanocephala (Sugarcane rust) | High RH | Tendency of small influence on the disease |
| Ustilago scitaminae (Smut) | |||
| Xanthomonas albilineans (Leaf scald) | |||
| Mycovellosiella koepkei (Yellow spot) | 28 °C and RH > 80% | The disease does not find favorable conditions | |
| Pothvirus—SCMV (Streak mosaic) | Above average rains | Reduce | |
| Winter cereals in southern Brazil—[186] | Bipolaris sorokiniana (Brown blotch or spot) | 20–25 °C and >18 h and wetting of the leaves | Increase |
| B. sorokiniana (Common root rot) | 20–25 °C and >18 h and wetting of the leaves | Remain similar | |
| Blumeria graminis (Powdery mildew) | 15–22 °C | Increase | |
| Drechslera tritici-repentis (Yellow spot) | 20 °C and > 24 h wetting of the leaves | Reduce | |
| Gaeumannomyces gramins var. tritici (Take-all) | 12–18 °C | Reduce | |
| Gibberella zeae (Fusarium head blight) | 25–30 °C and >48 h wetting of the leaves | Reduce | |
| Puccinia triticina (Leaf rust) | 15–20 °C and >10 h wetting of the leaves | Reduce | |
| Puccinia graminis (Stem rust) | 15–30 °C and >10 h wetting of the leaves | Reduce | |
| P. grisea (Blast) | 21–27 °C and 10–14 h wetting of the leaves | Increase | |
| Septoria tritici (Septoria tritici blotch) | 22–26 °C and 72–96 h wetting of the leaves | Reduce | |
| Septoria nodorum (Glume blotch) | 20–24 °C and 48–72 h wetting of the leaves | Reduce | |
| Forest | |||
| Black wattle—[187] | Phytophthora nicotianae (Gummosis) | 24–28 °C | Increase |
| Eucaliptus in São Paulo state—[188] | Puccinia psidii (Rust) | Mild temperatures, high RH and long leaf wetness | Reduce |
| Eucalyptus—[189] | Botrytis cinerea (Gray mold) | 20–24 °C and high RH | Remain similar |
| Ceratocystis fimbriata (Ceratocystis wilt) | 18–28 °C and high RH | Increase | |
| Chrysoporthe cubensis (Canker) | ≥23 °C and precipitation ≥ 1200 mm/year | Increase | |
| Coniothyrium eucalypti (Coniothyrium canker) | Hydric stress | Remain similar | |
| Cylindrocladium spp. (Leaf spot, blight) | High temperature and RH. Wetting of the leaves | Increase | |
| Erythricium salmonicolor (Pink disease) | Precipitation ≥ 1200 mm/year | Remain similar | |
| Hypoxylon spp. (Black stromata) | 30 °C and high RH | Increase | |
| Oidium eucalypti (Powdery mildew) | 20–25 °C and high RH | Remain similar | |
| P. psidii (Rust) | 18–25 °C and wetting of the leaves | Remain similar | |
| Quambalaria eucalypti (Leaf and shoot blight) | 27 °C and high RH | Increase | |
| Ralstonia solanacearum (Bacterial wilt) | 28–30 °C and high RH | Increase | |
| R. solani | Increase | ||
| Teratosphaeria nubilosa (Mycosphaerella leaf) | Remain similar | ||
| X. axonopodis (Bacterial leaf blight) | 26–30 °C and wetting of the leaves | Increase | |
| Pine—[190] | Cylindrocladium pteridis (Pine needle blight) | 30–33 °C and high precipitation | Increase |
| Sphaeropsis sapinea (Sphaeropsis blight, Tip blight) | 24–26 °C and high RH | Increase | |
| Rubber tree in São Paulo state—[191] | Ceratocystis frimbriata (Moldy rot) | Low temperature e and high RH | Reduce |
| Colletotrichum gloeosporioides (Panel anthracnose) | Low temperature | Reduce | |
| C. gloeosporioides (Anthracnose) | 21 °C and RH > 90% | Reduce | |
| F. moniliforme (Bark dryness) | Increase | ||
| Hevea pauciflora (Pink disease) | Increase | ||
| Lasiodiplodia theobromae (Stem diseases) | Increase | ||
| Microcyclus ulei (Southern American leaf blight) | Prolonged wetness, RH > 95% for 10 h | Reduce | |
| Oidium heveae (Powdery mildew) | Increase | ||
| Phytophthora citrophthora (Patch canker) | Mild temperature and high RH | Reduce | |
| Fruits | |||
| Banana—[192] | F. oxysporum f. sp. cubense (Panama disease, Fusarium wilt) | Increase | |
| Mycosphaerella fijiensis (Black sigatoka) | 25–28 °C and high RH | Increase in South and Vale do Ribeira Valley in São Paulo state, and reduce in Amazon state | |
| R. solanacearum race 2 (Moko) | Reduce | ||
| Cashew—[193] | C. gloeosporioides (Anthracnose) | Rain and high RH | Increase |
| Lasiodiplodia theobromae (Gummosis) | Hydric stress | ||
| Oidium anacardii (Powdery mildew) | 26–28 °C | ||
| Pilgeriella anacardii (Black mould) | Rain | ||
| Xanthomonas campestris pv. mangifereaeindicae (Bacterial leaf, Fruit spot) | |||
| Citrus in São Paulo state—[194] | Colletotrichum acutatum (Citrus postbloom frui drop disease) | 23–27 °C and leaf wetness between 10 to 12 h | Remain similar |
| Guignardia citricarpa (Phyllosticta citricarpa (Citrus black spot) | 21–32 °C and leaf wetness between 24 to 48 h | Increase | |
| Candidatus Liberibacter spp. (Huanglongbing = Greening) | High temperatures favor the Diaphorina citri vector | In the North and Northwest regions, the tendency is to remain similar its importance. In the central and southern regions, the tendency is for an increase in importance | |
| Xanthomonas axonopodis pv. citri (Citrus canker) | 30–35 °C and wetting of the leaves for 24 h | Increase | |
| Xylella fastidiosa (Citrus variegated chlorosis) | High temperatures and water deficit. | Increase | |
| Coconut—[195] | Bipolaris incurvata (Leaf spot, Bipolaris leaf blight) | 18–27 °C and high RH | Reduce |
| Botryosphaeria cocogena (Leaf blight) | Rain between 25–80 mm | ||
| Camarotella torrendiella and Camarotella acrocomiae (Tar spot, black leaf spot) | High RH | ||
| Phytophthora spp. (Bud rot, nutfall) | 25–28 °C and high RH in poorly drained soils | ||
| Thielaviopsis (Ceratocystis) paradoxa (Stem bleeding disease) | Increase | ||
| Grape—[196] | Elsinoe ampelina (Anthracnose) | 24–26 °C and RH > 90% | Remain similar |
| Phakopsora euvitis (Rust) | 16–30 °C and wetting of the leaves | ||
| Plasmopara viticola (Downy mildew) | 20–25 °C, high RH and wetting of the leaves | ||
| Phomopsis viticola (Leaf spot, Phomopsis cane) | 23–25 °C and wetting of the leaves | ||
| Pseudocercospora vitis (Leaf blight) | High temperature and RH | ||
| Uncinula necator (Powdery mildew) | 25 °C and RH between 40-60% | Remain similar, with an increasing trend in some regions | |
| Mango—[197] | Ceratocystis fimbriata (Mango wilt) | High temperature and rainy periods | Remain similar |
| C. gloeosporioides (Anhtracnose) | >25 °C, RH > 95% and wetting of the leaves | Increase | |
| Elsinoe (Sphaceloma) mangiferae (Mango scab) | High RH | Remain similar | |
| Fusarium spp. (Mango malformation) | Rain | ||
| L. theobromae (Stem end rot, Die back, Gummosis) | 27–32 °C and RH > 80% | Increase | |
| Oidium mangiferae (Erysiphe polygoni) (Powdery mildew) | 20–25 °C and RH between 20–65% | Increase in São Paulo, Minas Gerais, Espírito Santo and Bahia states | |
| X. campestris pv. mangiferaindica (Bacterial black spot) | High temperature and rainy periods | Remain similar | |
| Melon—[198] | C. gloeosporioides (Anthracnose) | 21–27 °C and high RH | Increase |
| Corynespora cassiicola (Corynespora leaf disease) | 25–35 °C and high RH | Increase | |
| Didymella bryoniae (Gummy stem blight) | 22–32 °C and high RH | Increase | |
| Monosporascus cannobalus and M. phaseolina (root rot, vine decline, sudden wilt, sudden death, melon collpase) | 30–35 °C, low soil moisture and and alkaline pH | Disease has assumed significant importance at the moment. Tendency of indefinite importance. | |
| Phodosphaera xanthii, Golovinomyces cichoracearum (Powdery mildew) | 10–32 °C and high RH | Remain similar | |
| Pseudoperonospora cubensis (Downy mildew) | 5–30 °C and water film by > 6 h | Increase | |
| Acidovorax avenae subsp. citrulli (Bacterial fruit blotch) | ±26 °C and high RH | Increase | |
| Papaya—[199] | Asperisporium caricae (Black spot) | 23–27 °C | Increase in Espírito Santo state, and will reduce in other regions |
| C. gloeosporioides (Anthracnose, charcoal spot) | 21–27 °C, RH > 97 and wetting of the leaves | Increase | |
| Corynespora cassiicola (Corynespora target spot) | High RH and temperature | ||
| L. theobromae (Stem end rot) | Remain similar | ||
| Oidium caricae, Ovulariopsis papayae (Powdery mildew) | 15–20 °C and RH between 60–70% | Reduce | |
| Phytophthora palmivora and Phytophthora parasitica (Papaya fruit rot) | 25 °C and high soil moisture | Increase in irrigate crops | |
| Phoma caricae papayae (Leaf spot) | Rainy days | Reduce | |
| Pythium, R. solani, Fusarium sp. and Phytophthora sp. (Damping-off) | High temperature and RH | ||
| Papaya lethal yellowing virus (PLYV) | Remain similar | ||
| Pineapple—[200] | Fusarium subglutinans f. sp. ananas (Gommusis) | 15–22 °C and high precipitation | Increase |
| P. nicotianae var. parasitica (Heart rot) | 25–36 °C and high precipitation | ||
| P. cinnamomi (Root rot) | 19–25 °C | Reduce | |
| Stone fruit—[201] | Armillaria mellea (Armillaria root rot) | Increase | |
| Botryosphaeria dothidea (Gommusis) | |||
| Cladosporium carpophilum (Scab) | 25–30 °C and high RH | Increase in South, and remain similar in Southeast region | |
| Glomerella cingulata (Anthracnose) | 25–30 °C and high RH | Increase in South, and remain similar in Southeast region | |
| Monilinia fructicola (Brown rot) | 25 °C and high RH | Increase | |
| Phomopsis amygdali (Twig canker) | 27–29 °C | ||
| Phytophthora spp. (Crown rot) | 30–32 °C | ||
| Rhyzopus stolonifer (Rhyzopuys rot) | 15–23 °C and high RH | ||
| Taphrina deformans (Peach leaf curl) | 18–20 °C and RH > 95% | ||
| Tranzschelia discolor (Rust) | 18–26 °C | ||
| Wilsonomyces carpophylus (Shot hole) | 15–20 °C | ||
| Xanthomonas arboricola pv. pruni (Bacterial spot | 30 °C and wetting of the leaves | ||
| Xyllela fastidiosa (Phony peach disease) | 20–25 °C | ||
| Strawberry—[202] | B. cinerea (Gray mold) | 20 °C, high RH and wetting of the leaves | Remain similar |
| Colletotrichum acutatum (Anthracsone fruit rot) | 18–23 °C | Reduce | |
| Colletotrichum fragariae (Anthracnose) | High temperature and RH | Increase during rainfall | |
| Mycosphaerella fragariae, Diplocarpon earlianum, Dendrophoma obscurans, Pestalotiopsis longisetula (Leaf spot) | 25–30 °C and high RH | Increase | |
| Phytophthora cactorum, S. sclerotiorum and R. solani (Root rot, fruit rot) | 15–22 °C, high RH and rain | Reduce | |
| Podosphaera aphanis (Sphaerotheca macularis) (Powdery mildew) | 15–30 °C | Increase | |
| R. solani, Fusarium, Pythium ultimum, Phytophthora (Root rot) | 25–27 °C and high soil moisture | Increase in soils with excessive moisture | |
| Verticillium dahliae (Verticillium wilt) | 20–25 °C and hydric stress | Increase | |
| Xanthomonas fragariae (Bacterial angular leaf spot) | 18–22 °C and high RH | ||
| Redness | Factors that cause plant stress | ||
| Vegetables | |||
| Brassicas—[203] | Alternaria brassicae and Alternaria brassicicola (Alternaria leaf spot) | 20–28 °C and high RH | Remain similar |
| Peronospora parasitica (Downy mildew) | 14–18 °C and high RH | Reduce | |
| Plasmodiophora brassicae (Clubroot) | 20–25 °C and high soil moisture | Increase | |
| Pseudomonas syringae pv. maculicola (Bacterial leaf spot) | 22–25 °C and high RH | Remain similar in South and Southeast, and will reduce in other regions | |
| R. solani (Wirestem) | 25–30 °C and high soil moisture | Increase | |
| Sclerotinia sclerotiorum (White mould) | 15–20 °C and high RH | Reduce | |
| Sclerotium rolfsii (Stem rot) | 22–30 °C | Increase | |
| Pectobacterium carotovorum subsp. carotovorum (Soft rot) | High soil moisture and high temperature | Increase | |
| X. campestris pv. campestres (Black rot) | 28–30 °C and high RH | Remain similar of favorability but with an upward trend | |
| Lettuce—[204] | Pythium spp. (Damping-off) | 20–30 °C and high RH | Increase in hydroponic systems |
| Bremia lactucae (Downy mildew) | 18–20 °C, high RH and wetting of the leaves | Increase in Rio Grande do Sul and Santa Catarina states during the winter, and will reduce with increase in temperature | |
| Cercospora longissima (Cercospora leaf spot) | 20–30 °C, high RH and wetting of the leaves | Increase | |
| Erysiphe cichoracearum (Powdery mildew) | 22–30 °C | Increase | |
| F. oxysporum f. sp. lactucae (Fusarium wilt) | >27 °C | Increase between October and May | |
| R. solani (Damping-off) | 25–30 °C and high RH | Increase between December and May | |
| S. sclerotiorum, S. minor (Leaf drop) | 15–21 °C and wetting of the leaves > 12h | Reduce | |
| S. rolfsii (Southern blight) | 25–35 °C and high RH | Increase between December and May | |
| Septoria lactucae (Septoria leaf spot) | 10–25 °C | Remain similar of the current winter scenario for Rio Grande do Sul, Santa Catarina, Paraná, Rio de Janeiro, and Minas Gerais states with the use of irrigation. Reduction for other periods and regions. | |
| Thielaviopsis basicola (Black root rot) | 23–30 °C | Increase | |
| P. carotovorum (Bacterial soft rot) | 25–30 °C and high RH | Increase between October and March | |
| Pseudomonas cichorii, X. axonopodis pv. vitians (Bacterial leaf spot) | 18–25 °C, high RH and wetting of the leaves | Reduce | |
| Onion—[205] | Alternaria porri (Purple blotch) | 21–30 °C and wetting of the leaves | Increase |
| Botrytis squamosa (Botrytis leaf blight) | 12–16 °C and high RH | Reduce | |
| Colletotrichum circinans (Anthracnose) | 26 °C | Increase in times with high temperatures | |
| C. gloeosporioides f. sp. cepae (Mal-de-sete-voltas) | 23–30 °C and high RH | Increase | |
| Fusarium oxysporum f. sp. cepae (Fusarium basal plate rot) | 20–30 °C and high soil moisture | Increase in times with high rainfall | |
| Peronospora destructor (Downy mildew) | 12 °C and RH > 80% | Reduce | |
| Pyrenochaeta terrestres (Pink root) | 24–28 °C and high soil moisture | Increase during rainfall | |
| P. nicotinae (Phytophthora neck) | High soil moisture and > 25 °C | Increase | |
| Sclerotium cepivorum (White rot) | Soil temperature between 10–20 °C | Reduce | |
| Burkholderia cepacia (Sour skin) | 30–35 °C and high RH | Increase | |
| P. carotovorum subsp. carotovorum (Soft rot) | 20–30 °C and high RH | Increase | |
| Potato—[206] | Alternaria solani (Early blight) | 20–24 °C | Remain similar, with a tendency to increase. |
| Helminthosporium solani (Silver scab) | High soil moisture (>90%) | Increase | |
| Phytophthora infestans (Late blight) | Zoospore production: 8–18 °C; sporangia germination: 18–25 °C. High humidity | Remain similar, with a tendency to reduce | |
| R. solani (Rhizoctonia) | <20 °C | Remain similar, with a tendency to reduce | |
| Spongospora subterrânea (powdery scab) | Soil temperature between 11–18 °C, with high humidity | Reduce | |
| S. sclerotiorum (White mold) | 15–21 °C and high humidity | Reduce | |
| S. rolfsii (Crown rot, Southern blight) | 28–30 °C and high soil moisture | Increase | |
| Pectobacterium (Erwinia) (Cinnamon black, and soft rot) | >30 °C | Increase | |
| R. solanacearum (Bacterial wilt) | Around 30 °C and high soil moisture | Increase | |
| Streptomyces (Commom scab) | 25–30 °C and low soil moisture | Remain similar, with a tendency to reduce | |
| Meloidogyne incognita (root knot nematode) | 25–32 °C | Remain similar, with a tendency to increase. | |
| Pepper—[207] | B. cinerea (Gray mold) | 18–23 °C and RH between 90%–95% | Reduce |
| Cercospora capsici and Stemphylium solani (Leaf spot) | 23–27 °C and RH > 90% | Remain similar, with a tendency to reduce | |
| Colletotrichum (Anthracnose) | 20–30 °C and high RH | Remain similar, with a tendency to reduce | |
| Oidiopsis taurica (Powdery mildew) | 10–35 °C and RH between 85%–95% | Remain similar, with a tendency to increase | |
| Phytophthora capsici (Phytophthora blight) | 22–28 °C and high RH | Remain similar, with a tendency to reduce | |
| S. sclerotiorum (White mold) | 16–22 °C and high RH | Reduce | |
| S. rolfsii (Southern blight) | 25–30 °C and high RH | Remain similar, with a tendency to increase | |
| C. michiganesis subsp. michiganensis (Bacterial canker) | 24–28 °C and high RH | Reduce | |
| Erwinia carotovora subsp. carotovora (Soft rot) | 28–30 °C and high RH | Increase | |
| R. solanacearum (Bacterial wilt) | 30–35 °C and high soil moisture | Increase | |
| X. campestris pv. vesicatoria (Bacterial spot) | 22–28 °C and RH between 95%–100% | Remain similar, with a tendency to increase | |
| Tomato mosaic virus (ToMV), Tobacco mosaic virus (TMV), Pepper mild mottle virus (PMMoV) (Mosaics—viruses transmitted mechanically) | Remain similar | ||
| Tomato—[208] | A. solani (Early blight) | 25–32 °C and free water on the surface of the leaves | Remain similar, with a tendency to increase |
| B. cinerea (Gray mold) | 18–23 °C, RH > 90% | Reduce | |
| F. oxysporum f. sp. lycopersici (Fusarium wilt) | 21–33 °C | Increase | |
| Leveilula taurica (Powdery mildew) | High temperature and low RH | Increase | |
| P. infestans (Late blight) | 12–18 °C and rain > 24 h | Reduce | |
| Septoria lycopersici (Septoria leaf spot) | 20–25 °C, mild temperatures and abundant rainfall | Remain similar, with a tendency to reduce | |
| Stemphylium solani (Gray leaf spot) | 25–28 °C and UR > 80% | Remain similar, with a tendency to increase | |
| S. sclerotiorum (White mold) | 15–21 °C and high humidity | Reduce | |
| S. rolfsii (Southern blight) | 25–35 °C and high humidity | Increase | |
| Verticillium albo-atrum, V. dahliae (Verticillium wilt) | 22–25 °C | Reduce | |
| Clavibacter michiganensis subsp. michiganensis (Bacterial canker) | 18–25 °C and high RH | Reduce | |
| Erwinia spp. (Soft rot) | 25–30 °C and RH around 100% | Reduce | |
| Pseudomonas corrugata (Pith necrosis) | Mild night temperatures and high RH | Reduce | |
| P. syringae pv. tomato (Bacterial speck) | 18–25 °C and high RH | Reduce | |
| R. solanacearum (Bacterial wilt) | 24–35 °C and high soil moisture | Increase | |
| Xanthomonas spp. (Bacterial spot) | 24–30 °C and high RH | Increase | |
| Tomato mosaic virus | Remain similar | ||
| Host | Pathogen (Disease) | Effects of Climate Change on Future | References |
|---|---|---|---|
| Banana | Mycosphaerella fijiensis (black Sigatoka) | There will be a reduction in the favorable area | [120,211] |
| Cacao | Moniliophthora roreri (frosty pod rot of cocoa) | Favorability will be increased | [212] |
| M. roreri (Moniliasis) | The potential risk will be reduced | [213] | |
| Coffee | Hemileia vastatrix (coffee leaf rust) | The severity will increase with the reduction in the incubation period in the states of Minas Gerais and São Paulo | [214] |
| H. vastatrix (coffee leaf rust) | The incubation period will be reduced | [121] | |
| Meloidogyne incognita (root disease) | The infestation of the nematode will be increased | [215] | |
| Mycena citricolor (American leaf spot) | There will be a reduction in favorability for the disease in future decades, except in southern Brazil during May and July | [216] | |
| Phoma sp. (Phoma leaf spot) | There will be a reduction in some areas, but there will still be potential risk in the Southern region | [217] | |
| Common beans | Fusarium solani species complex (root rot) | Strong convergence on the environmental requirements of both the host and the disease development. Climate change will probably move the disease toward cooler regions | [218] |
| Eucalyptus | Puccinia psidii (rust) | There will be a reduction in the favorable area | [219] |
| Grape | Glomerella cingulata (ripe rot) and Botrytis cinerea (gray mold) | There will be a reduction in the favorable area in Brazilian Northeast | [220] |
| Plasmopara viticola (downy mildew) | Favorability will be increased in Rio Grande do Sul and Santa Catarina states. There will be a reduction in the favorability in São Francisco Valley. For Northern Paraná state and Eastern São Paulo state, the condition will be the same as the current ones | [221] | |
| Uncinula necator (powdery mildew) | There will be an increase in the favorable area | [222] | |
| Papaya | Asperisporium caricae (smallpox) | There will be a reduction in the favorable area | [223] |
| Peanut | Cercosporidium personatum (black spot) | There will be an increase in the favorable area | [224] |
| Host | Pathogen (Disease) | Effects of Increased CO2 | References |
|---|---|---|---|
| Coffee | Hemileia vastatrix (coffee leaf rust) | The severity was reduced | [231] |
| H. vastatrix (coffee leaf rust) Cercospora coffeicola (Cercospora leaf spot) | There was no significant effect of CO2 on diseases incidence | [76] | |
| Leucoptera coffeella (leaf miner) | The incidence of leaf minor was lower under elevated CO2 | [76] | |
| H. vastatrix (coffee leaf rust) | The incidence of coffee leaf was the same in elevated and ambient CO2 | [126] | |
| L. coffeella (leaf miner) | The incidence of leaf minor was lower under elevated CO2 | [126] | |
| Eucalyptus | Cylindrocladium candelabrum (leafspot) | The severity and incidence were reduced | [226] |
| Puccinia psidii (rust) | The severity was reduced, and growth plant was stimulated | [73] | |
| Ceratocystis fimbriata | The severity was reduced, and growth plant was stimulated | [225] | |
| Melon | Oidium sp. (powdery mildew) | The severity will be reduced, and the incubation period will be increased | [227] |
| Rice | Bipolaris oryzae (brown spot) | The severity was reduced | [232,233] |
| Magnaporthe oryzae (rice blast) | The disease was more severe | [230] | |
| Soybean | Microsphaera diffusa (powdery mildew) | The severity was increased | [229] |
| Phytophthora sojae (stem canker) | Plant defense responses was changed | [228] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelotti, F.; Hamada, E.; Bettiol, W. A Comprehensive Review of Climate Change and Plant Diseases in Brazil. Plants 2024, 13, 2447. https://doi.org/10.3390/plants13172447
Angelotti F, Hamada E, Bettiol W. A Comprehensive Review of Climate Change and Plant Diseases in Brazil. Plants. 2024; 13(17):2447. https://doi.org/10.3390/plants13172447
Chicago/Turabian StyleAngelotti, Francislene, Emília Hamada, and Wagner Bettiol. 2024. "A Comprehensive Review of Climate Change and Plant Diseases in Brazil" Plants 13, no. 17: 2447. https://doi.org/10.3390/plants13172447







