Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum
Abstract
1. Introduction
2. Results
2.1. Visual Screening According to Aerial Symptoms
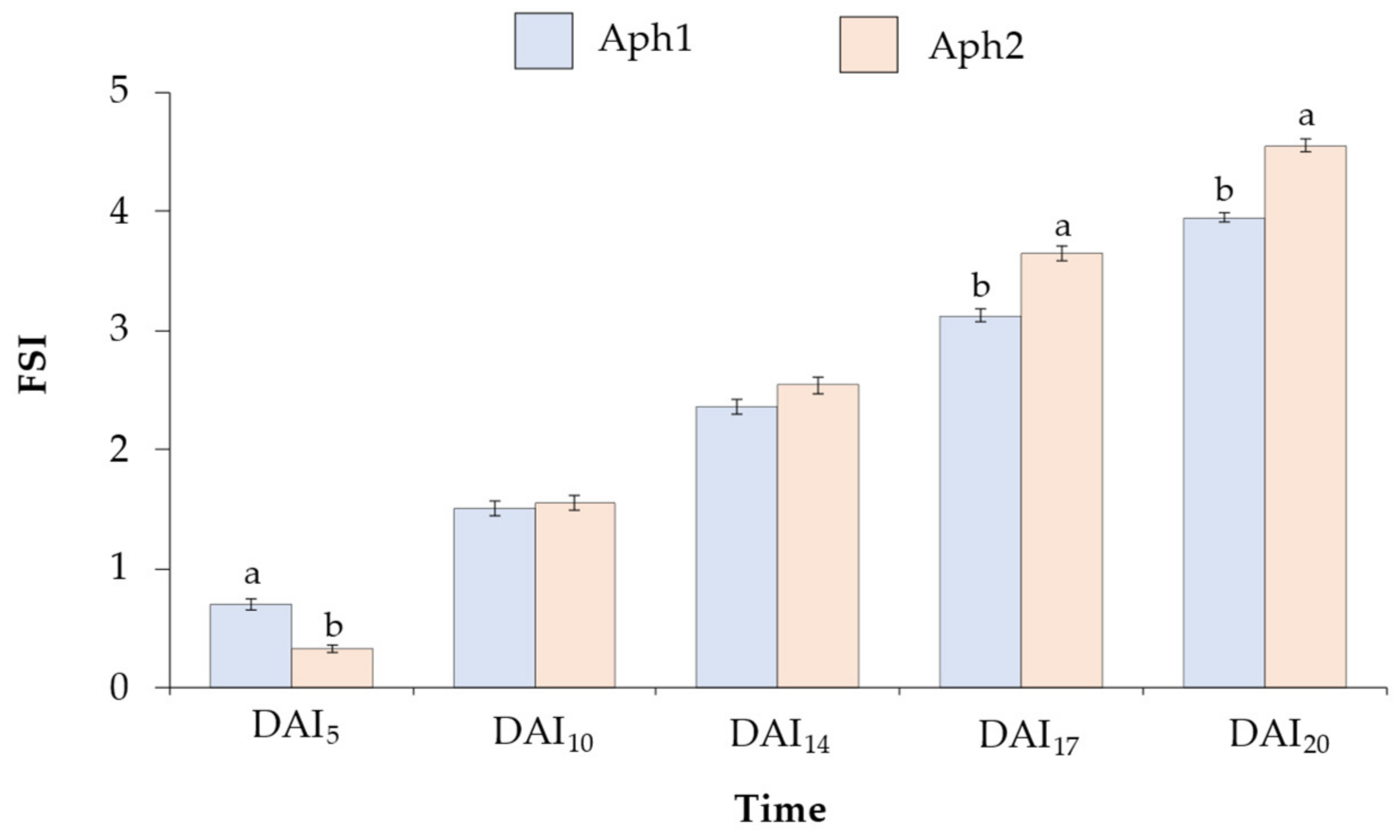
2.2. Root and Foliar Symptoms of Selected Accessions
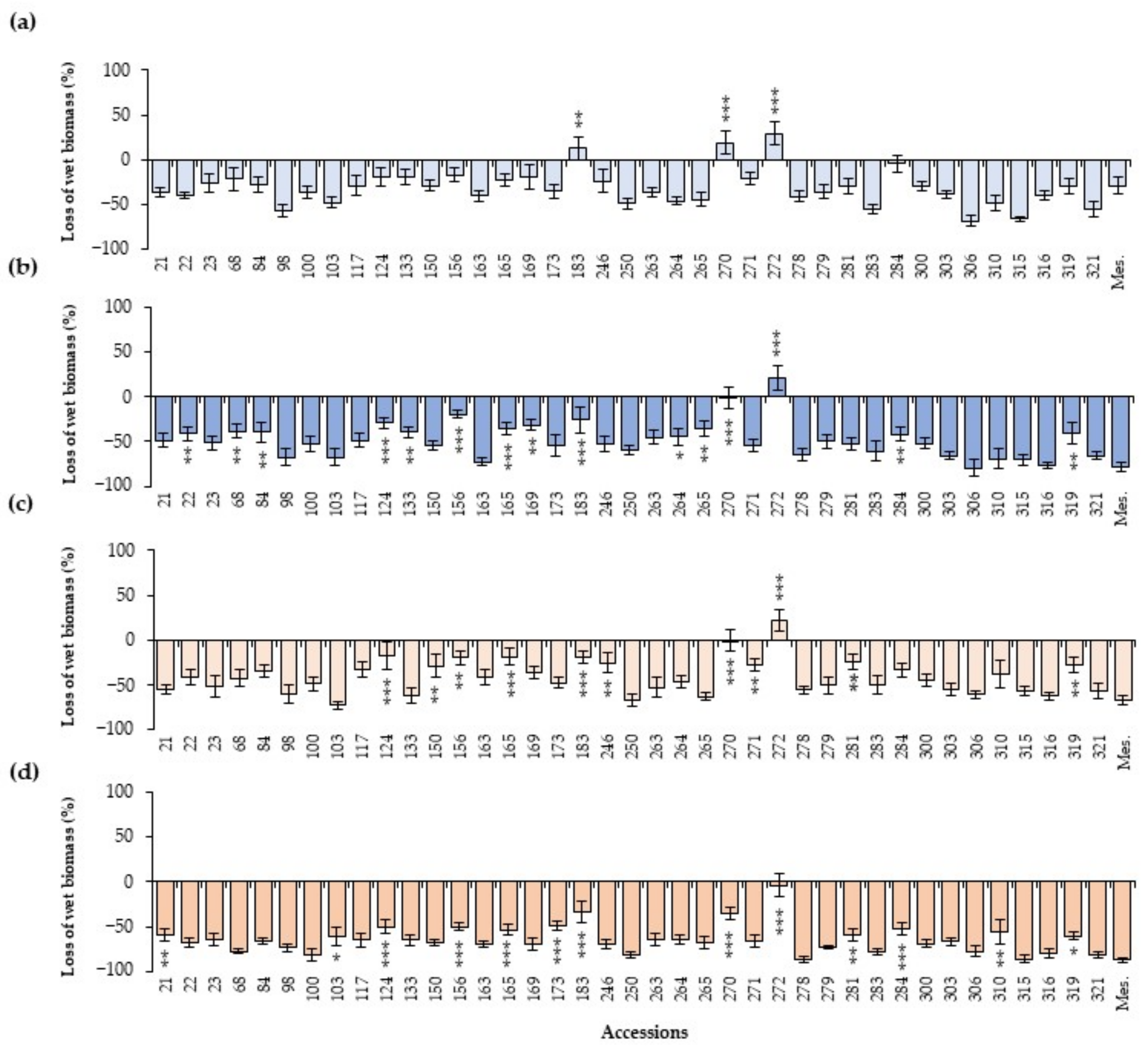
2.3. Effect of the Inoculation in Wet Biomass
3. Discussion
4. Materials and Methods
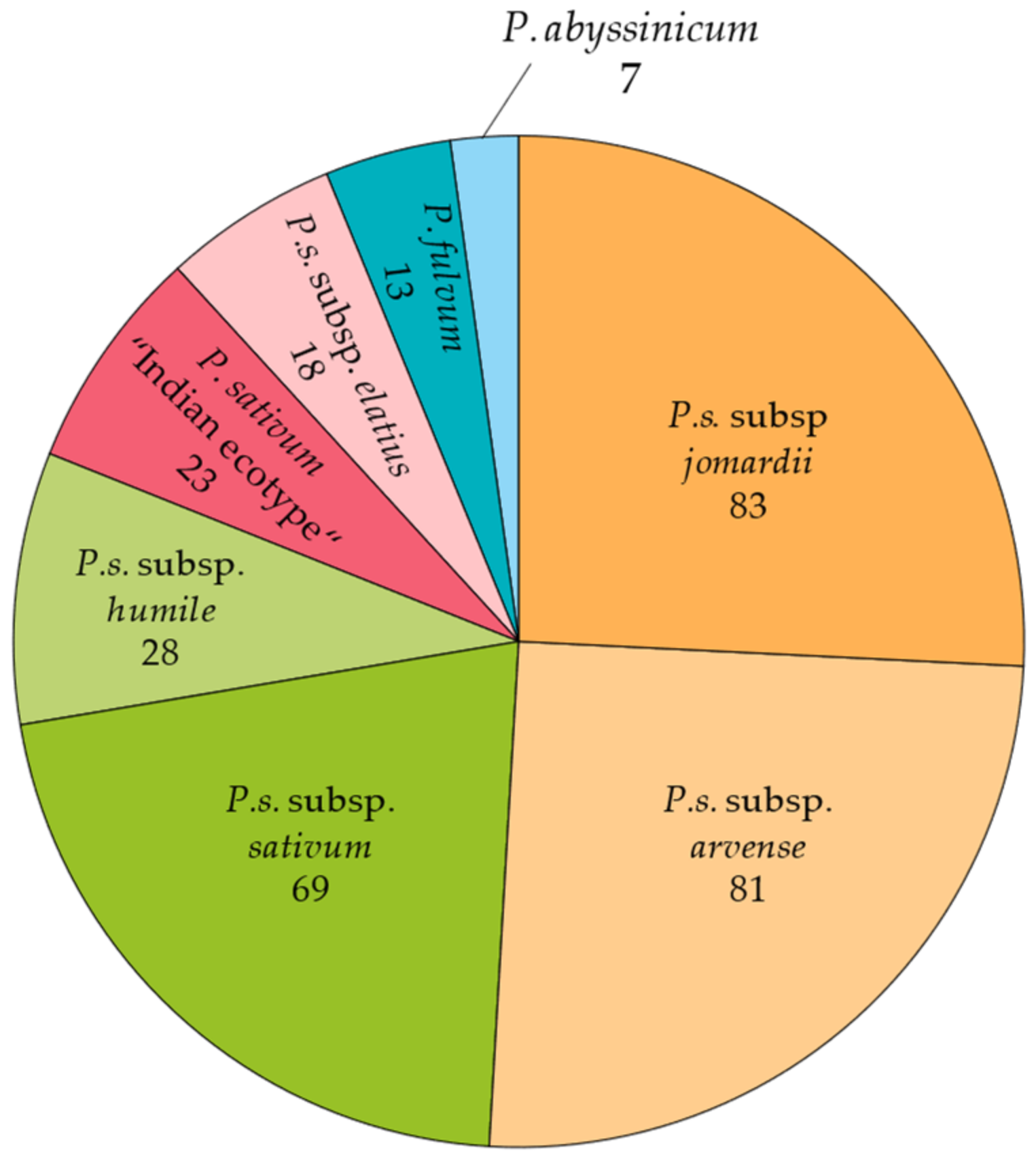
4.1. Plant Material
4.2. Aphanomyces euteiches Growth and Inoculation
4.3. Evaluation of Symptoms and Selection of Resistant Accessions
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iannetta, P.P.M.; Hawes, C.; Begg, G.S.; Maaß, H.; Ntatsi, G.; Savvas, D.; Vasconcelos, M.; Hamann, K.; Williams, M.; Styles, D.; et al. A Multifunctional Solution for Wicked Problems: Value-Chain Wide Facilitation of Legumes Cultivated at Bioregional Scales Is Necessary to Address the Climate-Biodiversity-Nutrition Nexus. Front. Sustain. Food Syst. 2021, 5, 692137. [Google Scholar] [CrossRef]
- Ditzler, L.; van Apeldoorn, D.F.; Pellegrini, F.; Antichi, D.; Bàrberi, P.; Rossing, W.A.H. Current research on the ecosystem service potential of legume inclusive cropping systems in Europe. A review. Agron. Sustain. Dev. 2021, 41, 26. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and Challenges in Legume Breeding for Pest and Disease Resistance. Crit. Rev. Plant Sci. 2014, 34, 195–236. [Google Scholar] [CrossRef]
- Wu, L. Occurrence and Management of Root Rot of Field Pea Cause by Aphanomyces euteiches. Master’s Thesis, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Canada, 2018. [Google Scholar]
- Quillévéré-Hamard, A.; Le Roy, G.; Moussart, A.; Baranger, A.; Andrivon, D.; Pilet-Nayel, M.-L.; Le May, C. Genetic and Pathogenicity Diversity of Aphanomyces euteiches Populations from Pea-Growing Regions in France. Front. Plant Sci. 2018, 9, 1673. [Google Scholar] [CrossRef] [PubMed]
- Abbo, S.; Gopher, A. Near Eastern Plant Domestication: A History of Thought. Trends Plant Sci. 2017, 22, 491–511. [Google Scholar] [CrossRef]
- Becking, T.; Kiselev, A.; Rossi, V.; Street-Jones, D.; Grandjean, F.; Gaulin, E. Pathogenicity of animal and plant parasitic Aphanomyces spp. and their economic impact on aquaculture and agriculture. Fungal Biol. Rev. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Papavizas, G.Z.; Ayers, W.A. Aphanomyces Species and Their Root Diseases and Sugar Beet: Review; HardPress Publishing: Miami, FL, USA, 1974; pp. 1–158. [Google Scholar]
- Moussart, A.; Even, M.N.; Tivoli, B. Reaction of genotypes from several species of grain and forage legumes to infection with a French pea isolate of the oomycete Aphanomyces euteiches. Eur. J. Plant Pathol. 2008, 122, 321–333. [Google Scholar] [CrossRef]
- Zitnick-Anderson, K.; Porter, L.D.; Hanson, L.E.; Pasche, J.S. Identification, Laboratory, Greenhouse, and Field Handling of Aphanomyces euteiches on Pea (Pisum sativum). Plant Health Prog. 2021, 22, 392–403. [Google Scholar] [CrossRef]
- Chatterton, S.; Schwinghamer, T.D.; Pagé, A.; Davidson, R.B.; Harding, M.W.; Banniza, S. Inoculum dose–disease response relationships for the pea root rot pathogen, Aphanomyces euteiches, are dependent on soil type and other pathogens. Front. Plant Sci. 2023, 14, 1115420. [Google Scholar] [CrossRef]
- Hossain, S.; Bergkvist, G.; Berglund, K.; Mårtensson, A.; Persson, P. Aphanomyces pea root rot disease and control with special reference to impact of Brassicaceae cover crops. Acta Agric. Scand. B Soil Plant Sci. 2012, 62, 477–487. [Google Scholar] [CrossRef]
- Heyman, F.; Lindahl, B.; Persson, L.; Wikström, M.; Stenlid, J. Calcium concentrations of soil affect suppressiveness against Aphanomyces root rot of pea. Soil Biol. Biochem. 2007, 39, 2222–2229. [Google Scholar] [CrossRef]
- Godebo, A.T.; Germida, J.J.; Walley, F.L. isolation, identification, and assessment of soil bacteria as biocontrol agents of pea root rot caused by Aphanomyces euteiches. Can. J. Soil Sci. 2020, 100, 206–216. [Google Scholar] [CrossRef]
- Heungens, K.; Parke, J.L. Postinfection Biological Control of Oomycete Pathogens of Pea by Burkholderia cepacia AMMDR1. Phytopathology 2001, 91, 383–391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakelin, S.A.; Walter, M.; Jaspers, M.; Stewart, A. Biological control of Aphanomyces euteiches root-rot of pea with spore-forming bacteria. Australas. Plant Pathol. 2002, 31, 401–407. [Google Scholar] [CrossRef]
- Vandemark, G.J.; Kraft, J.M.; Larsen, R.C.; Gritsenko, M.A.; Boge, W.L. A PCR-Based Assay by Sequence-Characterized DNA Markers for the Identification and Detection of Aphanomyces euteiches. Phytopathology 2000, 90, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Pfender, W.F.; Hagedorn, D.J. Disease progress and yield loss in Aphanomyces root-rot of peas. Phytopathology 1983, 73, 1109–1113. [Google Scholar] [CrossRef]
- Conner, R.L.; Chang, K.F.; Hwang, S.F.; Warkentin, T.D.; McRae, K.B. Assessment of tolerance for reducing yield losses in field pea caused by Aphanomyces root rot. Can. J. Plant Sci. 2013, 93, 473–482. [Google Scholar] [CrossRef]
- Malvick, D.K.; Percich, J.A. Genotypic and Pathogenic Diversity Among Pea-Infecting Strains of Aphanomyces euteiches from the Central and Western United States. Phytopathology 1998, 88, 915–921. [Google Scholar] [CrossRef]
- Gritton, E.T. Registration of Five Root Rot Resistant Germplasm Lines of Processing Pea. Crop Sci. 1990, 30, 1166–1167. [Google Scholar] [CrossRef]
- Leprévost, T.; Boutet, G.; Lesné, A.; Rivière, J.-P.; Vetel, P.; Glory, I.; Miteul, H.; Le Rat, A.; Dufour, P.; Regnault-Kraut, C.; et al. Advanced backcross QTL analysis and comparative mapping with RIL QTL studies and GWAS provide an overview of QTL and marker haplotype diversity for resistance to Aphanomyces root rot in pea (Pisum sativum). Front. Plant Sci. 2023, 14, 1189289. [Google Scholar] [CrossRef]
- Desgroux, A.; L’Anthoëne, V.; Roux-Duparque, M.; Rivière, J.-P.; Aubert, G.; Tayeh, N.; Moussart, A.; Mangin, P.; Vetel, P.; Piriou, C.; et al. Genome-wide association mapping of partial resistance to Aphanomyces euteiches in pea. BMC Genomics 2016, 17, 124. [Google Scholar] [CrossRef]
- Hamon, C.; Baranger, A.; Coyne, C.J.; McGee, R.J.; Le Goff, I.; L’Anthoëne, V.; Esnault, R.; Rivière, J.-P.; Klein, A.; Mangin, P.; et al. New consistent QTL in pea associated with partial resistance to Aphanomyces euteiches in multiple French and American environments. Theor. App. Genet. 2011, 123, 261–281. [Google Scholar] [CrossRef]
- Pilet-Nayel, M.-L.; Muehlbauer, F.; McGee, R.; Kraft, J.; Baranger, A.; Coyne, C. Quantitative trait loci for partial resistance to Aphanomyces root rot in pea. Theor. Appl. Genet. 2002, 106, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Pilet-Nayel, M.-L.; Muehlbauer, F.J.; McGee, R.J.; Kraft, J.M.; Baranger, A.; Coyne, C.J. Consistent Quantitative Trait Loci in Pea for Partial Resistance to Aphanomyces euteiches Isolates from the United States and France. Phytopathology 2005, 95, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, C.; Lesné, A.; Piriou, C.; Le Roy, G.; Boutet, G.; Moussart, A.; Poncet, C.; Delourme, R.; Baranger, A.; Pilet-Nayel, M.-L. Validation of QTL for resistance to Aphanomyces euteiches in different pea genetic backgrounds using near-isogenic lines. Theor. App. Genet. 2015, 128, 2273–2288. [Google Scholar] [CrossRef] [PubMed]
- Tordsen, C.L.; Giles, J.M.; Sathoff, A.E. First Report of Aphanomyces euteiches Race 1 and Race 2 Causing Aphanomyces Root Rot on Alfalfa (Medicago sativa) in South Dakota. Plant Dis. 2022, 106, 771. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Guo, Z. Research Progress and Prospect of Alfalfa Resistance to Pathogens and Pests. Plants 2022, 11, 2008. [Google Scholar] [CrossRef]
- Quillévéré-Hamard, A.; Le Roy, G.; Lesné, A.; Le May, C.; Pilet-Nayel, M.-L. Aggressiveness of Diverse French Aphanomyces euteiches Isolates on Pea Near Isogenic Lines Differing in Resistance Quantitative Trait Loci. Phytopathology 2021, 111, 695–702. [Google Scholar] [CrossRef]
- Hamon, C.; Coyne, C.J.; McGee, R.J.; Lesné, A.; Esnault, R.; Mangin, P.; Hervé, M.; Le Goff, I.; Deniot, G.; Roux-Duparque, M.; et al. QTL meta-analysis provides a comprehensive view of loci controlling partial resistance to Aphanomyces euteiches in four sources of resistance in pea. BMC Plant Biol. 2013, 13, 45. [Google Scholar] [CrossRef]
- Kälin, C.; Kolodinska Brantestam, A.; Arvidsson, A.-K.; Dubey, M.; Elfstrand, M.; Karlsson, M. Evaluation of pea genotype PI180693 partial resistance towards Aphanomyces root rot in commercial pea breeding. Front. Plant Sci. 2023, 14, 1114408. [Google Scholar] [CrossRef]
- Wicker, E.; Rouxel, F. Specific Behaviour of French Aphanomyces euteiches Drechs. Populations for Virulence and Aggressiveness on Pea, Related to Isolates from Europe, America and New Zealand. Eur. J. Plant Pathol. 2001, 107, 919–929. [Google Scholar] [CrossRef]
- Peters, R.D.; Grau, C.R. Inoculation with Nonpathogenic Fusarium solani Increases Severity of Pea Root Rot Caused by Aphanomyces euteiches. Plant Dis. 2002, 86, 411–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sivachandra Kumar, N.T.; Cox, L.; Armstrong-Cho, C.; Banniza, S. Optimization of zoospore production and inoculum concentration of Aphanomyces euteiches for resistance screening of pea and lentil. Can. J. Plant Pathol. 2020, 42, 419–428. [Google Scholar] [CrossRef]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic Induction of the Defensin and Phytoalexin Pisatin Pathways in Pea (Pisum sativum) against Aphanomyces euteiches by Acetylated and Nonacetylated Oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef]
- Bari, M.A.A.; Fonseka, D.; Stenger, J.; Zitnick-Anderson, K.; Atanda, S.A.; Morales, M.; Worral, H.; Piche, L.; Kim, J.; Johnson, J.; et al. A greenhouse-based high-throughput phenotyping platform for identification and genetic dissection of resistance to Aphanomyces root rot in field pea. Plant Phenome J. 2023, 6, e20063. [Google Scholar] [CrossRef]
- Kim, K.-T.; Jeon, J.; Choi, J.; Cheong, K.; Song, H.; Choi, G.; Kang, S.; Lee, Y.-H. Kingdom-Wide Analysis of Fungal Small Secreted Proteins (SSPs) Reveals Their Potential Role in Host Association. Front. Plant Sci. 2016, 7, 186. [Google Scholar] [CrossRef]
- Kiselev, A.; Clemente, H.S.; Camborde, L.; Dumas, B.; Gaulin, E. A Comprehensive Assessment of the Secretome Responsible for Host Adaptation of the Legume Root Pathogen Aphanomyces euteiches. J. Fungi 2022, 8, 88. [Google Scholar] [CrossRef]
- Meyer, M.; Bourras, S.; Gervais, J.; Labadie, K.; Cruaud, C.; Balesdent, M.-H.; Rouxel, T. Impact of biotic and abiotic factors on the expression of fungal effector-encoding genes in axenic growth conditions. Fungal Genet. Biol. 2017, 99, 1–12. [Google Scholar] [CrossRef]
- Kälin, C.; Berlin, A.; Kolodinska Brantestam, A.; Dubey, M.; Arvidsson, A.; Riesinger, P.; Elfstrand, M.; Karlsson, M. Genetic diversity of the pea root pathogen Aphanomyces euteiches in Europe. Plant Pathol. 2022, 71, 1570–1578. [Google Scholar] [CrossRef]
- Gaulin, E.; Jacquet, C.; Bottin, A.; Dumas, B. Root rot disease of legumes caused by Aphanomyces euteiches. Mol. Plant Pathol. 2007, 8, 539–548. [Google Scholar] [CrossRef]
- Wicker, E.; Moussart, A.; Duparque, M.; Rouxel, F. Further Contributions to the Development of a Differential Set of Pea Cultivars (Pisum sativum) to Investigate the Virulence of Isolates of Aphanomyces euteiches. Eur. J. Plant Pathol. 2003, 109, 47–60. [Google Scholar] [CrossRef]
- Kraft, J.M.; Haware, M.P.; Jiménez-Díaz, R.M.; Bayaa, B.; Harrabi, M. Screening techniques and sources of resistance to root rots and wilts in cool season food legumes. Euphytica 1993, 73, 27–39. [Google Scholar] [CrossRef]
- Bonhomme, M.; Fariello, M.I.; Navier, H.; Hajri, A.; Badis, Y.; Miteul, H.; Samac, D.A.; Dumas, B.; Baranger, A.; Jacquet, C.; et al. A local score approach improves GWAS resolution and detects minor QTL: Application to Medicago truncatula quantitative disease resistance to multiple Aphanomyces euteiches isolates. Heredity 2019, 123, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Rispail, N.; Wohor, O.Z.; Osuna-Caballero, S.; Barilli, E.; Rubiales, D. Genetic Diversity and Population Structure of a Wide Pisum spp. Core Collection. Int. J. Mol. Sci. 2023, 24, 2470. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, V.S.; Kosterin, O.E.; Yadrikhinskiy, A.K. Wild peas vary in their cross-compatibility with cultivated pea (Pisum sativum subsp. sativum L.) depending on alleles of a nuclear–cytoplasmic incompatibility locus. Theor. Appl. Genet. 2014, 127, 1163–1172. [Google Scholar] [CrossRef]
- Rubiales, D.; Barilli, E.; Rispail, N. Breeding for Biotic Stress Resistance in Pea. Agriculture 2023, 13, 1825. [Google Scholar] [CrossRef]
- Malvick, D.K.; Percich, J.A. Identification of Pisum sativum Germ Plasm with Resistance to Root Rot Caused by Multiple Strains of Aphanomyces euteiches. Plant Dis. 1999, 83, 51–54. [Google Scholar] [CrossRef]
- Davis, D.W.; Fritz, V.A.; Pfleger, F.L.; Percich, J.A.; Malvick, D.K. MN 144, MN 313, and MN 314: Garden Pea Lines Resistant to Root Rot Caused by Aphanomyces euteiches Drechs. Hort. Science 1995, 30, 639–640. [Google Scholar] [CrossRef]
- Sharma, A.; Rani, M.; Lata, H.; Thakur, A.; Sharma, P.; Kumar, P.; Jayswal, D.K.; Rana, R.N. Global dimension of root rot complex in garden pea: Current status and breeding prospective. Crop Prot. 2022, 158, 106004. [Google Scholar] [CrossRef]
- Lockwood, J.L. Pea Introductions with Partial Resistance to Aphanomyces Root Rot. Phytopathology 1960, 50, 621–624. [Google Scholar]
- Coyne, C.J.; Porter, L.D.; Boutet, G.; Ma, Y.; McGee, R.J.; Lesné, A.; Baranger, A.; Pilet-Nayel, M.-L. Confirmation of Fusarium root rot resistance QTL Fsp-Ps 2.1 of pea under controlled conditions. BMC Plant Biol. 2019, 19, 98. [Google Scholar] [CrossRef]
- Infantino, A.; Kharrat, M.; Riccioni, L.; Coyne, C.J.; McPhee, K.E.; Grünwald, N.J. Screening techniques and sources of resistance to root diseases in cool season food legumes. Euphytica 2006, 147, 201–221. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Coffman, V.A.; Kraft, J.M. Sources of Partial Resistance to Fusarium Root Rot in the Pisum Core Collection. Plant Dis. 2003, 87, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.J.; Satovic, Z.; Rubiales, D.; Fondevilla, S. Er3 Gene, conferring resistance to powdery mildew in pea, is located in pea LGIV. Euphytica 2018, 214, 203. [Google Scholar] [CrossRef]
- Rubiales, D.; Osuna-Caballero, S.; González-Bernal, M.J.; Cobos, M.J.; Flores, F. Pea Breeding Lines Adapted to Autumn Sowings in Broomrape Prone Mediterranean Environments. Agronomy 2021, 11, 769. [Google Scholar] [CrossRef]
- Fondevilla, S.; Flores, F.; Emeran, A.A.; Kharrat, M.; Rubiales, D. High productivity of dry pea genotypes resistant to crenate broomrape in Mediterranean environments. Agron. Sustain. Dev. 2017, 37, 61. [Google Scholar] [CrossRef]
- Kosterin, O.E.; Bogdanova, V.S. Relationship of wild and cultivated forms of Pisum L. as inferred from an analysis of three markers, of the plastid, mitochondrial and nuclear genomes. Genet. Resour. Crop Evol. 2008, 55, 735–755. [Google Scholar] [CrossRef]
- Kosterin, O.E.; Zaytseva, O.O.; Bogdanova, V.S.; Ambrose, M.J. New data on three molecular markers from different cellular genomes in mediterranean accessions reveal new insights into phylogeography of Pisum sativum L. subsp. elatius (Bieb.). Schmalh. Genet. Resour. Crop Evol. 2010, 57, 733–739. [Google Scholar] [CrossRef]
- Clemente, A.; Arques, M.C.; Dalmais, M.; Le Signor, C.; Chinoy, C.; Olias, R.; Rayner, T.; Isaac, P.G.; Lawson, D.M.; Bendahmane, A.; et al. Eliminating Anti-Nutritional Plant Food Proteins: The Case of Seed Protease Inhibitors in Pea. PLoS ONE 2015, 10, e0134634. [Google Scholar] [CrossRef]
- Osuna-Caballero, S.; Rispail, N.; Barilli, E.; Rubiales, D. Identification and Characterization of Novel Sources of Resistance to Rust Caused by Uromyces pisi in Pisum spp. Plants 2022, 11, 2268. [Google Scholar] [CrossRef]
- Barilli, E.; Cobos, M.J.; Carrillo, E.; Kilian, A.; Carling, J.; Rubiales, D. A High-Density Integrated DArTseq SNP-Based Genetic Map of Pisum fulvum and Identification of QTLs Controlling Rust Resistance. Front. Plant Sci. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Warkentin, T.D.; Gurusamy, V.; Tar’an, B.; Banniza, S. Identification of Mycosphaerella Blight Resistance in Wild Pisum Species for Use in Pea Breeding. Crop Sci. 2012, 52, 2462–2468. [Google Scholar] [CrossRef]
- Fondevilla, S.; Torres, A.M.; Moreno, M.T.; Rubiales, D. Identification of a New Gene for Resistance to Powdery Mildew in Pisum fulvum, a Wild Relative of Pea. Breed. Sci. 2007, 57, 181–184. [Google Scholar] [CrossRef]
- Rubiales, D.; Moreno, M.T.; Sillero, J.C. Search for Resistance to Crenate Broomrape (Orobanche crenata Forsk.) in Pea Germplasm. Genet. Resour. Crop. Evol. 2005, 52, 853–861. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M.; Pérez-de-Luque, A.; Castillejo, M.A.; Prats, E.; Sillero, J.C.; Rispail, N.; Fondevilla, S. Breeding approaches for crenate broomrape (Orobanche crenata Forsk.) management in pea (Pisum sativum L.). Pest Manag. Sci. 2009, 65, 553–559. [Google Scholar] [CrossRef]
- Barilli, E.; Carrillo-Perdomo, E.; Cobos, M.J.; Kilian, A.; Carling, J.; Rubiales, D. identification of potential candidate genes controlling pea aphid tolerance in a Pisum fulvum high-density integrated DArTseq SNP-based genetic map. Pest Manag. Sci. 2020, 76, 1731–1742. [Google Scholar] [CrossRef]
- Byrne, O.M.; Hardie, D.C.; Khan, T.N.; Speijers, J.; Yan, G. Genetic analysis of pod and seed resistance to pea weevil in a Pisum sativum × P. fulvum interspecific cross. Aust. J. Agric. Res. 2008, 59, 854–862. [Google Scholar] [CrossRef]
- Aznar-Fernández, T.; Barilli, E.; Cobos, M.J.; Kilian, A.; Carling, J.; Rubiales, D. Identification of quantitative trait loci (QTL) controlling resistance to pea weevil (Bruchus pisorum) in a high-density integrated DArTseq SNP-based genetic map of pea. Sci. Rep. 2020, 10, 33. [Google Scholar] [CrossRef]
- Wohor, O.Z.; Rispail, N.; Ojiewo, C.O.; Rubiales, D. Pea Breeding for Resistance to Rhizospheric Pathogens. Plants 2022, 11, 2664. [Google Scholar] [CrossRef]
- Awodele, S.O.; Gali, K.K.; Sivachandra Kumar, N.T.; De Silva, D.; Chatterton, S.; Banniza, S.; Warkentin, T.D. Evaluation of Pea Accessions Differing in Flower and Seed Coat Pigmentation for Resistance to Fusarium avenaceum Root Rot. Legum. Sci. 2024, 6, e230. [Google Scholar] [CrossRef]
- Loinsigh, B.Ò. Detection and Distribution of Aphanomyces euteiches in the United Kingdom. In International Congress of Plant Pathology (ICPP) 2018: Plant Health in A Global Economy; The American Phytopathological Society (APS): St. Paul, MN, USA, 2018. [Google Scholar]
- Moussart, A.; Onfroy, C.; Lesne, A.; Esquibet, M.; Grenier, E.; Tivoli, B. Host status and reaction of Medicago truncatula accessions to infection by three major pathogens of pea (Pisum sativum) and alfalfa (Medicago sativa). Eur. J. Plant Pathol. 2006, 117, 57–69. [Google Scholar] [CrossRef]
- Parke, J.L.; Grau, C.R. Aphanomyces. In Methods for Research on Soilborne Phytopathogenic Fungi; Singleton, L.L., Mihail, J.D., Rush, C.M., Eds.; The American Phytopathological Society (APS): St. Paul, MN, USA, 1992; pp. 27–30. [Google Scholar]
- Xue, A.G. Effect of seed-borne Mycosphaerella pinodes and seed treatments on emergence, foot rot severity, and yield of field pea. Can. J. Plant Pathol. 2000, 22, 248–253. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Mena, S.; Rubiales, D.; González, M. Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum. Plants 2024, 13, 2454. https://doi.org/10.3390/plants13172454
Rodriguez-Mena S, Rubiales D, González M. Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum. Plants. 2024; 13(17):2454. https://doi.org/10.3390/plants13172454
Chicago/Turabian StyleRodriguez-Mena, Sara, Diego Rubiales, and Mario González. 2024. "Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum" Plants 13, no. 17: 2454. https://doi.org/10.3390/plants13172454
APA StyleRodriguez-Mena, S., Rubiales, D., & González, M. (2024). Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum. Plants, 13(17), 2454. https://doi.org/10.3390/plants13172454






