Complex of Defense Polypeptides of Wheatgrass (Elytrigia elongata) Associated with Plant Immunity to Biotic and Abiotic Stress Factors
Abstract
:1. Introduction
2. Results
2.1. Isolation of Protein Extract from Elytrigia elongata Spikelets
2.2. Biological Activity of PE from E. elongata Spikelets
2.2.1. Antifungal Action
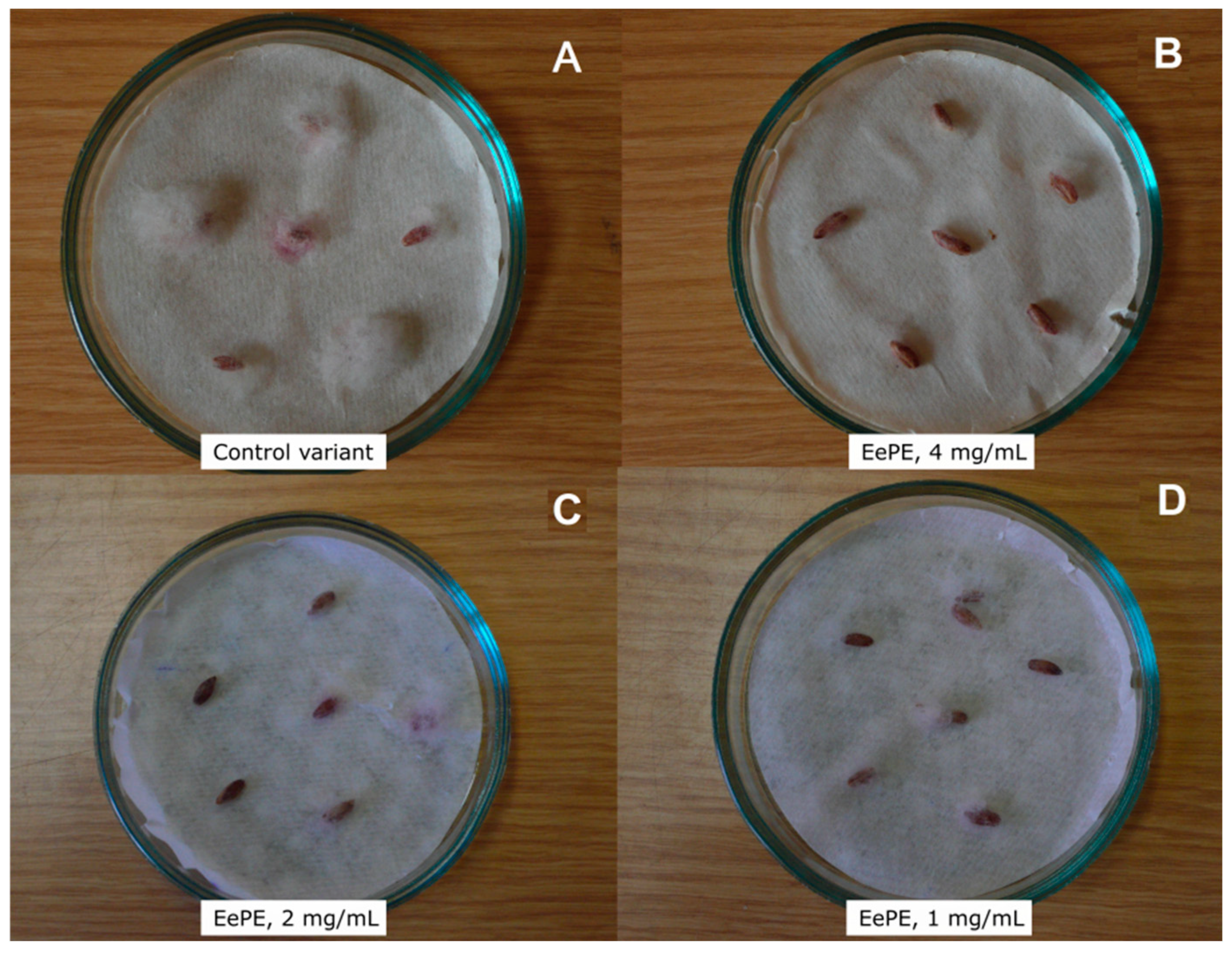
2.2.2. Protective Activity
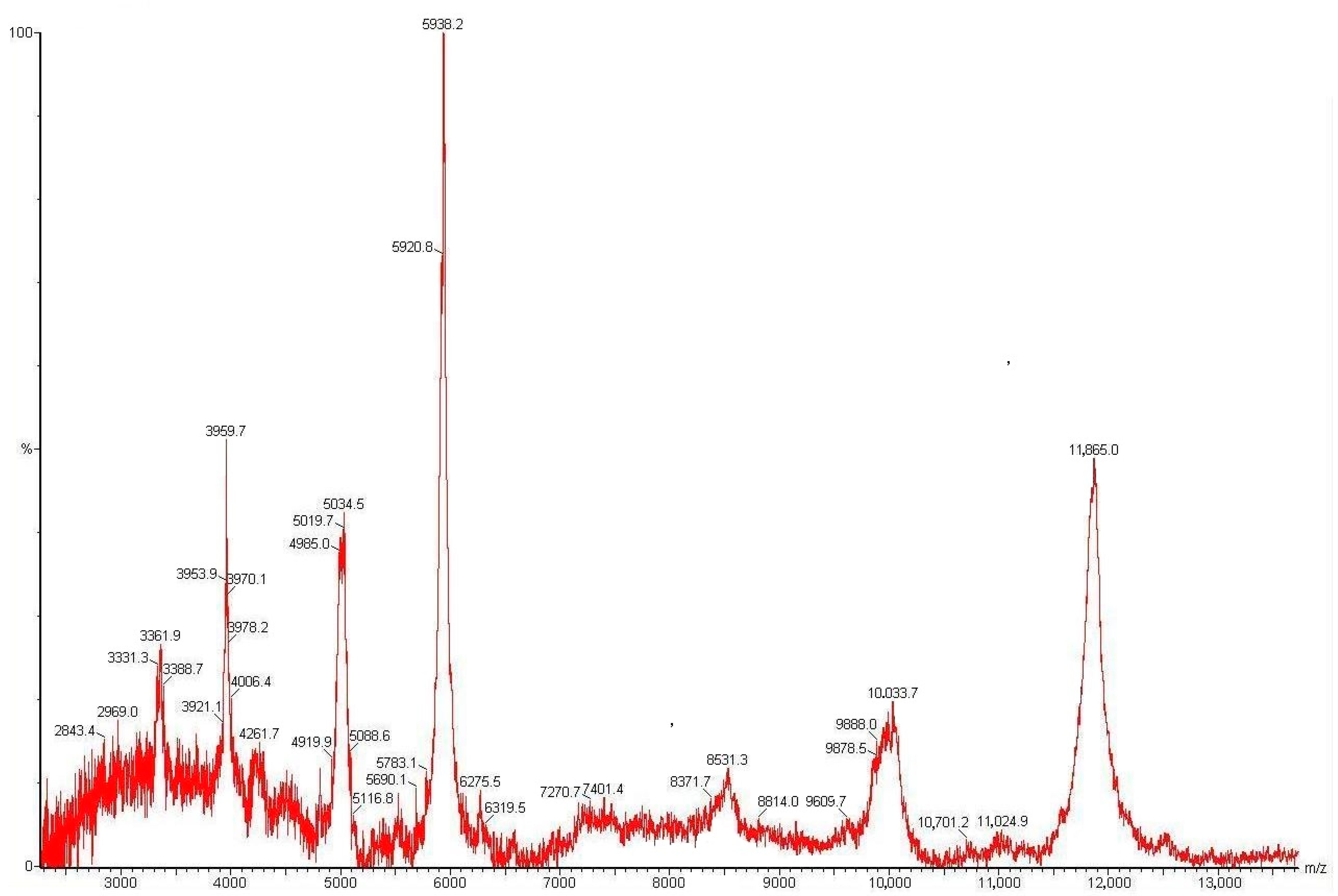
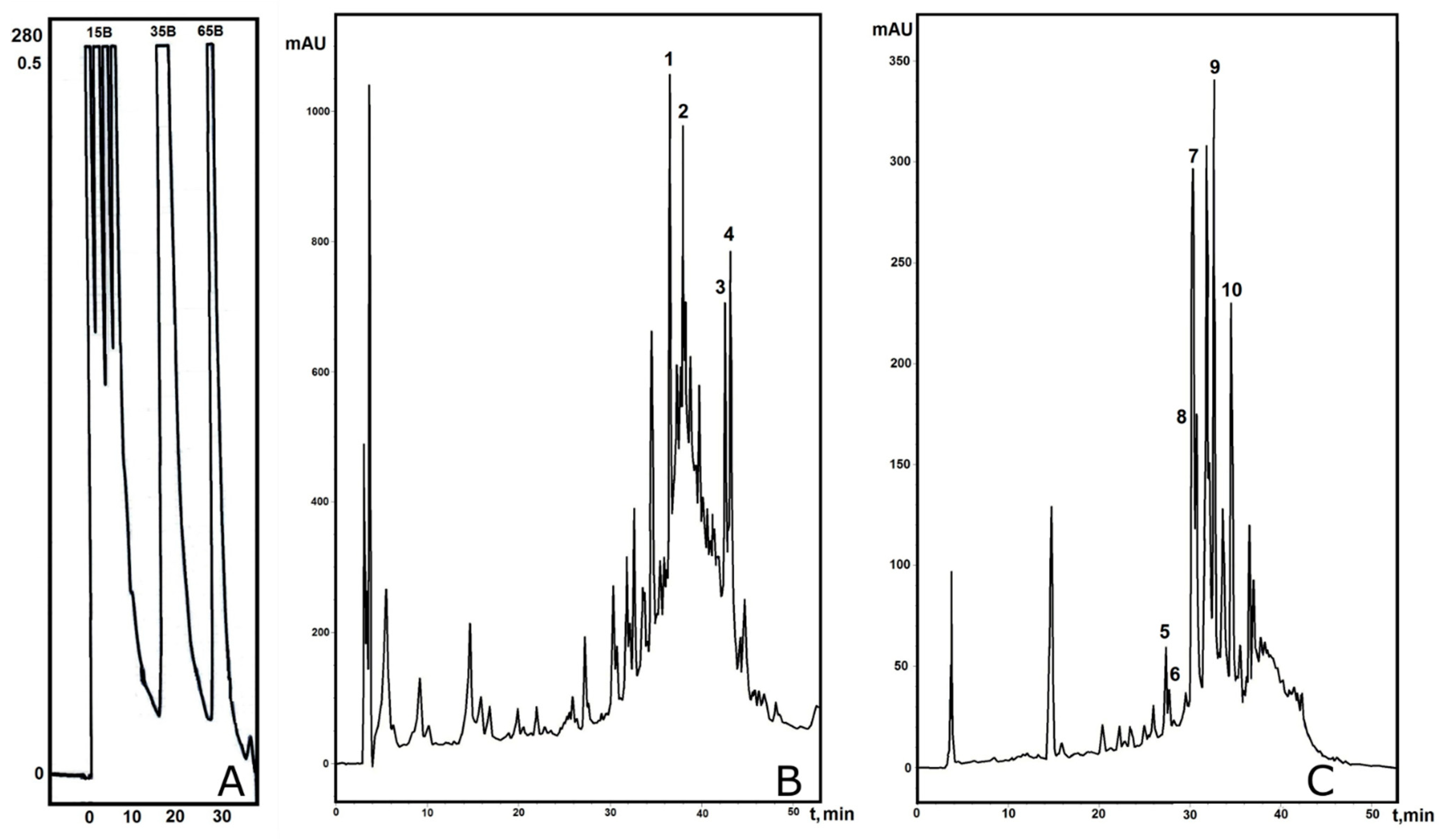
2.3. Isolation and Structure Analysis of Individual Polypeptides from E. elongata Spikelets
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Biological Material
5.1.1. Plant
5.1.2. Microorganisms
5.2. Preparation of Total Protein Extract from E. elongata Spikelets
5.3. Medium-Pressure Affinity Chromatography
5.4. Stepwise RP-HPLC
5.5. Analytical RP-HPLC
5.6. SDS-PAGE and Electro-Blotting
5.7. MALDI-TOF/TOF MS
5.8. N-Terminal Sequencing
5.9. Antifungal Activity In Vitro
5.10. Treatment of Wheat Grain
5.11. Protection Assays In Vitro
5.12. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahl, T.W.; Arens, S.K.M. The impacts of land plant evolution on Earth’s climate and oxygenation state—An interdisciplinary review. Chem. Geol. 2020, 547, 119665. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. The Role of Plants in the Effects of Global Change on Nutrient Availability and Stoichiometry in the Plant-Soil System. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef]
- Campos, M.L.; De Souza, C.M.; De Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The role of antimicrobial peptides in plant immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Yang, X.; Qi, F. Distinct Responses to Pathogenic and Symbionic Microorganisms: The Role of Plant Immunity. Int. J. Mol. Sci. 2022, 23, 10427. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, Y.T.; Wu, Y.Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Shelenkov, A.A.; Odintsova, T.I. Prediction of Leymus arenarius (L.) antimicrobial peptides based on de novo transcriptome assembly. Plant Mol. Biol. 2015, 89, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Tavormina, P.; De Coninck, B.; Nikonorova, N.; De Smet, I.; Cammuea, B.P.A. The Plant Peptidome: An Expanding Repertoire of Structural Features and Biological Functions. Plant Cell 2015, 27, 2095–2118. [Google Scholar] [CrossRef]
- Tang, S.S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef]
- Stec, B. Plant thionins—The structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef]
- Bogdanov, I.V.; Shenkarev, Z.O.; Finkina, E.I.; Melnikova, D.N.; Rumynskiy, E.I.; Arseniev, A.S.; Ovchinnikova, T.V. A novel lipid transfer protein from the pea Pisum sativum: Isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic roperties. BMC Plant Biol. 2016, 16, 107. [Google Scholar] [CrossRef]
- Franco, O.L.; Rigden, D.J.; Melo, F.R.; Grossi-de-Sá, M.F. Plant α-amylase inhibitors and their interaction with insect α-amylases. Eur. J. Biochem. 2002, 269, 397–412. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Rogozhin, E.A.; Musolyamov, A.K.; Andreev, Y.A.; Oparin, P.B.; Berkut, A.A.; Vassilevski, A.A.; Egorov, T.A.; Grishin, E.V.; Odintsova, T.I. Novel antifungal α-hairpinin peptide from Stellaria media seeds: Structure, biosynthesis, gene structure and evolution. Plant Mol. Biol. 2014, 84, 189–202. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.S.; Gomes, V.M.; Taveira, G.B.; de Azevedo dos Santos, L.; Maracahipes, Á.C.; Rodrigues, R.; de Oliveira Carvalho, A.; Fernandes, K.V.S.; Oliveira, A.E.A. Bifunctional Inhibitors from Capsicum chinense Seeds with Antimicrobial Activity and Specific Mechanism of Action Against Phytopathogenic Fungi. Protein Pept. Lett. 2020, 28, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Bao, H.; Ng, T.B.; Chan, H.H.L.; Ng, C.C.W.; Man, G.C.W.; Wang, H.; Guan, S.; Zhao, S.; Fang, E.F.; et al. New ribosome-inactivating proteins and other proteins with protein synthesis–inhibiting activities. Appl. Microbiol. Biotechnol. 2020, 104, 4211–4226. [Google Scholar] [CrossRef]
- Kushmerick, C.; De Souza Castro, M.; Cruz, J.S.; Bloch, C.; Beirão, P.S.L. Functional and structural features of γ-zeathionins, a new class of sodium channel blockers. FEBS Lett. 1998, 440, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential Antifungal and Calcium Channel-Blocking Activity among Structurally Related Plant Defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef]
- Lima, S.; Benko-Iseppon, A.; Neto, J.; Amorim, L.; Neto, J.; Crovella, S.; Pandolfi, V. Plants Defense-related Cyclic Peptides: Diversity, Structure and Applications. Curr. Protein Pept. Sci. 2017, 18, 375–390. [Google Scholar] [CrossRef]
- Bajpai, A.; Jackson, M.A.; Huang, Y.H.; Yap, K.; Du, Q.; Chau, T.C.Y.; Craik, D.J.; Gilding, E.K. Nematicidal Activity of Cyclotides: Toxicity Against Caenorhabditis elegans. J. Nat. Prod. 2023, 86, 1222–1229. [Google Scholar] [CrossRef]
- Goyal, R.K.; Mattoo, A.K. Multitasking antimicrobial peptides in plant development and host defense against biotic/abiotic stress. Plant Sci. 2014, 228, 135–149. [Google Scholar] [CrossRef]
- dos Santos-Silva, C.A.; Vilela, L.M.B.; de Oliveira-Silva, R.L.; da Silva, J.B.; Machado, A.R.; Bezerra-Neto, J.P.; Crovella, S.; Benko-Iseppon, A.M. Cassava (Manihot esculenta) defensins: Prospection, structural analysis and tissue-specific expression under biotic/abiotic stresses. Biochimie 2021, 186, 1–12. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Vilcinskas, A.; Rahnamaeian, M. Cooperative interaction of antimicrobial peptides with the interrelated immune pathways in plants. Mol. Plant Pathol. 2016, 17, 464–471. [Google Scholar] [CrossRef]
- Aerts, A.M.; Carmona-Gutierrez, D.; Lefevre, S.; Govaert, G.; François, I.E.J.A.; Madeo, F.; Santos, R.; Cammue, B.P.A.; Thevissen, K. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 2009, 583, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weerden, N.L.; Lay, F.T.; Anderson, M.A. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J. Biol. Chem. 2008, 283, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; García-Olmedo, F.; Molina, A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002, 128, 951–961. [Google Scholar] [CrossRef]
- Tesfaye, M.; Silverstein, K.A.T.; Nallu, S.; Wang, L.; Botanga, C.J.; Gomez, S.K.; Costa, L.M.; Harrison, M.J.; Samac, D.A.; Glazebrook, J.; et al. Spatio-Temporal Expression Patterns of Arabidopsis thaliana and Medicago truncatula Defensin-Like Genes. PLoS ONE 2013, 8, e58992. [Google Scholar] [CrossRef]
- Kiba, A.; Saitoh, H.; Nishihara, M.; Omiya, K.; Yamamura, S. C-Terminal Domain of a Hevein-Like Protein from Wasabia japonica has Potent Antimicrobial Activity. Plant Cell Physiol. 2003, 44, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F.N. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef]
- Langridge, P.; Paltridge, N.; Fincher, G. Functional genomics of abiotic stress tolerance in cereals. Brief. Funct. Genom. Proteomic. 2006, 4, 343–354. [Google Scholar] [CrossRef]
- Gharaghanipor, N.; Arzani, A.; Rahimmalek, M.; Ravash, R. Physiological and Transcriptome Indicators of Salt Tolerance in Wild and Cultivated Barley. Front. Plant Sci. 2022, 13, 819282. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Z.; Ding, Y.; Guo, M. Characterization of HSP70 family in watermelon (Citrullus lanatus): Identification, structure, evolution, and potential function in response to ABA, cold and drought stress. Front. Genet. 2023, 14, 1201535. [Google Scholar] [CrossRef]
- Chan, Z.; Blakeslee, J.; Lata, C.; Guerriero, G.; Esposito, S.; Landi, S.; Hausman, J.-F. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 1, 1214. [Google Scholar]
- Clemente, M.; Corigliano, M.G.; Pariani, S.A.; Sánchez-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming. Int. J. Mol. Sci. 2019, 20, 1345. [Google Scholar] [CrossRef]
- Li, S.S.; Claeson, P. Cys/Gly-rich proteins with a putative single chitin-binding domain from oat (Avena sativa) seeds. Phytochemistry 2003, 63, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Pyati, P.; Chellamuthu, A.; Gatehouse, A.M.R.; Fitches, E.; Gatehouse, J.A. Insecticidal activity of wheat Hessian fly responsive proteins HFR-1 and HFR-3 towards a non-target wheat pest, cereal aphid (Sitobion avenae F.). J. Insect Physiol. 2012, 58, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach Barroso Coelho, L.C.; Marcelino dos Santos Silva, P.; Felix de Oliveira, W.; de Moura, M.C.; Viana Pontual, E.; Soares Gomes, F.; Guedes Paiva, P.M.; Napoleão, T.H.; dos Santos Correia, M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.C.; Hwang, I.; Cheong, H.; Nah, J.W.; Hahm, K.S.; Park, Y. Protease Inhibitors from Plants with Antimicrobial Activity. Int. J. Mol. Sci. 2009, 10, 2860. [Google Scholar] [CrossRef] [PubMed]
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J.G.M. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant–pathogen interactions. Front. Plant Sci. 2015, 6, 151188. [Google Scholar] [CrossRef]
- Rogozhin, E.; Ryazantsev, D.; Smirnov, A.; Zavriev, S. Primary structure analysis of antifungal peptides from cultivated and wild cereals. Plants 2018, 7, 74. [Google Scholar] [CrossRef]
- Srivastava, S.; Dashora, K.; Ameta, K.L.; Singh, N.P.; El-Enshasy, H.A.; Pagano, M.C.; Hesham, A.E.L.; Sharma, G.D.; Sharma, M.; Bhargava, A. Cysteine-rich antimicrobial peptides from plants: The future of antimicrobial therapy. Phyther. Res. 2021, 35, 256–277. [Google Scholar] [CrossRef]
- Struyfs, C.; Cammue, B.P.A.; Thevissen, K. Membrane-Interacting Antifungal Peptides. Front. Cell Dev. Biol. 2021, 9, 706. [Google Scholar] [CrossRef]
- Hesler, L.S. Resistance to Rhopalosiphum padi (Homoptera: Aphididae) in Three Triticale Accessions. J. Econ. Entomol. 2005, 98, 603–610. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xing, P.; Bao, Y.; Ren, M.; Liu, S.; Wang, Y.; Li, X.; Wang, H. Chromosome pairing in hybrid progeny between triticum aestivum and elytrigia elongata. Front. Plant Sci. 2017, 8, 297408. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A.; Ryazantsev, D.Y.; Grishin, E.V.; Egorov, T.A.; Zavriev, S.K. Defense peptides from barnyard grass (Echinochloa crusgalli L.) seeds. Peptides 2012, 38, 33–40. [Google Scholar] [CrossRef]
- Rogozhin, E.A.; Vasilchenko, A.S.; Barashkova, A.S.; Smirnov, A.N.; Zavriev, S.K.; Demushkin, V.P. Peptide Extracts from Seven Medicinal Plants Discovered to Inhibit Oomycete Phytophthora infestans, a Causative Agent of Potato Late Blight Disease. Plants 2020, 9, 1294. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Egorov, T.A.; Odintsova, T.I.; Pukhalsky, V.A.; Grishin, E.V. Diversity of wheat anti-microbial peptides. Peptides 2005, 26, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A. Defensins of Grasses: A Systematic Review. Biomolecules 2020, 10, 1029. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Rogozhin, E.A.; Baranov, Y.; Musolyamov, A.K.; Yalpani, N.; Egorov, T.A.; Grishin, E.V. Seed defensins of barnyard grass Echinochloa crusgalli (L.) Beauv. Biochimie 2008, 90, 1667–1673. [Google Scholar] [CrossRef]
- Utkina, L.L.; Zhabon, E.O.; Slavokhotova, A.A.; Rogozhin, E.A.; Shiyan, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I.; Pukhal’skiy, V.A. Heterologous expression of a synthetic gene encoding a novel hevein-type antimicrobial peptide of Leymusarenarius in Escherichia coli cells. Russ. J. Genet. 2010, 46, 1449–1454. [Google Scholar] [CrossRef]
- Rampitsch, C.; Huang, M.; Djuric-Cignaovic, S.; Wang, X.; Fernando, U. Temporal Quantitative Changes in the Resistant and Susceptible Wheat Leaf Apoplastic Proteome During Infection by Wheat Leaf Rust (Puccinia triticina). Front. Plant Sci. 2019, 10, 1291. [Google Scholar] [CrossRef]
- Numan, M.; Bukhari, S.A.; Rehman, M.U.; Mustafa, G.; Sadia, B. Phylogenetic analyses, protein modeling and active site prediction of two pathogenesis related (PR2 and PR3) genes from bread wheat. PLoS ONE 2021, 16, e0257392. [Google Scholar] [CrossRef]
- Alkan, M.; Bayraktar, H.; İmren, M.; Özdemir, F.; Lahlali, R.; Mokrini, F.; Paulitz, T.; Dababat, A.A.; Özer, G. Monitoring of Host Suitability and Defense-Related Genes in Wheat to Bipolaris sorokiniana. J. Fungi 2022, 8, 149. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Recio, I.; Amigo, L. Beta-lactoglobulin as source of bioactive peptides. Amino Acids 2008, 35, 257–265. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Xing, Y. Next-Generation Sequencing Promoted the Release of Reference Genomes and Discovered Genome Evolution in Cereal Crops. Curr. Issues Mol. Biol. 2017, 27, 37–50. [Google Scholar]
- Zuluaga, D.L.; Blanco, E.; Mangini, G.; Sonnante, G.; Curci, P.L. A Survey of the Transcriptomic Resources in Durum Wheat: Stress Responses, Data Integration and Exploitation. Plants 2023, 12, 1267. [Google Scholar] [CrossRef]
- Slezina, M.P.; Korostyleva, T.V.; Slavokhotova, A.A.; Istomina, E.A.; Shcherbakova, L.A.; Pukhalskij, V.A.; Odintsova, T.I. Genes Encoding Hevein-Like Antimicrobial Peptides from Elytrigia repens (L.) Desv. ex Nevski. Russ. J. Genet. 2018, 54, 1152–1159. [Google Scholar] [CrossRef]
- Ryazantsev, D.Y.; Khodzhaev, E.Y.; Kuvarina, A.E.; Barashkova, A.S.; Rogozhin, E.A. The Antifungal and Reactivation Activities of a Novel Glycine/Histidine-Rich Linear Peptide from Dog-Grass (Elytrigia repens (L.) Desv. Ex Nevski) Ears. Appl. Biochem. Microbiol. 2023, 59, 41–47. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Egorov, T.A.; Musolyamov, A.K.; Odintsova, M.S.; Pukhalsky, V.A.; Grishin, E.V. Seed defensins from T. kiharae and related species: Genome localization of defensin-encoding genes. Biochimie 2007, 89, 605–612. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Korostyleva, T.V.; Odintsova, M.S.; Pukhalsky, V.A.; Grishin, E.V.; Egorov, T.A. Analysis of Triticum boeoticum and Triticum urartu seed defensins: To the problem of the origin of polyploid wheat genomes. Biochimie 2008, 90, 939–946. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef]
- Angulo, V.; Beriot, N.; Garcia-Hernandez, E.; Li, E.; Masteling, R.; Lau, J.A. Plant–microbe eco-evolutionary dynamics in a changing world. New Phytol. 2022, 234, 1919–1928. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, L.; Yin, Z.; Feng, Q.; Xi, H.; Zhang, C.; Gan, K.; Yong, T. Differences in soil fungal communities under salinity gradients in arid and semiarid regions. Glob. Planet. Change 2024, 236, 104425. [Google Scholar] [CrossRef]
- Khalid, A.; Hameed, A.; Tahir, M.F. Wheat quality: A review on chemical composition, nutritional attributes, grain anatomy, types, classification, and function of seed storage proteins in bread making quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef]
- Bloch, C.; Richardson, M. A new family of small (5 kDa) protein inhibitors of insect alpha-amylases from seeds or sorghum (Sorghum bicolar (L) Moench) have sequence homologies with wheat gamma-purothionins. FEBS Lett. 1991, 279, 101–104. [Google Scholar] [CrossRef]
- Hussain, S.; Güzel, Y.; Schönbichler, S.A.; Rainer, M.; Huck, C.W.; Bonn, G.K. Solid-phase extraction method for the isolation of plant thionins from European mistletoe, wheat and barley using zirconium silicate embedded in poly(styrene-co-divinylbenzene) hollow-monoliths. Anal. Bioanal. Chem. 2013, 405, 7509–7521. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A.; Korostyleva, T.V.; Kovtun, A.S.; Kasianov, A.S.; Shcherbakova, L.A.; Kudryavtsev, A.M. Non-Specific Lipid Transfer Proteins in Triticum kiharae Dorof. et Migush.: Identification, Characterization and Expression Profiling in Response to Pathogens and Resistance Inducers. Pathogens 2019, 8, 221. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A.; Korostyleva, T.V.; Kasianov, A.S.; Kovtun, A.S.; Makeev, V.J.; Shcherbakova, L.A.; Kudryavtsev, A.M. Defensin-like peptides in wheat analyzed by whole-transcriptome sequencing: A focus on structural diversity and role in induced resistance. PeerJ 2019, 7, e6125. [Google Scholar] [CrossRef]
- Shelenkov, A.; Slavokhotova, A.; Odintsova, T. Predicting antimicrobial and other cysteine-rich peptides in 1267 plant transcriptomes. Antibiotics 2020, 9, 60. [Google Scholar] [CrossRef]
- Marinaccio, L.; Zengin, G.; Pieretti, S.; Minosi, P.; Szucs, E.; Benyhe, S.; Novellino, E.; Masci, D.; Stefanucci, A.; Mollica, A. Food-inspired peptides from spinach Rubisco endowed with antioxidant, antinociceptive and anti-inflammatory properties. Food Chem. X 2023, 18, 100640. [Google Scholar] [CrossRef]
- Stintzi, A.; Schaller, A. Biogenesis of post-translationally modified peptide signals for plant reproductive development. Curr. Opin. Plant Biol. 2022, 69, 102274. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, C.; Shan, R.; Li, X.; TsekeInkabanga, A.; Li, L.; Jiang, H.; Chai, Y. Genome-Wide Identification and Expression Analysis of nsLTP Gene Family in Rapeseed (Brassica napus) Reveals Their Critical Roles in Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2022, 23, 8372. [Google Scholar] [CrossRef]
- Yang, Y.; Song, H.; Yao, P.; Zhang, S.; Jia, H.; Ye, X. NtLTPI.38, a plasma membrane-localized protein, mediates lipid metabolism and salt tolerance in Nicotiana tabacum. Int. J. Biol. Macromol. 2023, 242, 125007. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Molecular characterization and functional analysis of PR-l-like proteins identified from the wheat head blight fungus fusarium graminearum. Phytopathology 2018, 108, 510–520. [Google Scholar] [CrossRef]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as alternative fungicides for controlling plant diseases: A comprehensive review of their efficacy, commercial representatives, advantages, challenges for adoption, and possible solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Rogozhin, E.A. Isolation of antimicrobial peptides from different plant sources: Does a general extraction method exist? Plant Methods 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Vorob’eva, L.I.; Khodzhaev, E.Y.; Rogozhin, E.A.; Cherdyntseva, T.A.; Netrusov, A.I. Characterization of extracellular yeast peptide factors and their stress-protective effect on probiotic lactic acid bacteria. Microbiology 2016, 85, 411–419. [Google Scholar] [CrossRef]

| Fungal Species | Concentration of PE, mg/mL | Amphotericin B | |||
|---|---|---|---|---|---|
| 0.25 | 0.5 | 1.0 | 2.0 | ||
| B. sorokiniana | - | - | - | - | +(30 ± 2) |
| F. graminearum | - | - | +(14 ± 1) | +(20 ± 2) | +(34 ± 4) |
| F. culmorum | - | - | +(15 ± 2) | +(22 ± 3) | +(28 ± 1) |
| A. niger | - | - | - | - | +(26 ± 2) |
| Peak | Average Molecular Mass Measured, kDa | Peptide Title | N-Terminal Amino Acid Sequence | Annotation |
|---|---|---|---|---|
| 1 | 11.865 | Ee-BFTI | 1SSPSTCVAGEAIPGRP16 | Bifunctional trypsin/alpha-amylase inhibitor from Poaceae |
| 2 | 5.612 | Ee-D5 | 1KECKTGSAGYKGPC14 | Plant defensin |
| 3 | 10.038 | Ee-LTP1 | 1ISCLPYVDGQGKSP14 | Lipid-transfer protein (the 9 kDa subfamily) |
| 4 | 9.895 | Ee-LTP2 | 1IICLPYVDGQTKSP14 | Lipid-transfer protein (the 9 kDa subfamily) |
| 5 | 5.938 | Ee-D1 | 1KICRQKSAGVIGPC14 | Plant defensin |
| 6 | 5.920 | Ee-D2 | 1KICRNRSAGFRGPC14 | Plant defensin |
| 7 | 5.034 | Ee-D3 | 1KVCTGKGQDHSFPC14 | Plant defensin |
| 8 | 5.019 | Ee-D4 | 1KVCTGKSQDHSFPC14 | Plant defensin |
| 9 | 5.728 | Ee-D6 | 1KFCRTRSAGYRGPC14 | Plant defensin |
| 10 | 6.903 | Ee-BBTI | 1KKKGCCNNCQSWSG14 | Bowman–Birk trypsin inhibitor |
| 11 | >100.0 | Ee-P1 | 1LETITSQHRSFS12 | Disease resistance protein RGA2-like isoform X1 [Triticum aestivum] homologue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barashkova, A.S.; Smirnov, A.N.; Rogozhin, E.A. Complex of Defense Polypeptides of Wheatgrass (Elytrigia elongata) Associated with Plant Immunity to Biotic and Abiotic Stress Factors. Plants 2024, 13, 2459. https://doi.org/10.3390/plants13172459
Barashkova AS, Smirnov AN, Rogozhin EA. Complex of Defense Polypeptides of Wheatgrass (Elytrigia elongata) Associated with Plant Immunity to Biotic and Abiotic Stress Factors. Plants. 2024; 13(17):2459. https://doi.org/10.3390/plants13172459
Chicago/Turabian StyleBarashkova, Anna S., Alexey N. Smirnov, and Eugene A. Rogozhin. 2024. "Complex of Defense Polypeptides of Wheatgrass (Elytrigia elongata) Associated with Plant Immunity to Biotic and Abiotic Stress Factors" Plants 13, no. 17: 2459. https://doi.org/10.3390/plants13172459
APA StyleBarashkova, A. S., Smirnov, A. N., & Rogozhin, E. A. (2024). Complex of Defense Polypeptides of Wheatgrass (Elytrigia elongata) Associated with Plant Immunity to Biotic and Abiotic Stress Factors. Plants, 13(17), 2459. https://doi.org/10.3390/plants13172459






