Fine Root Traits across Different Root Orders and Their Associations with Leaf Traits in 15 Co-Occurring Plant Species from the Desert–Oasis Transition Zone in the Hexi Corridor, Gansu Province, China
Abstract
1. Introduction
2. Materials and Methods
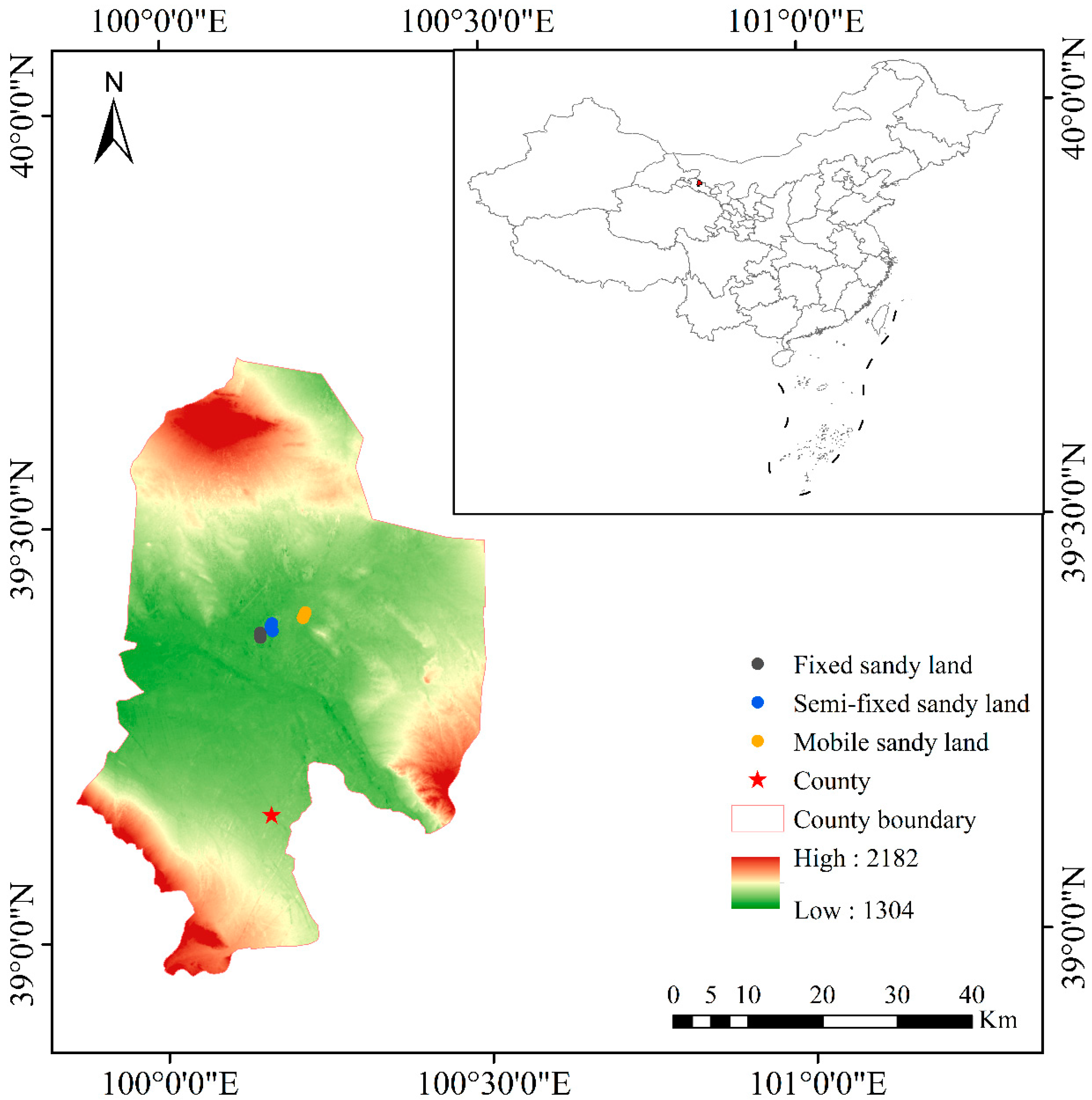
2.1. Study Site and Species Selection
2.2. Sample Collection
2.3. Plant Trait Measurements
2.4. Data Analysis
3. Results
3.1. Differences in Fine Root Traits among Root Orders
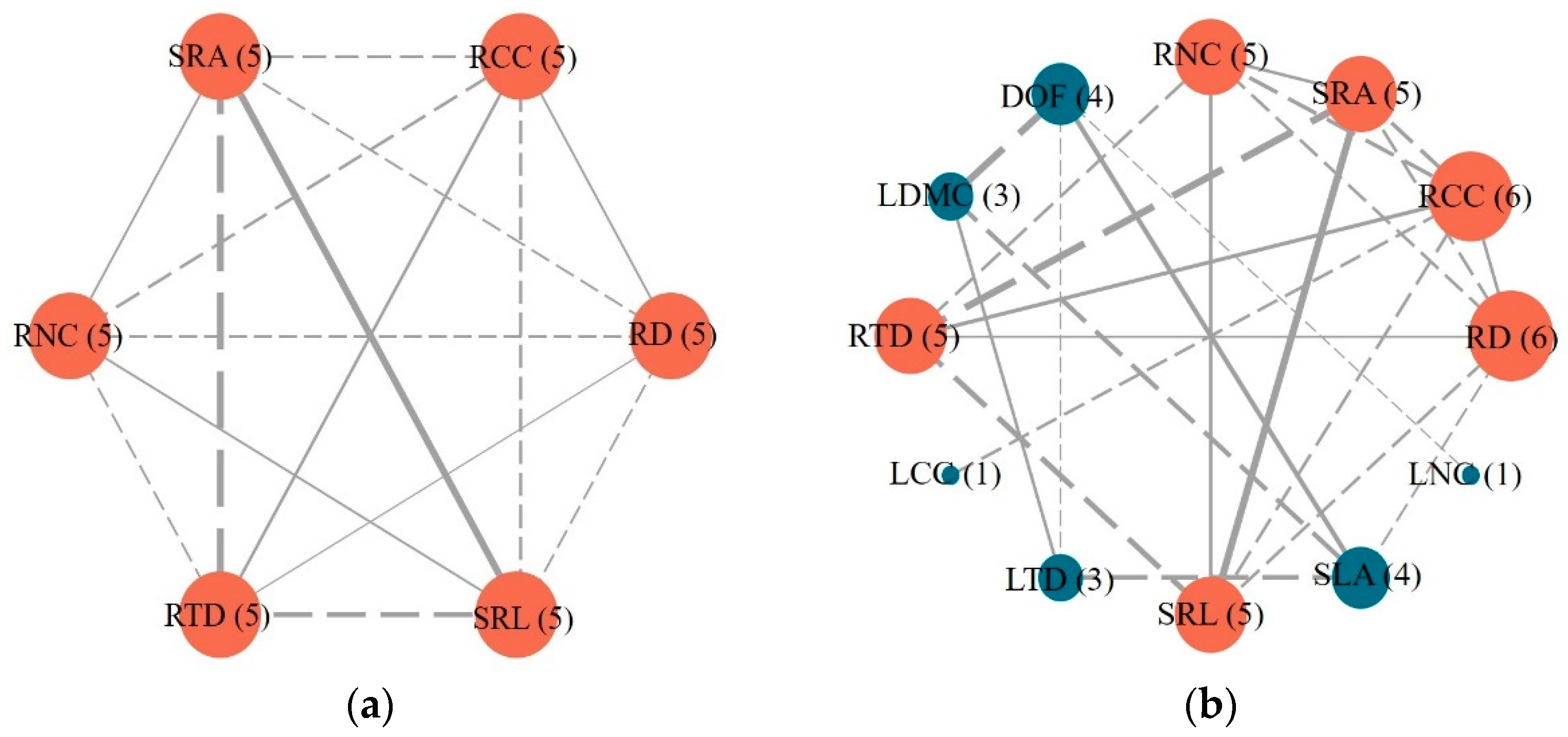
3.2. Trait Linkages between the Fine Roots of Different Orders and Leaves
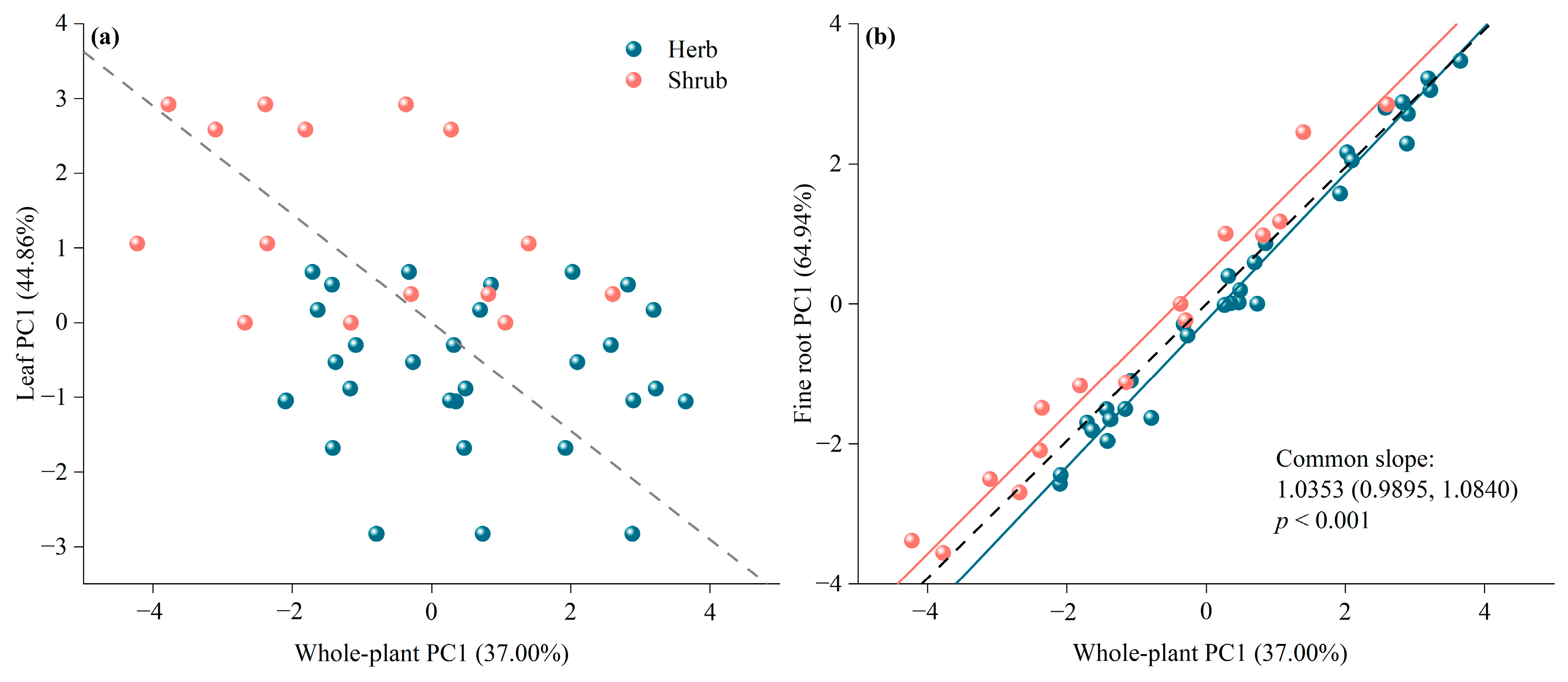
3.3. Leaf, Root, and Whole Plant Economics Spectrum
3.4. Linkages among Roots, Leaves, and Whole Plant Economics Spectrum of 15 Plant Species
4. Discussion
4.1. Differences and Associations between Fine Root Traits and Leaf Traits in Different Root Orders
4.2. Characterization of the Economics Spectrum of 15 Plant Resources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.T.; Morgan, H.D.; Heijden, M.G.A.V.D.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Huang, Z.; Wang, D.; Liu, G. Variations in the traits of fine roots of different orders and their associations with leaf traits in 12 co-occuring plant species in a semiarid inland dune. Plant Soil 2022, 472, 193–206. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Q.; Ge, W.; Liu, Q.; Kong, D.; Yin, H. Coordination of leaf and root economic space in alpine coniferous forests on the Tibetan Plateau. Plant Soil 2024, 496, 555–568. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.T.; Pan, X.; Cornelissen, J.H.C.; Li, Y.; Dong, M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, C.; Fine, P.V.A.; Baraloto, C. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M. Patterns in root trait variation among 25 co-existing North American forest species. New Phytol. 2009, 182, 919–928. [Google Scholar] [CrossRef]
- Li, J.; Le, X.; Chen, X.; Reich, P.B.; Niklas, K.J.; Li, X.; Wu, P.; Zhou, Y.; Zhong, Q.; Hu, D.; et al. Divergent intra- and interspecific root order variability identifies a two-dimensional root economics spectrum. Plant Soil 2024, 499, 473–490. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef]
- Shen, Y.; Gilbert, G.S.; Li, W.; Fang, M.; Lu, H.; Yu, S. Linking aboveground traits to root traits and local environment: Implications of the plant economics spectrum. Front. Plant Sci. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; Van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Hendrick, R.L.; Pregitzer, K.S. The demography of fine roots in a Northern hardwood forest. Ecology 1992, 73, 1094–1104. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Weemstra, M.; Zambrano, J.; Allen, D.; Umaña, M.N. Tree growth increases through opposing above-ground and below-ground resource strategies. J. Ecol. 2021, 109, 3502–3512. [Google Scholar] [CrossRef]
- Long, Y.; Kong, D.; Chen, Z.; Zeng, H. Variation of the linkage of root function with root branch order. PLoS ONE 2013, 8, e57153. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Deforest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Beyer, F.; Hertel, D.; Leuschner, C. Fine root morphological and functional traits in Fagus sylvatica and Fraxinus excelsior saplings as dependent on species, root order and competition. Plant Soil 2013, 373, 143–156. [Google Scholar] [CrossRef]
- McCormack, M.L.; Kaproth, M.A.; Cavender-Bares, J.; Carlson, E.; Hipp, A.L.; Han, Y.; Kennedy, P.G. Climate and phylogenetic history structure morphological and architectural trait variation among fine-root orders. New Phytol. 2020, 228, 1824–1834. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, D.; Bona, C.; Carta, A. Coordination between leaf and root traits in Mediterranean coastal dune plants. Plant Biol. J. 2023, 25, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Valverde-Barrantes, O.J.; Weemstra, M.; Cahill, J.F.; Wang, Z.; He, D.; Chen, Y.; Chu, C.; Wang, Y. Leaf and root traits are partially coordinated but they show contrasting multi-trait-based community trait dispersion patterns in a subtropical forest. J. Plant Ecol. 2024, 17, rtad045. [Google Scholar] [CrossRef]
- Jiang, X.; Jia, X.; Gao, S.; Jiang, Y.; Wei, N.; Han, C.; Zha, T.; Liu, P.; Tian, Y.; Qin, S. Plant nutrient contents rather than physical traits are coordinated between leaves and roots in a desert shrubland. Front. Plant Sci. 2021, 12, 734775. [Google Scholar] [CrossRef]
- Weemstra, M.; Roumet, C.; Cruz-Maldonado, N.; Anthelme, F.; Stokes, A.; Freschet, G.T. Environmental variation drives the decoupling of leaf and root traits within species along an elevation gradient. Ann. Bot. 2022, 130, 419–430. [Google Scholar] [CrossRef]
- Burton, J.I.; Perakis, S.S.; Brooks, J.R.; Puettmann, K.J. Trait integration and functional differentiation among co-existing plant species. Am. J. Bot. 2020, 107, 628–638. [Google Scholar] [CrossRef]
- Isaac, M.E.; Martin, A.R.; De Melo Virginio Filho, E.; Rapidel, B.; Roupsard, O.; Van Den Meersche, K. Intraspecific trait variation and coordination: Root and leaf economics spectra in coffee across environmental gradients. Front. Plant Sci. 2017, 8, 1196. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate lne-fitting methods for allometry. Biol. Rev. 2006, 81, 259–291. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Niklas, K.J.; Sun, J.; Wang, Z.; Zhong, Q.; Hu, D.; Cheng, D. A Whole-plant economics spectrum including bark functional traits for 59 subtropical woody plant species. J. Ecol. 2022, 110, 248–261. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Miyatani, K.; Tanikawa, T.; Makita, N.; Hirano, Y. Relationships between specific root length and respiration rate of fine roots across stands and seasons in Chamaecyparis obtusa. Plant Soil 2018, 423, 215–227. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, H.; Eissenstat, D.M.; Guo, D. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Glob. Ecol. Biogeogr. 2013, 22, 846–856. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Sun, J.H.; Shi, H.L.; Chen, K.Y.; Ji, B.M.; Zhang, J. Research advances on trade-off relationships of plant fine root functional traits. Chin. J. Plant Ecol. 2023, 47, 1055–1070. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, X.; Yang, X.; Liu, G.; Liu, X.; Song, Y.; Zhang, M.; Cui, L.; Dong, M. Is there coordination of leaf and fine root traits at local scales? A test in temperate forest swamps. Ecol. Evol. 2019, 9, 8714–8723. [Google Scholar] [CrossRef]
- Chen, G.; Hobbie, S.E.; Reich, P.B.; Yang, Y.; Robinson, D. Allometry of fine roots in forest ecosystems. Ecol. Lett. 2019, 22, 322–331. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Blackwood, C.B. Fine root morphology is phylogenetically structured, but nitrogen is related to the plant economics spectrum in temperate trees. Funct. Ecol. 2015, 29, 796–807. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chu, P.; Chen, D.; Bai, Y. Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Funct. Ecol. 2016, 30, 985–997. [Google Scholar] [CrossRef]
- Han, F.; Zhang, J.; Xia, L.; Gao, J.; Ji, H.; Li, H.; Liu, B. Effects of mycorrhizal type on the correlation of root and leaf functional traits of main tree species in subtropical forests. Chin. J. Ecol. 2023, 42, 838–845. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cahill, J.F. Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS ONE 2011, 6, e19992. [Google Scholar] [CrossRef]
- Holdaway, R.J.; Richardson, S.J.; Dickie, I.A.; Peltzer, D.A.; Coomes, D.A. Species- and community-level patterns in fine root traits along a 120,000-year soil chronosequence in temperate rain forest. J. Ecol. 2011, 99, 954–963. [Google Scholar] [CrossRef]
- Wang, M.; Wan, P.; Guo, J.; Xu, J.; Chai, Y.; Yue, M. Relationships among leaf, stem and root traits of the dominant shrubs from four vegetation zones in Shaanxi Province, China. Isr. J. Ecol. Evol. 2017, 63, 25–32. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Oleksyn, J.; Eissenstat, D.M.; Reich, P.B. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 2010, 162, 505–513. [Google Scholar] [CrossRef]
- Kurze, S.; Engelbrecht, B.M.J.; Bilton, M.C.; Tielbörger, K.; Álvarez-Cansino, L. Rethinking the plant economics spectrum for annuals: A multi-species study. Front. Plant Sci. 2021, 12, 640862. [Google Scholar] [CrossRef]
- Yu, J.; Song, Z.; Hou, J. Life form-dependent nitrogen–phosphorous allocation strategies of leaf and fine root in a temperate natural forest under long-term nitrogen addition. J. Plant Ecol. 2023, 16, rtad013. [Google Scholar] [CrossRef]
- Comas, L.H.; Mueller, K.E.; Taylor, L.L.; Midford, P.E.; Callahan, H.S.; Beerling, D.J. Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int. J. Plant Sci. 2012, 173, 584–595. [Google Scholar] [CrossRef]
- Lu, B.; Qian, J.; Hu, J.; Wang, P.; Jin, W.; Tang, S.; He, Y.; Zhang, C. The role of fine root morphology in nitrogen uptake by riparian plants. Plant Soil 2022, 472, 527–542. [Google Scholar] [CrossRef]
- Kong, D.L.; Wang, J.J.; Kardol, P.; Wu, H.F.; Zeng, H.; Deng, X.B.; Deng, Y. Economic strategies of plant absorptive roots vary with root diameter. Biogeosciences 2016, 13, 415–424. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Lu, Q.; Ma, J.; Shen, Y.; Wang, G. Different responses of leaf and root economics spectrum to grazing time at the community level in desert steppe, China. Sci. Total Environ. 2024, 909, 168547. [Google Scholar] [CrossRef] [PubMed]
- He, J.S.; Wang, L.; Flynn, D.F.B.; Wang, X.; Ma, W.; Fang, J. Leaf nitrogen: Phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhao, W.; Zhou, H.; Wang, Z. The features of main osmolytes, silicon and their coupling effects in improving drought resistance of the typical xerophytes in the desert areas of Northwest China. Land. Degrad. Dev. 2020, 31, 2720–2733. [Google Scholar] [CrossRef]
- Ren, L.; Huang, Y.; Pan, Y.; Xiang, X.; Huo, J.; Meng, D.; Wang, Y.; Yu, C. Differential investment strategies in leaf economic traits across climate regions worldwide. Front. Plant Sci. 2022, 13, 798035. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Guo, S.; Li, Z.; He, M.; Zhang, Y.; Li, G.; Han, X.; Yang, G. Divergent patterns and drivers of leaf functional traits of Robinia pseudoacacia and Pinus tabulaeformis plantations along a precipitation gradient in the Loess plateau, China. J. Environ. Manag. 2023, 348, 119318. [Google Scholar] [CrossRef]
- Vleminckx, J.; Fortunel, C.; Valverde-Barrantes, O.; Paine, C.E.T.; Engel, J.; Petronelli, P.; Dourdain, A.K.; Guevara, J.; Béroujon, S.; Baraloto, C. Resolving whole-plant economics from leaf, stem and root traits of 1467 Amazonian tree species. Oikos 2021, 130, 1193–1208. [Google Scholar] [CrossRef]
- Dorrepaal, E. Are plant growth-form-based classifications useful in predicting northern ecosystem carbon cycling feedbacks to climate change? J. Ecol. 2007, 95, 1167–1180. [Google Scholar] [CrossRef]
- Carmona, C.P.; Bueno, C.G.; Toussaint, A.; Träger, S.; Díaz, S.; Moora, M.; Munson, A.D.; Pärtel, M.; Zobel, M.; Tamme, R. Fine-root traits in the global spectrum of plant form and function. Nature 2021, 597, 683–687. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Lin, Z.; Zhao, Y.; Yan, Z.; Zhang, K.; Visser, M.; Townsend, P.A.; Wu, J. Spectra-phenology integration for high-resolution, accurate, and scalable mapping of foliar functional traits using time-series sentinel-2 data. Remote Sens. Environ. 2024, 305, 114082. [Google Scholar] [CrossRef]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Lusk, C.H. Predicting leaf physiology from simple plant and climate attributes: A global Glopnet analysis. Ecol. Appl. 2007, 17, 1982–1988. [Google Scholar] [CrossRef]
- Rowe, N.; Speck, T. Plant growth forms: An ecological and evolutionary perspective. New Phytol. 2005, 166, 61–72. [Google Scholar] [CrossRef]


| Organ | Functional Trait | Functions | Acronym | Unit |
|---|---|---|---|---|
| Leaf | Leaf dry matter content | Defense | LDMC | mg/g |
| Specific leaf area | Resource capture | SLA | cm2/g | |
| Leaf tissue density | Defense | LTD | g/cm3 | |
| Degree of fleshiness | Defense | DOF | / | |
| Leaf carbon concentration | Defense | LCC | mg/g | |
| Leaf nitrogen concentration | Resource capture | LNC | mg/g | |
| Fine root | Root diameter | Transport, construction, defense | RD | mm |
| Specific root length | Resource capture | SRL | cm/g | |
| Specific root area | Resource capture | SRA | cm2/g | |
| Root tissue density | Transport, construction, defense | RTD | g/cm3 | |
| Root carbon concentration | Transport, construction, defense | RCC | mg/g | |
| Root nitrogen concentration | Resource capture | RNC | mg/g |
| Root Trait | Root Order (RO) | Growth Form (GF) | RO × GF | |||
|---|---|---|---|---|---|---|
| F | p Value | F | p Value | F | p Value | |
| RD | 10.864 | <0.001 | 7.337 | 0.010 | 0.710 | 0.498 |
| SRL | 34.548 | <0.001 | 5.523 | 0.024 | 1.777 | 0.183 |
| SRA | 95.946 | <0.001 | 12.582 | 0.001 | 2.219 | 0.122 |
| RTD | 41.99 | <0.001 | 2.400 | 0.129 | 1.029 | 0.367 |
| RCC | 8.128 | 0.001 | 5.232 | 0.028 | 0.016 | 0.984 |
| RNC | 7.307 | 0.002 | 0.056 | 0.814 | 0.014 | 0.986 |
| Traits | Fine Root | Leaf | Whole-Plant | |||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | |
| RD | −0.659 ** | 0.434 ** | / | / | −0.698 ** | 0.183 |
| SRL | 0.899 ** | 0.367 * | / | / | 0.867 ** | 0.198 |
| SRA | 0.920 ** | 0.351 * | / | / | 0.893 ** | 0.196 |
| RTD | −0.858 ** | −0.272 | / | / | −0.815 ** | −0.339 * |
| RCC | −0.760 ** | 0.364 * | / | / | −0.748 ** | −0.237 |
| RNC | 0.702 ** | −0.460 ** | / | / | 0.690 ** | 0.066 |
| LDMC | / | / | 0.840 ** | 0.097 | −0.215 | 0.799 ** |
| SLA | / | / | −0.889 ** | 0.019 | 0.377 * | −0.815 ** |
| LTD | / | / | 0.833 ** | −0.205 | −0.360 * | 0.728 ** |
| LCC | / | / | −0.058 | 0.785 ** | 0.123 | 0.094 |
| LNC | / | / | 0.224 | 0.681 ** | 0.038 | 0.290 |
| Growth Form (GF) | Root Order (RO) | GF × RO | |||||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Root traits | PC1 | 15.398 | <0.001 | 95.871 | <0.001 | 0.341 | 0.713 |
| PC2 | 0.162 | 0.689 | 0.867 | 0.428 | 0.666 | 0.519 | |
| Leaf traits | PC1 | 11.146 | 0.005 | / | / | / | / |
| PC2 | 3.209 | 0.097 | / | / | / | / | |
| Whole-plant traits | PC1 | 36.552 | <0.001 | 77.035 | <0.001 | 0.248 | 0.782 |
| PC2 | 18.473 | <0.001 | 2.604 | 0.087 | 0.049 | 0.952 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ma, J.; Wang, H.; Xie, T.; Li, Q.; Shan, L. Fine Root Traits across Different Root Orders and Their Associations with Leaf Traits in 15 Co-Occurring Plant Species from the Desert–Oasis Transition Zone in the Hexi Corridor, Gansu Province, China. Plants 2024, 13, 2472. https://doi.org/10.3390/plants13172472
Chen Y, Ma J, Wang H, Xie T, Li Q, Shan L. Fine Root Traits across Different Root Orders and Their Associations with Leaf Traits in 15 Co-Occurring Plant Species from the Desert–Oasis Transition Zone in the Hexi Corridor, Gansu Province, China. Plants. 2024; 13(17):2472. https://doi.org/10.3390/plants13172472
Chicago/Turabian StyleChen, Yiming, Jing Ma, Hongyong Wang, Tingting Xie, Quangang Li, and Lishan Shan. 2024. "Fine Root Traits across Different Root Orders and Their Associations with Leaf Traits in 15 Co-Occurring Plant Species from the Desert–Oasis Transition Zone in the Hexi Corridor, Gansu Province, China" Plants 13, no. 17: 2472. https://doi.org/10.3390/plants13172472
APA StyleChen, Y., Ma, J., Wang, H., Xie, T., Li, Q., & Shan, L. (2024). Fine Root Traits across Different Root Orders and Their Associations with Leaf Traits in 15 Co-Occurring Plant Species from the Desert–Oasis Transition Zone in the Hexi Corridor, Gansu Province, China. Plants, 13(17), 2472. https://doi.org/10.3390/plants13172472






