Ergothioneine Improves Seed Yield and Flower Number through FLOWERING LOCUS T Gene Expression in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
2.1. Effect of EGT on Seed Yield of A. thaliana
2.2. Growth of A. thaliana at the Reproductive Growth Stage
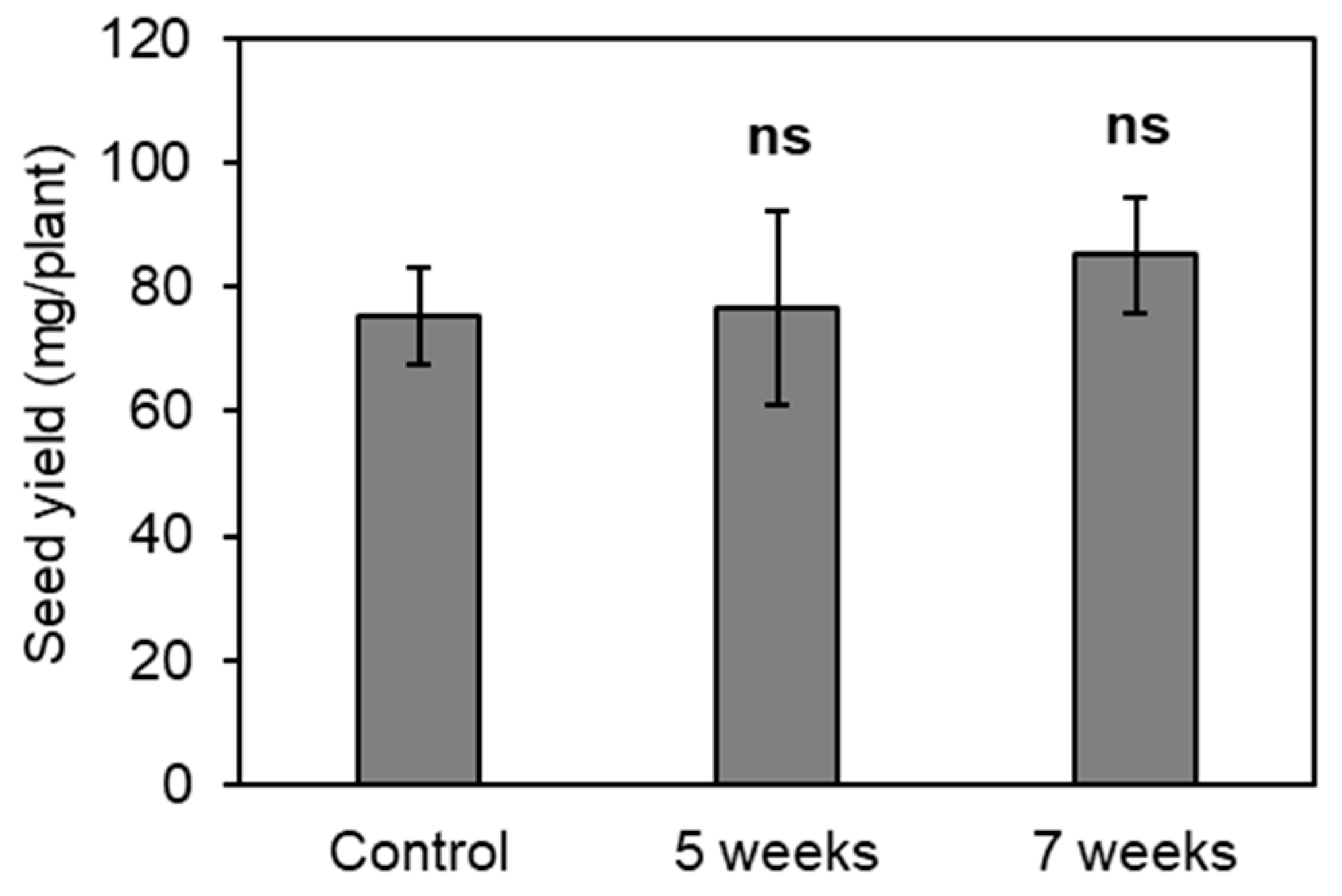
2.3. Effect of EGT Application at the Reproductive Growth Stage on Seed Yield
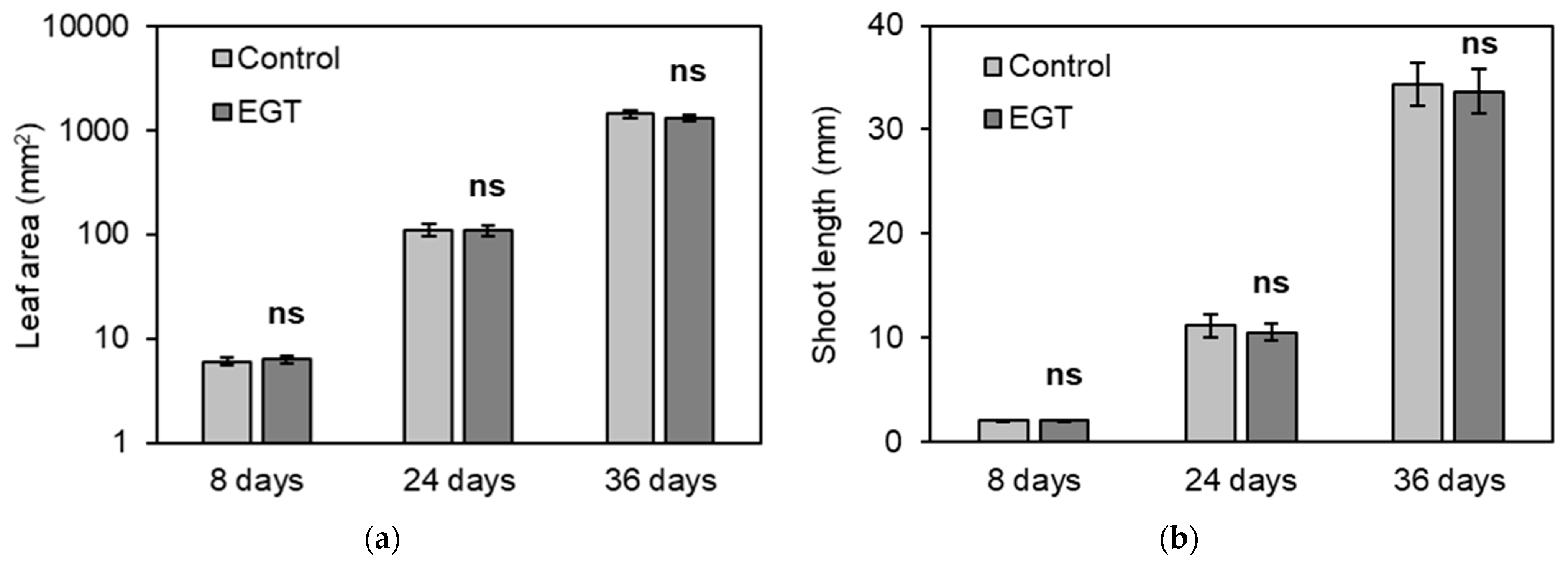
2.4. Effects of EGT on Vegetative Organs of A. thaliana at the Vegetative Growth Stage
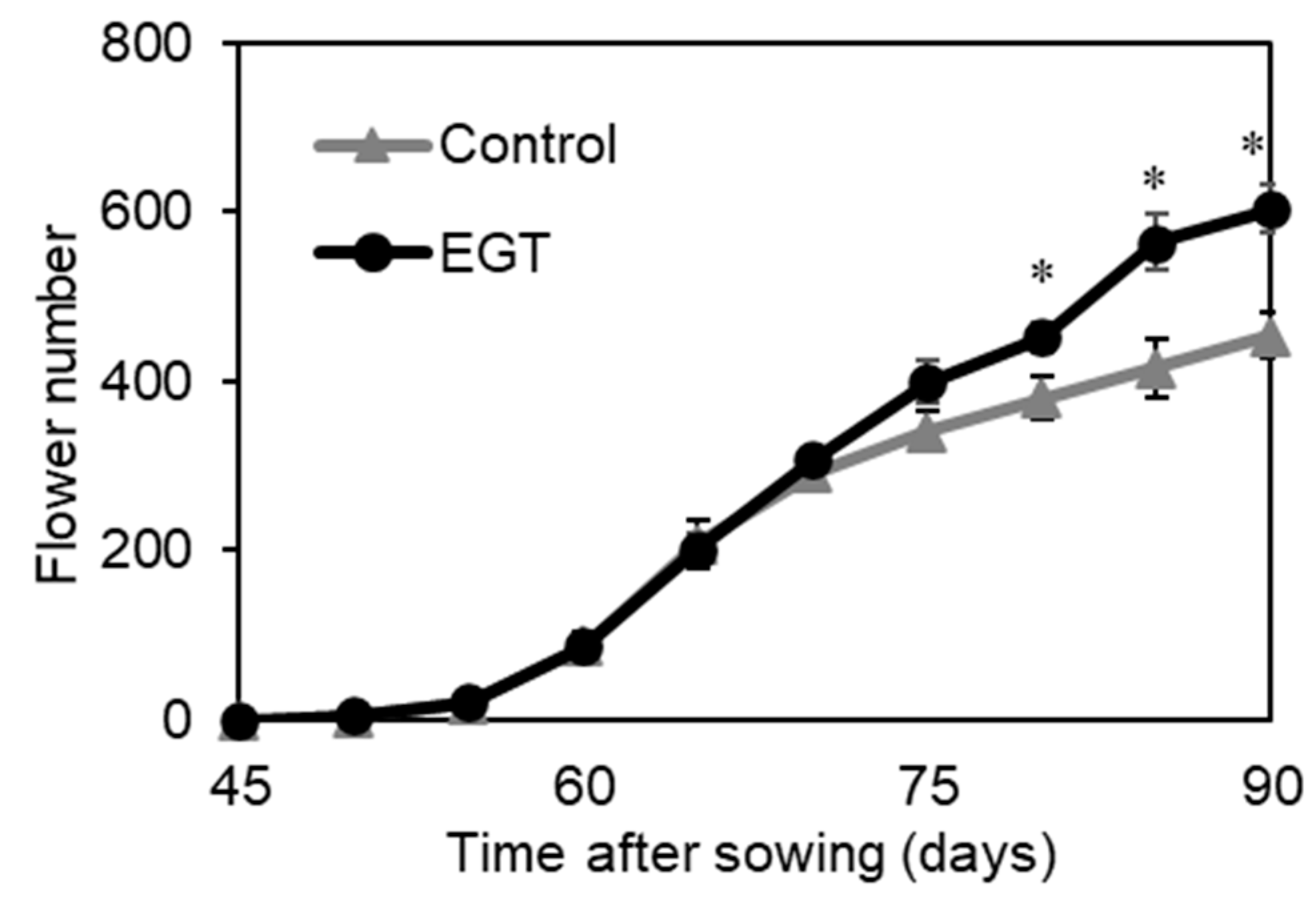
2.5. Effect of EGT on Flower Number
2.6. Effect of EGT on Gene Expression of Flowering Hormone
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Application Conditions
4.3. Growth Parameters
4.4. RNA Extraction and cDNA Synthesiss
4.5. RT-qPCR Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The Futuristic Sustainable Approach for Alleviating Crop Productivity and Abiotic Stress Tolerance. J. Plant Growth Regul. 2023, 43, 659–674. [Google Scholar] [CrossRef]
- Rakkammal, K.; Maharajan, T.; Ceasar, S.A.; Ramesh, M. Biostimulants and their role in improving plant growth under drought and salinity. Cereal Res. Commun. 2023, 51, 61–74. [Google Scholar] [CrossRef]
- García-García, A.L.; García-Machado, F.J.; Borges, A.A.; Morales-Sierra, S.; Boto, A.; Jiménez-Arias, D. Pure Organic Active Compounds Against Abiotic Stress: A Biostimulant Overview. Front. Plant Sci. 2020, 11, 575829. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino Acids Biostimulants and Protein Hydrolysates in Agricultural Sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Lardos, M.; Marmagne, A.; Bottino, N.B.; Caris, Q.; Béal, B.; Chardon, F.; Masclaux-Daubresse, C. Discovery of the biostimulant effect of asparagine and glutamine on plant growth in Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1281495. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; García-García, A.L.; Herrera, A.J.; Valdés, F.; Luis, J.C.; Borges, A.A. A Beginner’s Guide to Osmoprotection by Biostimulants. Plants 2021, 10, 363. [Google Scholar] [CrossRef]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef]
- Sato, S.; Saika, A.; Ushimaru, K.; Koshiyama, T.; Higashiyama, Y.; Fukuoka, T.; Morita, T. Biosynthetic ability of diverse basidiomycetous yeast strains to produce the natural antioxidant ergothioneine. AMB Express 2024, 14, 20. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta 2012, 1822, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; Castaldo, D.; Casale, R.; D’Onofrio, N.; Giovane, A.; Cautela, D.; Balestrieri, M.L. An uncommon redox behavior sheds light on the cellular antioxidant properties of ergothioneine. Free Radic. Biol. Med. 2015, 79, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.T.; Shen, L. Ergothioneine as a Natural Antioxidant Against Oxidative Stress-Related Diseases. Front. Pharmacol. 2022, 13, 850813. [Google Scholar] [CrossRef] [PubMed]
- Melville, D.B.; Eich, S. The occurrence of ergothioneine in plant material. J. Biol. Chem. 1956, 218, 647–651. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, W.Y.; Kim, S.T.; Ahn, J.K.; Bae, E.K. Ergothioneine accumulation in a medicinal plant Gastrodia elata. J. Med. Plant Res. 2010, 4, 1141–1147. [Google Scholar] [CrossRef]
- Matsuo, M.; Satomura, Y. Physiological Effect of Sclerothionine on Plant Growth and Phospholipases Formation. Agric. Biol. Chem. 1968, 32, 611–616. [Google Scholar] [CrossRef]
- Leijten, W.; Koes, R.; Roobeek, I.; Frugis, G. Translating Flowering Time from Arabidopsis thaliana to Brassicaceae and Asteraceae Crop Species. Plants 2018, 7, 111. [Google Scholar] [CrossRef]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Krienger, U.; Lippman, Z.B.; Zamir, D. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield tomato. Nat. Genet. 2010, 42, 459–463. [Google Scholar] [CrossRef]
- Dookie, M.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci. Hortic. 2021, 276, 109715. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Trovato, M. Proline Affects Flowering Time in Arabidopsis by Modulating FLC Expression: A Clue of Epigenetic Regulation? Plants 2022, 11, 2348. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.S.; Wong, S.K.; Ismail, N.H.; Zengin, G.; Duangjai, A.; Saokaew, S.; Phisalprapa, P.; Tan, K.W.; Goh, B.H.; Tang, S.Y. Mitigation of Environmental Stress-Impacts in Plants: Role of Sole and Combinatory Exogenous Application of Glutathione. Front. Plant Sci. 2021, 12, 791205. [Google Scholar] [CrossRef]
- Ogawa, K.; Tasaka, Y.; Mino, M.; Tanaka, Y.; Iwabuchi, M. Association of Glutathione with Flowering in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Ionescu, I.A.; Møller, B.L.; Sánchez-Pérez, R. Chemical control of flowering time. J. Exp. Bot. 2017, 68, 369–382. [Google Scholar] [CrossRef]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering. Agronomy 2019, 9, 482. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]




| Gene Name | Locus | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| FLOWERING LOCUS T | AT1G65480 | CTACAACTGGAACAACCTTTGGC | CGAGTGTTGAAGTTCTGGCG |
| UBC21 | AT5G25760 | TCCTCTTAACTGCGACTCAGG | GCGAGGCGTGTATACATTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshiyama, T.; Higashiyama, Y.; Mochizuki, I.; Yamada, T.; Kanekatsu, M. Ergothioneine Improves Seed Yield and Flower Number through FLOWERING LOCUS T Gene Expression in Arabidopsis thaliana. Plants 2024, 13, 2487. https://doi.org/10.3390/plants13172487
Koshiyama T, Higashiyama Y, Mochizuki I, Yamada T, Kanekatsu M. Ergothioneine Improves Seed Yield and Flower Number through FLOWERING LOCUS T Gene Expression in Arabidopsis thaliana. Plants. 2024; 13(17):2487. https://doi.org/10.3390/plants13172487
Chicago/Turabian StyleKoshiyama, Tatsuyuki, Yukihiro Higashiyama, Izumi Mochizuki, Tetsuya Yamada, and Motoki Kanekatsu. 2024. "Ergothioneine Improves Seed Yield and Flower Number through FLOWERING LOCUS T Gene Expression in Arabidopsis thaliana" Plants 13, no. 17: 2487. https://doi.org/10.3390/plants13172487




