Development and Characterization of a New TILLING Population for Forward and Reverse Genetics in Barley (Hordeum vulgare L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Generation of EMS-Mutagenized Population
2.3. Establishment of the CEL I Enzyme Digestion System
2.4. Screening of the Mutation Lines
2.5. Phenotype Experiment
3. Results
3.1. Generation of a TILLING Population in the Barley Cultivar “TX9425”
3.2. Mutant Types and Mutation Frequency Observed in the M2 Population
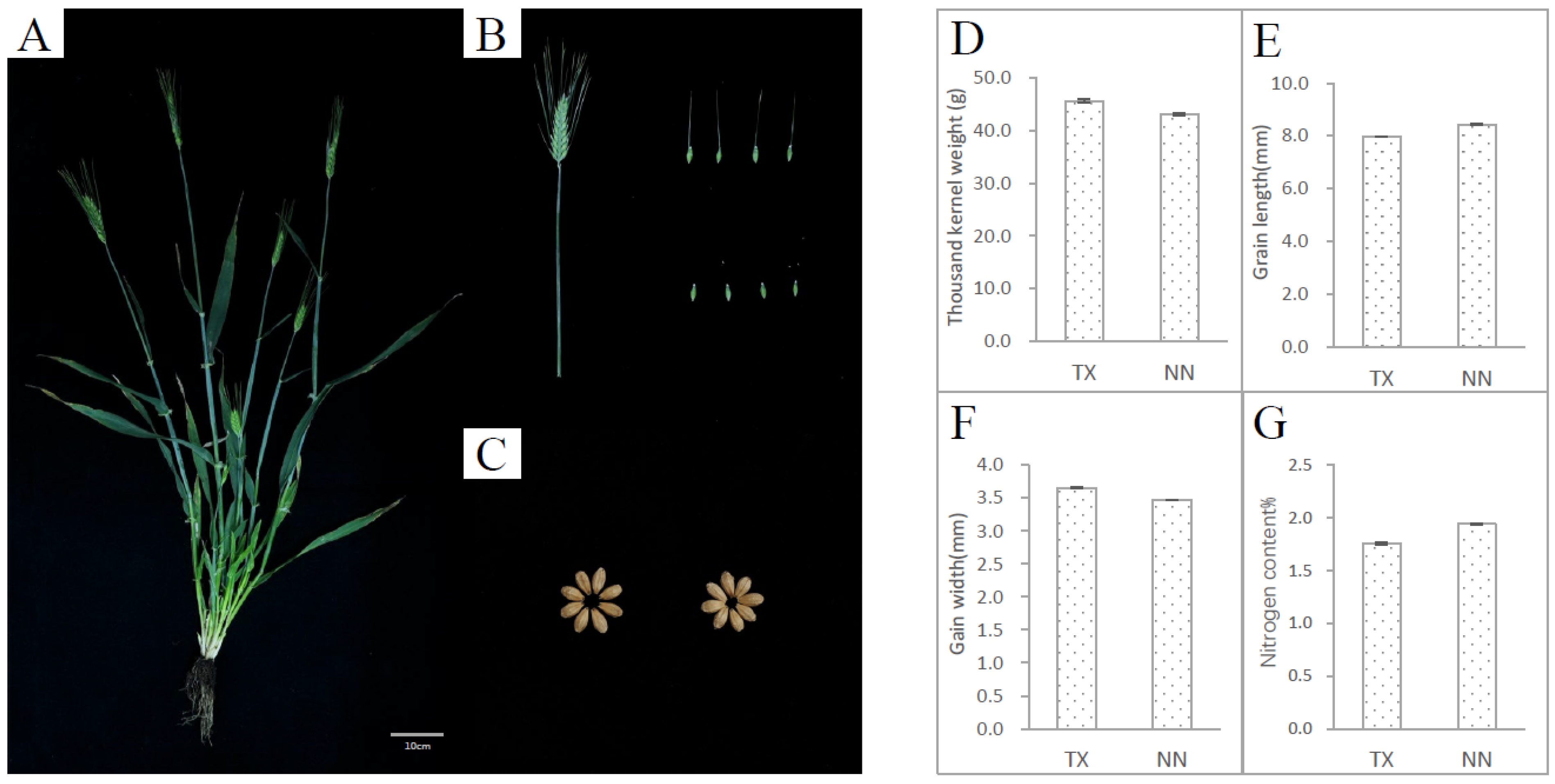
3.3. The Main Agronomic Traits of Stable Mutant Lines in the M3 Generation
3.4. Mutant Screening
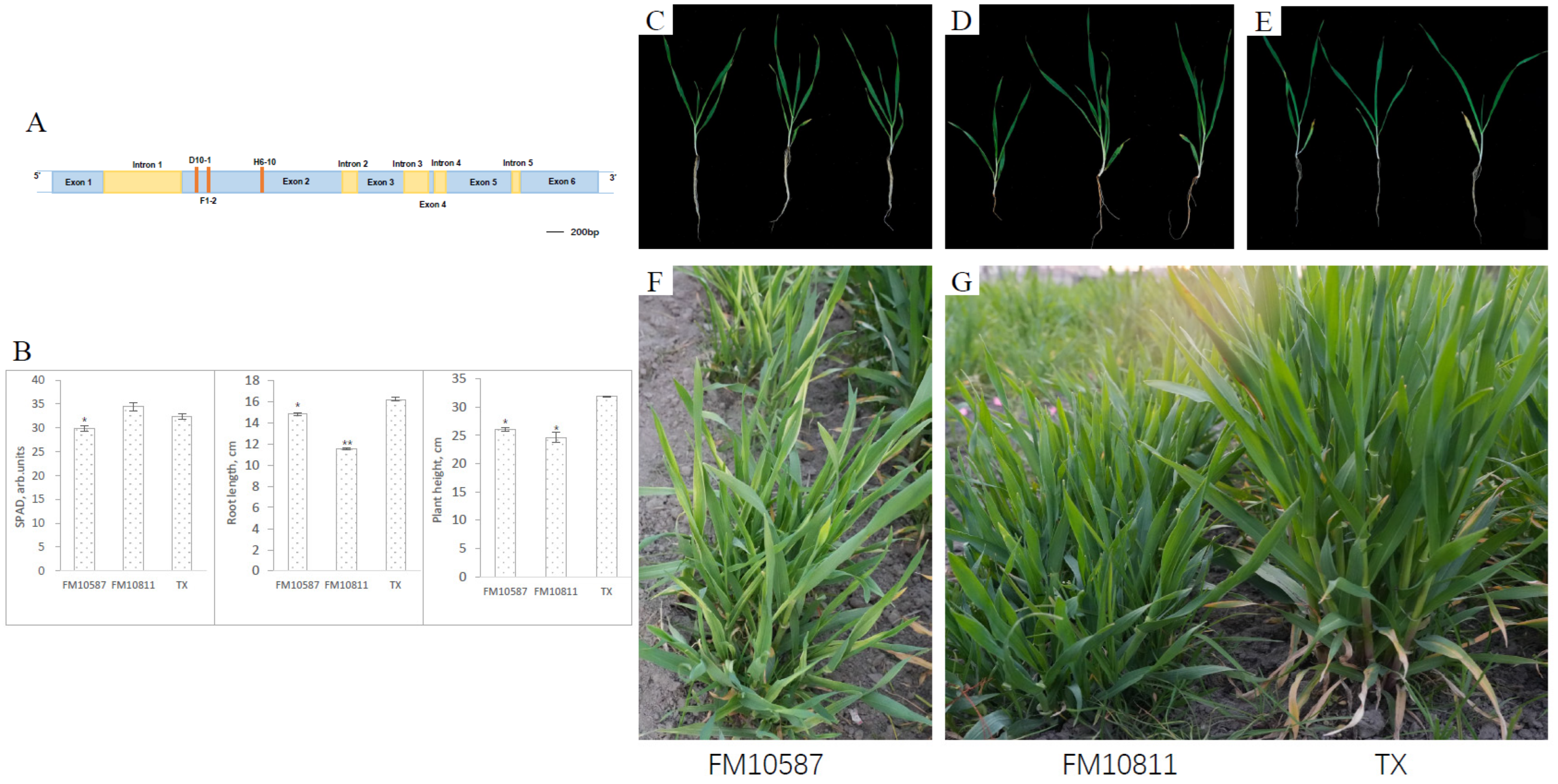
3.5. New Alleles of Functional Mutations in the Gene HvGLR3.5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bothmer, R.V.; Jacobsen, N.H.G.; Baden, C.; Jørgensen, R.B.; Linde-Laursen, I.B. An Ecogeographical Study of the Genus Hordeum; IBPGR: Rome, Italy, 1992. [Google Scholar]
- Dai, F.; Zhang, G. Domestication and Improvement of Cultivated Barley. In Exploration, Identification and Utilization of Barley Germplasm; Zhang, G., Li, C., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Knüpffer, H. Triticeae Genetic Resources in ex situ Genebank Collections. In Genetics and Genomics of the Triticeae; Muehlbauer, G.J., Feuillet, C., Eds.; Springer: New York, NY, USA, 2009; pp. 31–79. [Google Scholar] [CrossRef]
- Stanca, A.M.; Romagosa, I.; Takeda, K.; Lundborg, T.; Terzi, V.; Cattivelli, L. Chapter 9 Diversity in Abiotic Stress Tolerances. Dev. Plant Genet. Breed. 2003, 7, 179–199. [Google Scholar] [CrossRef]
- Kharkwal, M.C. History of Plant Mutation Breeding and Global Impact of Mutant Varieties. In Mutation Breeding for Sustainable Food Production and Climate Resilience; Penna, S., Jain, S.M., Eds.; Springer: Singapore, 2023; pp. 25–55. [Google Scholar] [CrossRef]
- Harten, A.M.V. Mutation Breeding: Theory and Practical Applications; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Shahwar, D.; Ahn, N.; Kim, D.; Ahn, W.; Park, Y. Mutagenesis-based plant breeding approaches and genome engineering: A review focused on tomato. Mutat. Res./Rev. Mutat. Res. 2023, 792, 108473. [Google Scholar] [CrossRef] [PubMed]
- McCallum, C.M.; Comai, L.; Greene, E.A.; Henikoff, S. Targeted screening for induced mutations. Nat. Biotechnol. 2000, 18, 455–457. [Google Scholar] [CrossRef]
- Till, B.J.; Zerr, T.; Comai, L.; Henikoff, S. A protocol for TILLING and ecotilling in plants and animals. Nat. Protoc. 2006, 1, 2465–2477. [Google Scholar] [CrossRef]
- Caldwell, D.G.; McCallum, N.; Shaw, P.; Muehlbauer, G.J.; Marshall, D.F.; Waugh, R. A structured mutant population for forward and reverse genetics in barley (Hordeum vulgare L.). Plant J. Cell Mol. Biol. 2004, 40, 143–150. [Google Scholar] [CrossRef]
- Schreiber, M.; Barakate, A.; Uzrek, N.; Macaulay, M.; Sourdille, A.; Morris, J.; Hedley, P.E.; Ramsay, L.; Waugh, R. A highly mutagenised barley (cv. Golden Promise) TILLING population coupled with strategies for screening-by-sequencing. Plant Methods 2019, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, S.; Bauer, P.; Komatsuda, T.; Lundqvist, U.; Stein, N. TILLING in the two-rowed barley cultivar ‘Barke’ reveals preferred sites of functional diversity in the gene HvHox1. BMC Res. Notes 2009, 2, 258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lei, M.; Guo, Y.; Gao, G.; Shi, L.; Jin, Y.; Cai, Y.; Himmelbach, A.; Zhou, S.; He, Q.; et al. A reference-guided TILLING by amplicon-sequencing platform supports forward and reverse genetics in barley. Plant Commun. 2022, 3, 100317. [Google Scholar] [CrossRef]
- Yang, P.; Lüpken, T.; Habekuss, A.; Hensel, G.; Steuernagel, B.; Kilian, B.; Ariyadasa, R.; Himmelbach, A.; Kumlehn, J.; Scholz, U.; et al. PROTEIN DISULFIDE ISOMERASE LIKE 5-1 is a susceptibility factor to plant viruses. Proc. Natl. Acad. Sci. USA 2014, 111, 2104–2109. [Google Scholar] [CrossRef]
- van Esse, G.W.; Walla, A.; Finke, A.; Koornneef, M.; Pecinka, A.; von Korff, M. Six-owed Spike3 (VRS3) is a histone demethylase that controls lateral spikelet development in barley. Plant Physiol. 2017, 174, 2397–2408. [Google Scholar] [CrossRef]
- Poursarebani, N.; Trautewig, C.; Melzer, M.; Nussbaumer, T.; Lundqvist, U.; Rutten, T.; Schmutzer, T.; Brandt, R.; Himmelbach, A.; Altschmied, L.; et al. COMPOSITUM 1 contributes to the architectural simplification of barley inflorescence via meristem identity signals. Nat. Commun. 2020, 11, 5138. [Google Scholar] [CrossRef]
- Kjeldahl, J.Z. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Li, C.; Johnson, P.; Lu, C.; Zhou, M. A single locus is responsible for salinity tolerance in a Chinese landrace barley (Hordeum vulgare L.). PLoS ONE 2012, 7, e43079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.X.; Johnson, P.; Zhou, G.F.; Li, C.D.; Lance, R. Quantitative trait loci for waterlogging tolerance in a barley cross of Franklin x YuYaoXiangTian Erleng and the relationship between waterlogging and salinity tolerance. Crop Sci. 2012, 52, 2082–2088. [Google Scholar] [CrossRef]
- Pang, J.Y.; Zhou, M.X.; Mendham, N.; Shabala, S. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust. J. Agric. Res. 2004, 55, 895–906. [Google Scholar] [CrossRef]
- Zhou, M.X.; Li, H.B.; Mendham, N.J. Combining ability of waterlogging tolerance in barley. Crop Sci. 2007, 47, 278–284. [Google Scholar] [CrossRef]
- Li, H.B.; Zhou, M.X.; Liu, C.J. A major QTL conferring crown rot resistance in barley and its association with plant height. Theor. Appl. Genet. 2009, 118, 903–910. [Google Scholar] [CrossRef]
- Li, H.B.; Zhou, M.X. Quantitative trait loci controlling barley powdery mildew and scald resistances in two different barley doubled haploid populations. Mol. Breed. 2011, 27, 479–490. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; McNeil, D.L.; Zhou, M. Identification and molecular mapping of a dwarfing gene in barley (Hordeum vulgare L.) and its correlation with other agronomic traits. Euphytica 2010, 175, 331–342. [Google Scholar] [CrossRef]
- Chen, G.D.; Li, H.B.; Zheng, Z.; Wei, Y.M.; Zheng, Y.L.; McIntyre, C.L.; Zhou, M.X.; Liu, C.J. Characterization of a QTL affecting spike morphology on the long arm of chromosome 3H in barley (Hordeum vulgare L.) based on near isogenic lines and a NIL-derived population. Theor. Appl. Genet. 2012, 125, 1385–1392. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Zhang, Q.; Zhu, J.; Jia, Q.; Hua, W.; Shang, Y.; Li, C.; Zhou, M. Mapping a major QTL for malt extract of barley from a cross between TX9425 × Naso Nijo. Theor. Appl. Genet. 2015, 128, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.-M.; Chiu, J.; Hsieh, M.-H.; Meisel, L.; Oliveira, I.C.; Shin, M.; Coruzzi, G. Glutamate-receptor genes in plants. Nature 1998, 396, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Recatalà, V. New roles for the GLUTAMATE RECEPTOR-LIKE 3.3, 3.5, and 3.6 genes as on/off switches of wound-induced systemic electrical signals. Plant Signal. Behav. 2016, 11, e1161879. [Google Scholar] [CrossRef]
- Parry, M.A.; Madgwick, P.J.; Bayon, C.; Tearall, K.; Hernandez-Lopez, A.; Baudo, M.; Rakszegi, M.; Hamada, W.; Al-Yassin, A.; Ouabbou, H.; et al. Mutation discovery for crop improvement. J. Exp. Bot. 2009, 60, 2817–2825. [Google Scholar] [CrossRef]
- Jankowicz-Cieslak, J.; Mba, C.; Till, B.J. Mutagenesis for Crop Breeding and Functional Genomics. In Biotechnologies for Plant Mutation Breeding: Protocols; Jankowicz-Cieslak, J., Tai, T.H., Kumlehn, J., Till, B.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar]
- Oleykowski, C.A.; Bronson Mullins, C.R.; Godwin, A.K.; Yeung, A.T. Mutation detection using a novel plant endonuclease. Nucleic Acids Res. 1998, 26, 4597–4602. [Google Scholar] [CrossRef]
- Colbert, T.; Till, B.J.; Tompa, R.; Reynolds, S.; Steine, M.N.; Yeung, A.T.; McCallum, C.M.; Comai, L.; Henikoff, S. High-throughput screening for induced point mutations. Plant Physiol. 2001, 126, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, S.; Till, B.J.; Comai, L. TILLING. Traditional mutagenesis meets functional genomics. Plant Physiol. 2004, 135, 630–636. [Google Scholar] [CrossRef]
- Sestili, F.; Botticella, E.; Bedo, Z.; Phillips, A.; Lafiandra, D. Production of novel allelic variation for genes involved in starch biosynthesis through mutagenesis. Mol. Breed. 2010, 25, 145–154. [Google Scholar] [CrossRef]
- Raghavan, C.; Naredo, M.E.B.; Wang, H.; Atienza, G.; Liu, B.; Qiu, F.; McNally, K.L.; Leung, H. Rapid method for detecting SNPs on agarose gels and its application in candidate gene mapping. Mol. Breed. 2007, 19, 87–101. [Google Scholar] [CrossRef]
- Uauy, C.; Paraiso, F.; Colasuonno, P.; Tran, R.K.; Tsai, H.; Berardi, S.; Comai, L.; Dubcovsky, J. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol. 2009, 9, 115. [Google Scholar] [CrossRef]
- Talamè, V.; Bovina, R.; Sanguineti, M.C.; Tuberosa, R.; Lundqvist, U.; Salvi, S. TILLMore, a resource for the discovery of chemically induced mutants in barley. Plant Biotechnol. J. 2008, 6, 477–485. [Google Scholar] [CrossRef]
- Sparla, F.; Falini, G.; Botticella, E.; Pirone, C.; Talamè, V.; Bovina, R.; Salvi, S.; Tuberosa, R.; Sestili, F.; Trost, P. New starch phenotypes produced by TILLING in barley. PLoS ONE 2014, 9, e107779. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-I.; Tai, T.H. Identification of novel rice low phytic acid mutations via TILLING by sequencing. Mol. Breed. 2014, 34, 1717–1729. [Google Scholar] [CrossRef]
- Moehs, C.P.; Austill, W.J.; Holm, A.; Large, T.A.G.; Loeffler, D.; Mullenberg, J.; Schnable, P.S.; Skinner, W.; van Boxtel, J.; Wu, L.; et al. Development of decreased-gluten wheat enabled by determination of the genetic basis of lys3a barley. Plant Physiol. 2019, 179, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Till, B.J.; Reynolds, S.H.; Weil, C.; Springer, N.; Burtner, C.; Young, K.; Bowers, E.; Codomo, C.A.; Enns, L.C.; Odden, A.R.; et al. Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biol. 2004, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, S.; Comai, L. Single-nucleotide mutations for plant functional genomics. Annu. Rev. Plant Biol. 2003, 54, 375–401. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Liu, X.; Colmer, T.D.; Zhou, M.; Shabala, S. Tissue-specific root ion profiling reveals essential roles of the CAX and ACA calcium transport systems in response to hypoxia in Arabidopsis. J. Exp. Bot. 2016, 67, 3747–3762. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Liu, X.; Colmer, T.D.; Shabala, L.; Salih, A.; Zhou, M.; Shabala, S. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot. 2017, 68, 3191–3204. [Google Scholar] [CrossRef]
- Wang, F.; Shabala, S.; Chen, Z.-H. Hypoxia snsing in plants: On a quest for ion channels as putative oxygen sensors. Plant Cell Physiol 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Sanmartín, M.; Rojo, E.; Kurenda, A.; Larruy-García, B.; Zamarreño, Á.M.; Delgadillo, M.O.; Brito-Gutiérrez, P.; García-Mina, J.M.; Farmer, E.E.; Sánchez-Serrano, J.J. GLR-dependent calcium and electrical signals are not coupled to systemic, oxylipin-based wound-induced gene expression in Marchantia polymorpha. New Phytol. 2024. [Google Scholar] [CrossRef]
- Yan, C.; Gao, Q.; Yang, M.; Shao, Q.; Xu, X.; Zhang, Y.; Luan, S. Ca2+/calmodulin-mediated desensitization of glutamate receptors shapes plant systemic wound signalling and anti-herbivore defence. Nat. Plants 2024, 10, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijo, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [PubMed]

| Categories | Phenotype | Number of Mutants | Mutation Frequency |
|---|---|---|---|
| Seedling stage | Albino | 2 | 0.03% |
| Etiolated | 12 | 0.17% | |
| Seedling tuft | 34 | 0.47% | |
| Leaves | Yellow striped leaves | 36 | 0.50% |
| Light leaf color | 17 | 0.23% | |
| Yellow | 41 | 0.57% | |
| White striped leaves | 4 | 0.06% | |
| Flag leaf deformation | 5 | 0.07% | |
| Leaf spot | 16 | 0.22% | |
| Wide | 4 | 0.06% | |
| Thin | 9 | 0.12% | |
| Curled | 4 | 0.06% | |
| Non-midrib | 5 | 0.07% | |
| Stems | Non-waxy | 11 | 0.15% |
| Non-tiller | 11 | 0.15% | |
| Multiple tillers | 15 | 0.21% | |
| Less tiller | 23 | 0.32% | |
| Dwarf | 9 | 0.12% | |
| Stem curvature | 6 | 0.08% | |
| Spikes | Six rows | 2 | 0.03% |
| Spike anomaly | 16 | 0.22% | |
| White glume | 13 | 0.18% | |
| Red glume | 4 | 0.06% | |
| Long awn | 3 | 0.04% | |
| Short awn | 6 | 0.08% | |
| Awnless | 5 | 0.07% | |
| Sterile | 6 | 0.08% |
| Item | Plant Height (cm) | Spike Length (cm) | Spacer Number | Spike Number | Thousand-Kernel Weight (g) |
|---|---|---|---|---|---|
| TX9425 | 90.50 | 5.90 | 4.00 | 10.08 | 45.61 |
| Mean of mutant lines | 56.66 | 5.31 | 4.00 | 8.59 | 30.60 |
| Maximum of mutant lines | 91.50 | 12.03 | 5.00 | 15.56 | 48.39 |
| Minimum of mutant lines | 27.72 | 2.50 | 3.00 | 2.78 | 15.63 |
| CV/% | 13.29 | 16.38 | 0.11 | 43.04 | 12.08 |
| Gene | Description | Mutation | |||
|---|---|---|---|---|---|
| Total | Intron | Synonymous | Non-Synonymous | ||
| HvRBOHB | Respiratory Burst Oxidase Homolog B | 1 | - | 1 | - |
| HvRBOHE | Respiratory Burst Oxidase Homolog E | 2 | - | 1 | 1 |
| HvDTX16 | protein DETOXIFICATION 16-like | 2 | 1 | - | 1 |
| HvGLR2.8 | Glutamate Receptor 2.8-like | 3 | - | 1 | 2 |
| HvGLR3.5 | Glutamate Receptor 3.5-like | 3 | - | 3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhu, L.; Zhou, Z.; Gu, Y.; Guo, B.; Lv, C.; Zhu, J.; Liu, X.; Xu, R. Development and Characterization of a New TILLING Population for Forward and Reverse Genetics in Barley (Hordeum vulgare L.). Plants 2024, 13, 2490. https://doi.org/10.3390/plants13172490
Wang F, Zhu L, Zhou Z, Gu Y, Guo B, Lv C, Zhu J, Liu X, Xu R. Development and Characterization of a New TILLING Population for Forward and Reverse Genetics in Barley (Hordeum vulgare L.). Plants. 2024; 13(17):2490. https://doi.org/10.3390/plants13172490
Chicago/Turabian StyleWang, Feifei, Liang Zhu, Zhenxiang Zhou, Yangyang Gu, Baojian Guo, Chao Lv, Juan Zhu, Xiaohui Liu, and Rugen Xu. 2024. "Development and Characterization of a New TILLING Population for Forward and Reverse Genetics in Barley (Hordeum vulgare L.)" Plants 13, no. 17: 2490. https://doi.org/10.3390/plants13172490
APA StyleWang, F., Zhu, L., Zhou, Z., Gu, Y., Guo, B., Lv, C., Zhu, J., Liu, X., & Xu, R. (2024). Development and Characterization of a New TILLING Population for Forward and Reverse Genetics in Barley (Hordeum vulgare L.). Plants, 13(17), 2490. https://doi.org/10.3390/plants13172490










