Overexpression of OsRbohH Enhances Heat and Drought Tolerance through ROS Homeostasis and ABA Mediated Pathways in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Expression Patterns of OsRbohH
2.2. Overexpression of OsRbohH Enhanced Heat Stress Tolerance
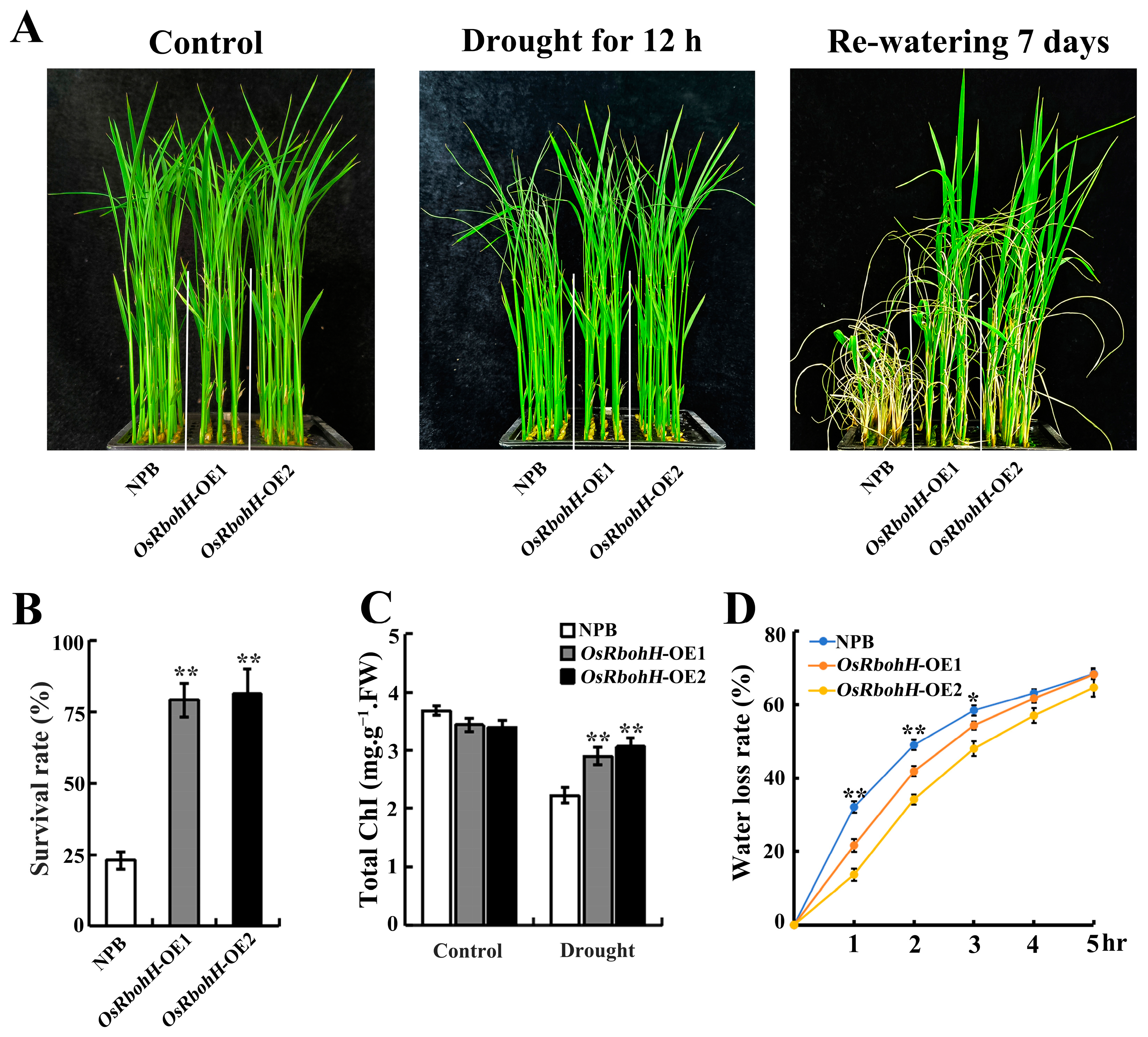
2.3. Overexpression of OsRbohH Enhanced Drought Stress Tolerance
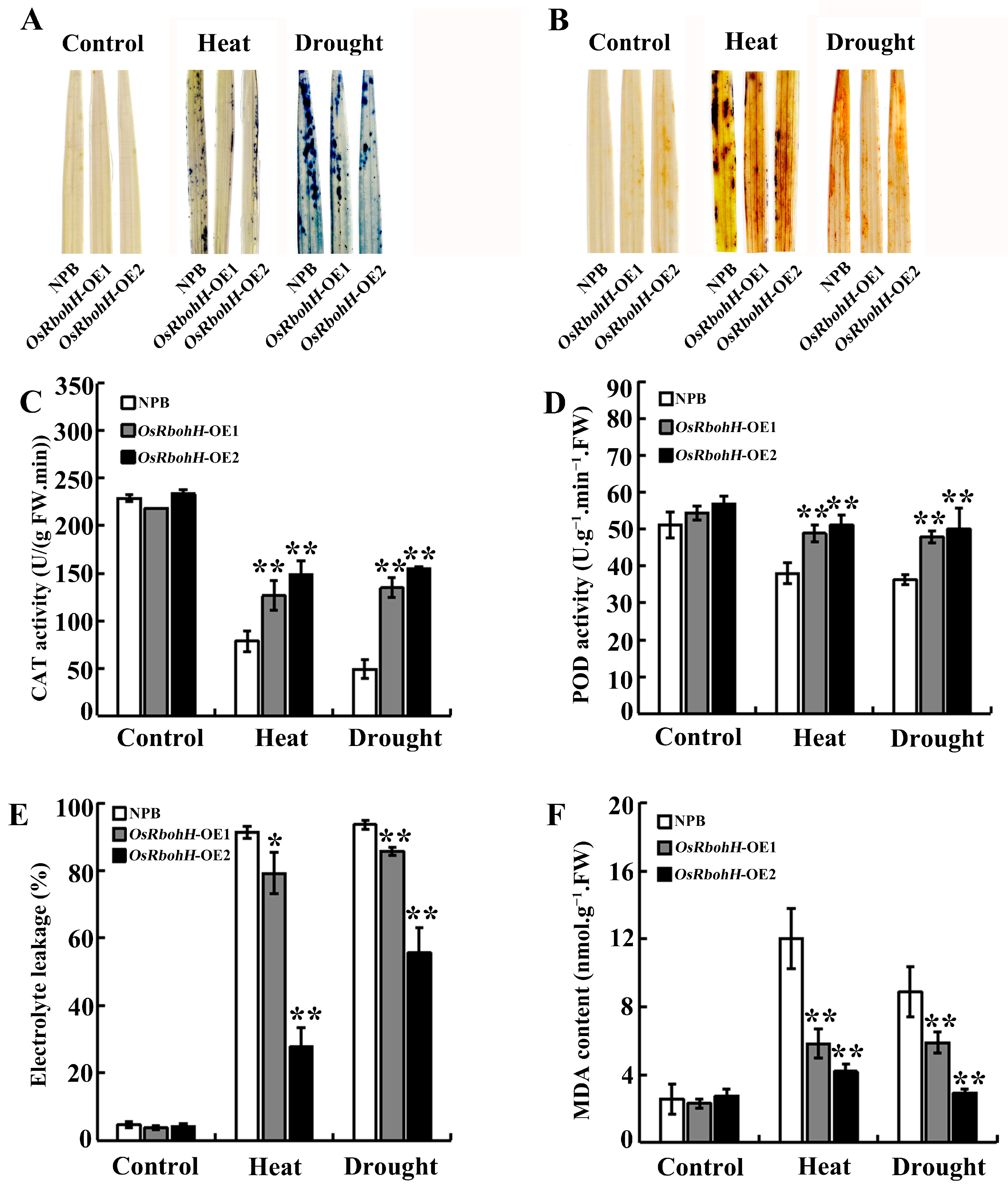
2.4. OsRbohH Modulates ROS Homeostasis in Heat and Drought Stress
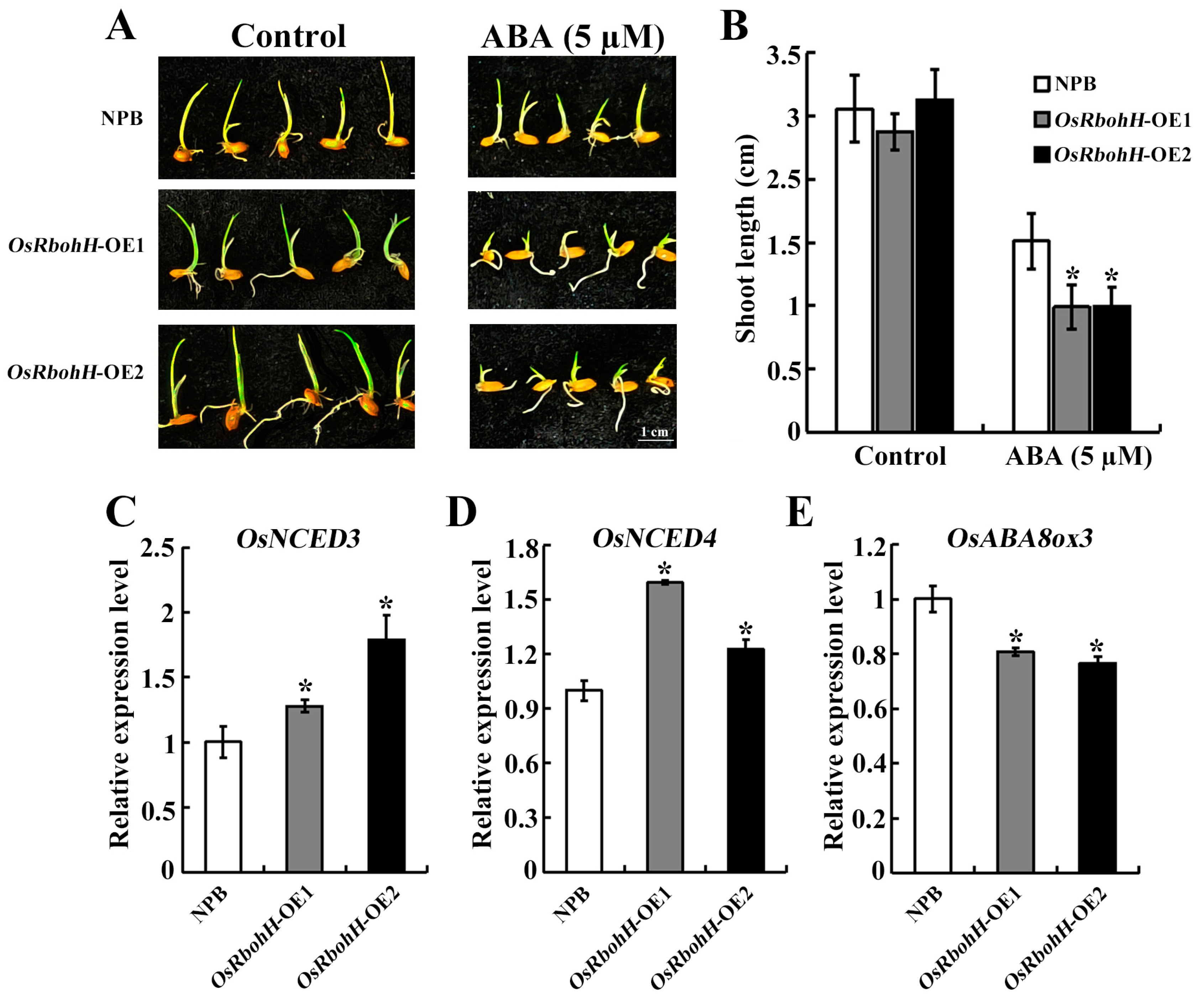
2.5. Overexpression of OsRbohH Enhanced Sensitivity to Exogenous ABA
2.6. OsRbohH Is Involved in the ABA-Mediated Regulatory Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation Conditions
4.2. Generation of Transgenic Plants
4.3. Expression Profile Analysis
4.4. Assays of Heat and Drought Treatment
4.5. Analysis of ABA Sensitivity Assays
4.6. Gene Expression Analysis
4.7. Measurement of Chlorophyll Concentrations
4.8. Measurement of Malondialdehyde Content
4.9. Electrolyte Leakage Measurements in Leaves
4.10. Measurements of Antioxidant Enzyme Activities
4.11. Detection of O2− and H2O2 In Situ
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Yu, W.; Wu, Y.; Li, Y. Roles of hydrogen gas in plants under abiotic stress: Current knowledge and perspectives. Antioxidants 2022, 11, 1999. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Kaushik, D.; Aryadeep, R. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Li, M.; Zhao, D.; Yang, J.F.; Fu, J.D.; Xu, Z.S. Maize WRKY transcription factor ZmWRKY106 confers drought and heat tolerance in transgenic plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Niu, C.Y.; Yang, C.R.; Jinn, T.L. The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Wang, A.; Xu, X.; Li, J. Ectopic overexpression of SlHsfA3, a heat stress transcription factor from tomato, confers increased thermotolerance and salt hypersensitivity in germination in transgenic Arabidopsis. PLoS ONE 2013, 8, e54880. [Google Scholar] [CrossRef]
- Xiang, J.; Zhou, X.; Zhang, X.; Liu, A.; Xiang, Y.; Yan, M.; Peng, Y.; Chen, X. The Arabidopsis AtUNC-93 acts as a positive regulator of abiotic stress tolerance and plant growth via modulation of ABA signaling and K+ homeostasis. Front. Plant Sci. 2018, 9, 718. [Google Scholar] [CrossRef]
- Yan, J.; Ninkuu, V.; Fu, Z.; Yang, T.; Ren, J.; Li, G.; Yang, X.; Zeng, H. OsOLP1 contributes to drought tolerance in rice by regulating ABA biosynthesis and lignin accumulation. Front. Plant Sci. 2023, 14, 1163939. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Hou, D.; Wan, J.; Ye, T.; Zhang, L.; Fan, J.; Li, C.; Dong, Y.; Chen, W.; Rong, S.; et al. OsWRKY97, an abiotic stress-induced gene of rice, plays a key role in drought tolerance. Plants 2023, 12, 3338. [Google Scholar] [CrossRef]
- Long, Q.; Qiu, S.; Man, J.; Ren, D.; Xu, N.; Luo, R. OsAAI1 increases rice yield and drought tolerance dependent on ABA-mediated regulatory and ROS scavenging pathway. Rice 2023, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Guruprasad, K.; Temple, B.R.S.; Shirvanyants, D.G.; Dokholyan, N.V.; Pati, P.K. Structural complexity and functional diversity of plant NADPH oxidases. Amino Acids 2018, 50, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, T.; Ni, L.; Chen, C.; Jiang, J.; Cui, Z.; Wang, S.; Xu, F.; Yan, R.; Jiang, M. Phosphorylation of OsRbohB by the protein kinase OsDMI3 promotes H2O2 production to potentiate ABA responses in rice. Mol. Plant 2023, 16, 882–902. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Yan, A.; Deng, J.; Xie, Y.; Liu, S.; Liu, D.; He, L.; Weng, J.; Xu, J. Evolutionary analysis of respiratory burst oxidase homolog (RBOH) genes in plants and characterization of ZmRBOHs. Int. J. Mol. Sci. 2023, 24, 3858. [Google Scholar] [CrossRef]
- Amicucci, E.; Ward, J.M.; Gaschler, K. NADPH oxidase genes from tomato (Lycopersicon esculentum) and curly-leaf pondweed (Potamogeton crispus). Plant Biol. 1999, 1, 524–528. [Google Scholar] [CrossRef]
- Yoshioka, H.; Numata, N.; Nakajima, K.; Katou, S.; Kawakita, K.; Rowland, O.; Jones, J.D.G.; Doke, N. Nicotiana benthamiama gp91 phox homologues NbrbohA and NbrbohB participatein H2O2 accumulationand resistanceto Phytophthora infestans. Plant Cell 2003, 15, 706–718. [Google Scholar] [CrossRef]
- Yoshioka, H.; Sugie, K.; Park, H.J.; Maeda, H.; Tsuda, N.; Kawakita, K.; Doke, N. Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol. Plant Microbe Interact. 2001, 14, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Andrio, E.; Danchin, E.G.; Oger, E.; Gucciardo, S.; Lambert, A.; Puppo, A.; Pauly, N. A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol. 2011, 189, 580–592. [Google Scholar] [CrossRef]
- Groom, Q.J.; Torres, M.A.; Fordham-Skelton, A.P.; Hammond-Kosack, K.E.; Robinson, N.J.; Jones, J.D. rbohA, a rice homologue of the mammalian gp91 phox respiratory burst oxidase gene. Plant J. 1996, 10, 515–522. [Google Scholar] [CrossRef]
- Nestler, J.; Liu, S.; Wen, T.J.; Paschold, A.; Marcon, C.; Tang, H.M.; Li, D.; Li, L.; Meeley, R.B.; Sakai, H. Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J. 2014, 79, 729–740. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, L.; Pan, X.; Yao, K.; Wei, W.; Liao, W.; Wang, C. The Characteristics and expression analysis of the tomato SlRBOH gene family under exogenous phytohormone treatments and abiotic stresses. Int. J. Mol. Sci. 2024, 25, 5780. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Tian, L.; Huang, L.; Liu, S.; Li, D.; Song, F. Tomato SlRbohB, a member of the NADPH oxidase family, is required for disease resistance against Botrytis cinerea and tolerance to drought stress. Front. Plant Sci. 2015, 6, 463. [Google Scholar] [CrossRef]
- Song, X.; Wang, T.; Zhang, Y.; Yu, J.Q.; Xia, X.J. S-Nitrosoglutathione reductase contributes to thermotolerance by modulating high temperature-induced apoplastic H2O2 in Solanum lycopersicum. Front. Plant Sci. 2022, 13, 862649. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Sun, L.; Jiao, Y.; Zhang, G.; Miao, C.; Hao, F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2012, 63, 305–317. [Google Scholar] [CrossRef]
- Müller, K.; Carstens, A.C.; Linkies, A.; Torres, M.A.; Leubner-Metzger, G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol. 2009, 184, 885–897. [Google Scholar] [CrossRef]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal production of reactive oxygen species by NADPH Oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- He, H.; Yan, J.; Yu, X.; Liang, Y.; Fang, L.; Scheller, H.V.; Zhang, A. The NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 491, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, H.; Liu, X.X.; Sun, J.F.; Xiang, L.; Liu, Y.J.; Hu, Z.W.; Xiong, X.Y.; Yang, X.M.; Bhutto, S.H.; et al. Identification of NADPH oxidase genes crucial for rice multiple disease resistance and yield traits. Rice 2024, 17, 1. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, L.; Cui, Z.; Jiang, J.; Chen, C.; Jiang, M. The NADPH oxidase OsRbohA increases salt tolerance by modulating K+ homeostasis in rice. Crop J. 2022, 10, 1611–1622. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.M.; Wang, Y.J.; Gao, Y.T.; Li, R.; Wang, G.F.; Li, W.Q.; Liu, W.T.; Chen, K.M. The plasma membrane NADPH oxidase OsRbohA plays a crucial role in developmental regulation and drought-stress response in rice. Physiol. Plant. 2016, 156, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chang, Y.L.; Wu, H.T.; Shalmani, A.; Liu, W.T.; Li, W.Q.; Xu, J.W.; Chen, K.M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef]
- Shen, T.; Wang, Q.; Chen, D.; Li, K.; Ni, L.; Jiang, M. The NADPH oxidase OsRbohD and OsRbohH negatively regulate saline-alkaline tolerance in rice. Environ. Exp. Bot. 2023, 213, 105445. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Bjerring, N.; Vrobel, O.; Akula, N.; De, N.; Tarkowski, P.; Ottosen, C.O.; Zhou, R. Stomatal effects and ABA metabolism mediate differential regulation of leaf and flower cooling in tomato cultivars exposed to heat and drought stress. J. Exp. Bot. 2024, 75, 2156–2175. [Google Scholar] [CrossRef]
- Lesk, C.; Anderson, W.; Rigden, A.; Coast, O.; Jägermeyr, J.; McDermid, S.; Davis, K.F.; Konar, M. Compound heat and moisture extreme impacts on global crop yields under climate change. Nat. Rev. Earth Environ. 2022, 3, 872–889. [Google Scholar] [CrossRef]
- Kaur, G.; Pati, P.K. Analysis of cis-acting regulatory elements of Respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016, 62, 104–118. [Google Scholar] [CrossRef]
- Wu, Q.; Lin, J.; Liu, J.Z.; Wang, X.; Lim, W.; Oh, M.; Park, J.; Rajashekar, C.B.; Whitham, S.A.; Cheng, N.H.; et al. Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol. J. 2012, 10, 945–955. [Google Scholar] [CrossRef]
- Nykiel, M.; Gietler, M.; Fidler, J.; Prabucka, B.; Labudda, M. Abiotic stress signaling and responses in plants. Plants 2023, 12, 3405. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Ji, E.; Hu, S.; Lu, Q.; Zhang, M.; Jiang, M. Hydrogen peroxide positively regulates ABA signaling via oxidative modification of the C2H2-type zinc finger protein ZFP36 in rice. Plant Physiol. Biochem. 2024, 213, 108844. [Google Scholar] [CrossRef]
- Manan, S.; Li, P.; Alfarraj, S.; Ansari, M.J.; Bilal, M.; Ullah, M.W.; Zhao, J. FUS3: Orchestrating soybean plant development and boosting stress tolerance through metabolic pathway regulation. Plant Physiol. Biochem. 2024, 213, 108803. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The interaction of ABA and ROS in plant growth and stress resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Ahammed, G.J.; Zhang, X.N.; Ying, L.; Zhang, L.; Yan, P.; Zhang, L.P.; Li, Q.Y.; Han, W.Y. RBOH1-dependent apoplastic H 2O2 mediates epigallocatechin-3-gallate-induced abiotic stress tolerance in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 357–366. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, W.; Zhang, Q.; Xu, Z.; Zhu, Z.; Duan, F.; Wu, R. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol. Biochem. 2011, 49, 1384–1391. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Y.; Tang, N.; Xiong, L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor. Appl. Genet. 2007, 115, 35–46. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, D.K.; Yu, I.J.; Kim, Y.S.; Choi, Y.D.; Kim, J.K. Overexpression of the osbzip66 transcription factor enhances drought tolerance of rice plants. Plant Biotechnol. Rep. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Lu, G.; Gao, C.; Zheng, X.; Han, B. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 2009, 229, 605–615. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Sinha, R.; Zandalinas, S.I.; Fichman, Y.; Sen, S.; Zeng, S.; Gómez-Cadenas, A.; Joshi, T.; Fritschi, F.B.; Mittler, R. Differential regulation of flower transpiration during abiotic stress in annual plants. New Phytol. 2022, 235, 611–629. [Google Scholar] [CrossRef]
- Zong, W.; Tang, N.; Yang, J.; Peng, L.; Ma, S.; Xu, Y.; Li, G.; Xiong, L. Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes. Plant Physiol. 2016, 171, 2810–2825. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, R.; Wang, R.; Li, J.; Wu, B.; Zhang, H.; Xiao, G. Overexpression of OsRbohH Enhances Heat and Drought Tolerance through ROS Homeostasis and ABA Mediated Pathways in Rice (Oryza sativa L.). Plants 2024, 13, 2494. https://doi.org/10.3390/plants13172494
Chen Y, Zhang R, Wang R, Li J, Wu B, Zhang H, Xiao G. Overexpression of OsRbohH Enhances Heat and Drought Tolerance through ROS Homeostasis and ABA Mediated Pathways in Rice (Oryza sativa L.). Plants. 2024; 13(17):2494. https://doi.org/10.3390/plants13172494
Chicago/Turabian StyleChen, Yating, Rui Zhang, Rujie Wang, Jiangdi Li, Bin Wu, Haiwen Zhang, and Guiqing Xiao. 2024. "Overexpression of OsRbohH Enhances Heat and Drought Tolerance through ROS Homeostasis and ABA Mediated Pathways in Rice (Oryza sativa L.)" Plants 13, no. 17: 2494. https://doi.org/10.3390/plants13172494





