From Leaves to Reproductive Organs: Chemodiversity and Chemophenetics of Essential Oils as Important Tools to Evaluate Piper mollicomum Kunth Chemical Ecology Relevance in the Neotropics
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of the Essential Oils of P. mollicomum
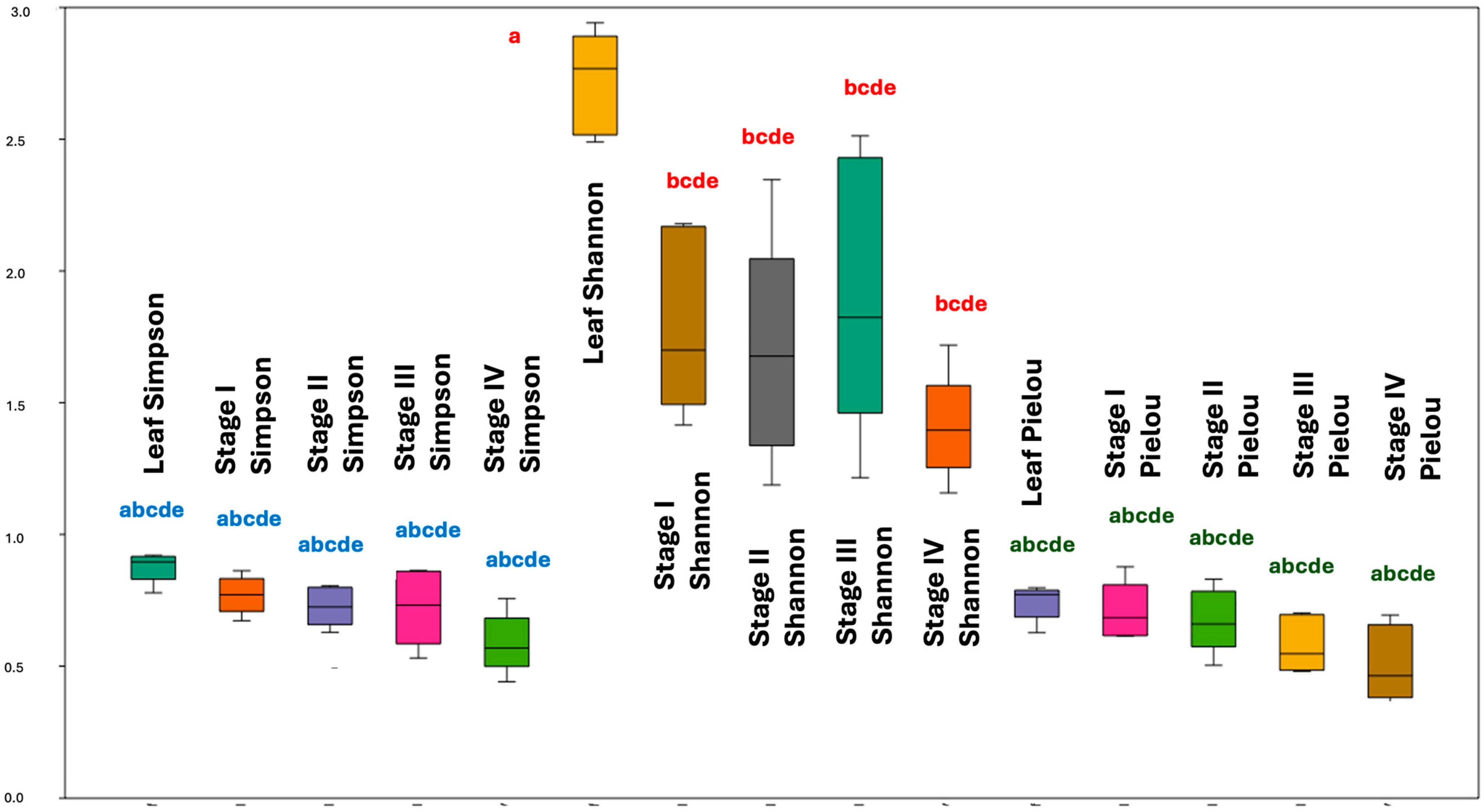
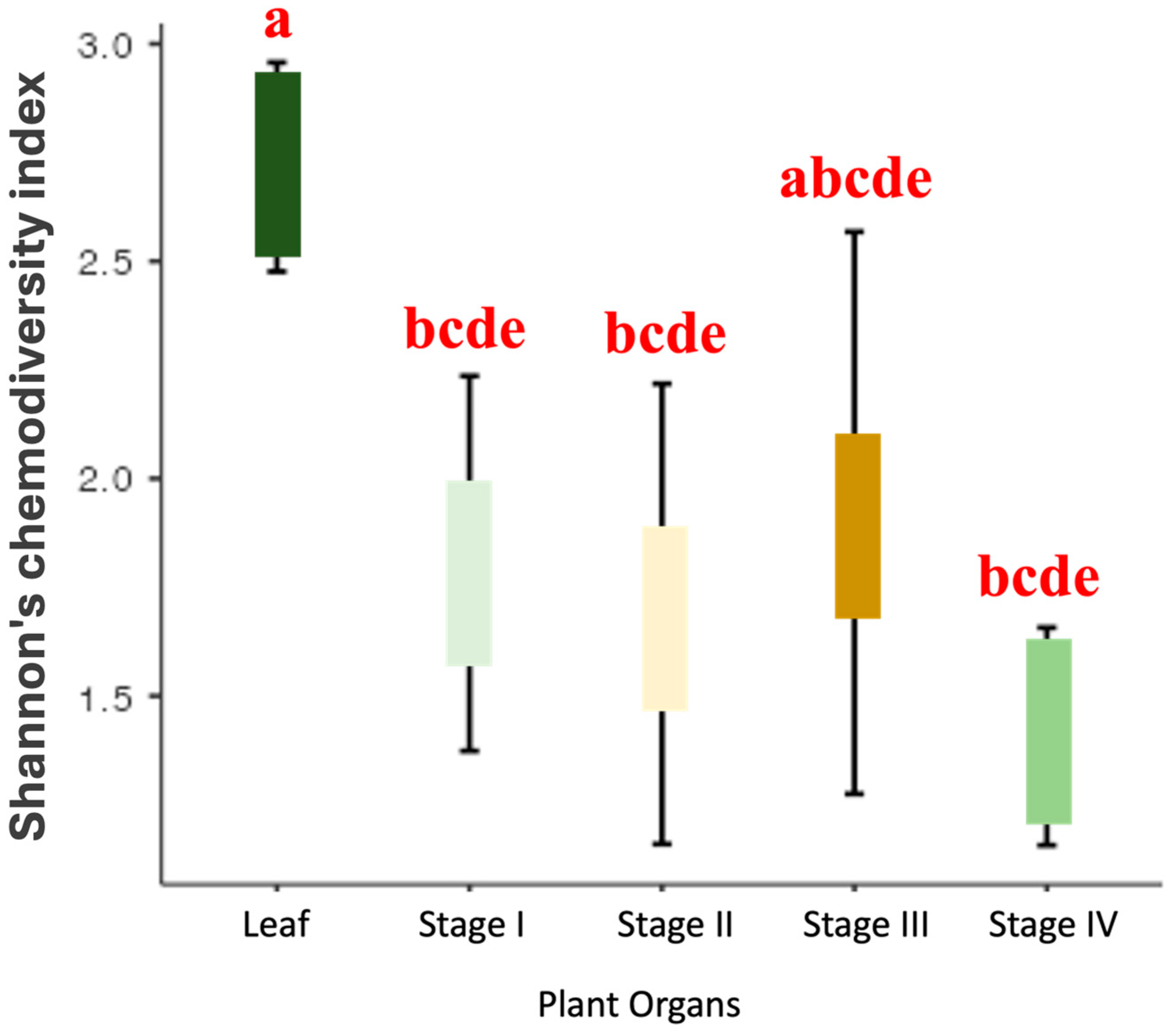
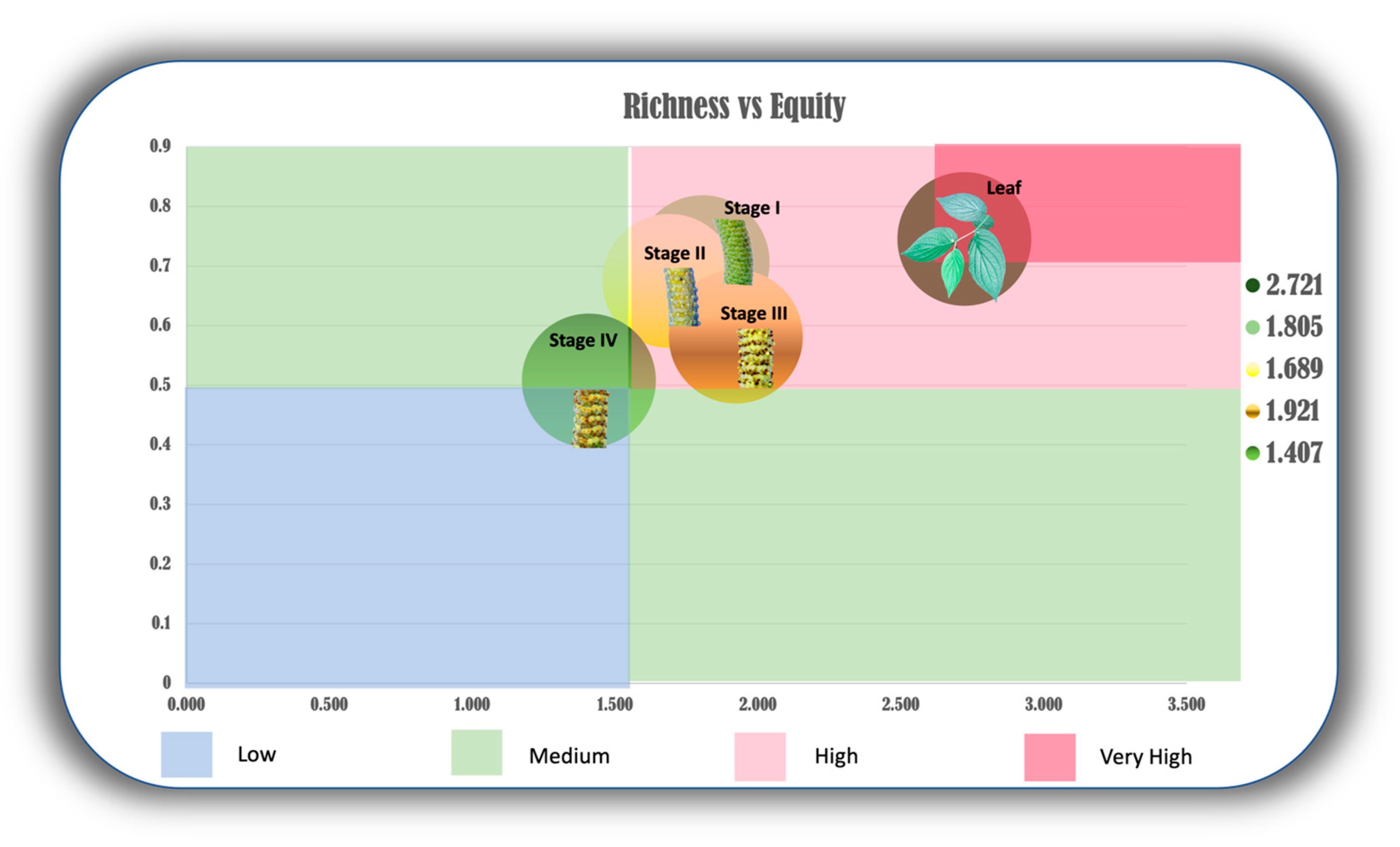
2.2. Chemodiversity Variability of the Organs of P. mollicomum: A Multifaceted Analysis of the Leaves and Reproductive Organs
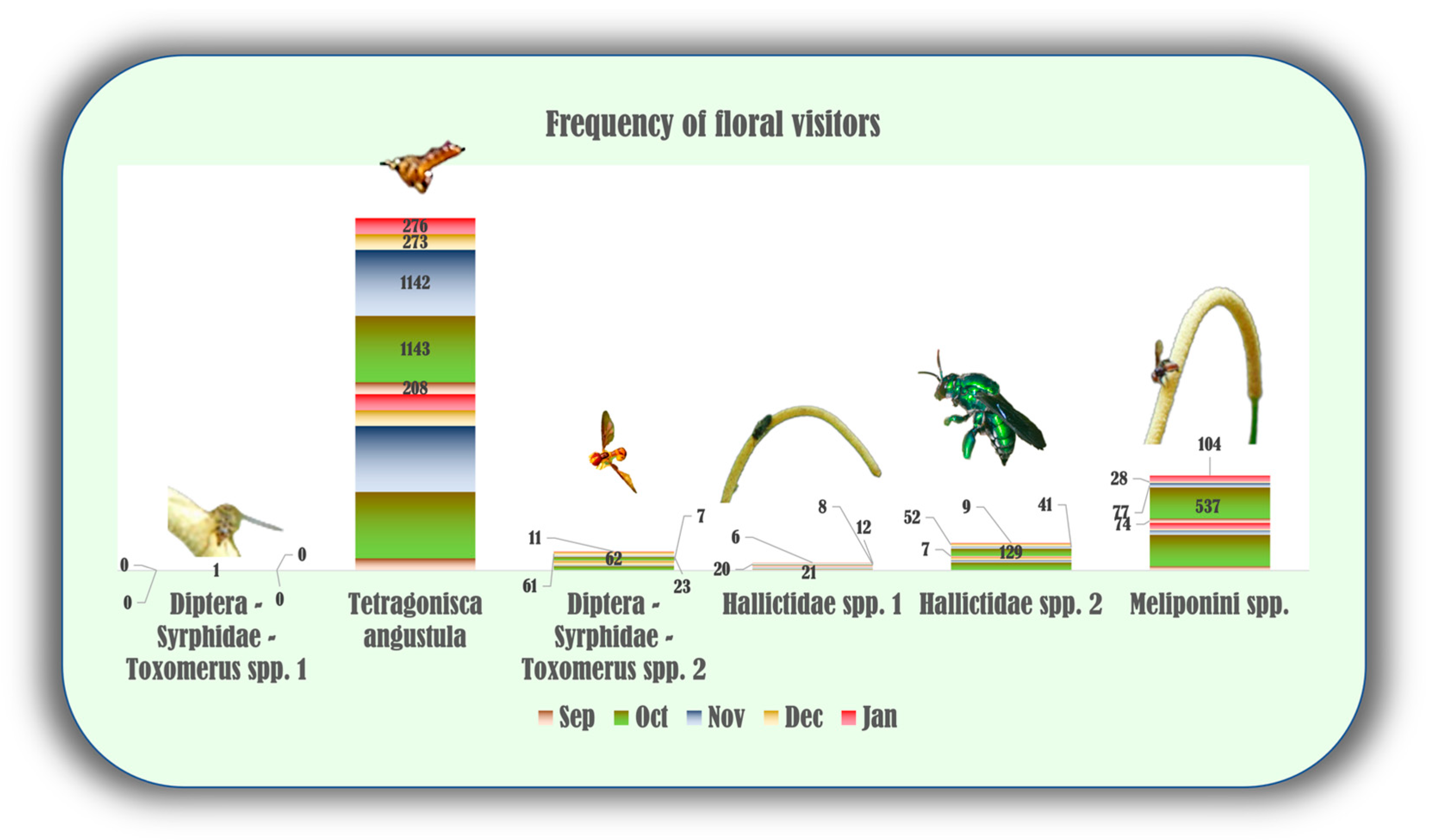
2.3. Chemical Ecology of P. mollicomum: Interactions between Chemical Diversity, Potential Pollinators, Volatile Compounds, and Microclimatic Variables
3. Discussion
3.1. Chemical Composition of P. mollicomum Essential Oils
3.2. Chemodiversity Analysis of P. mollicomum: Ecophysiological and Ecological Interconnections of Volatile Metabolites along Developmental Stages
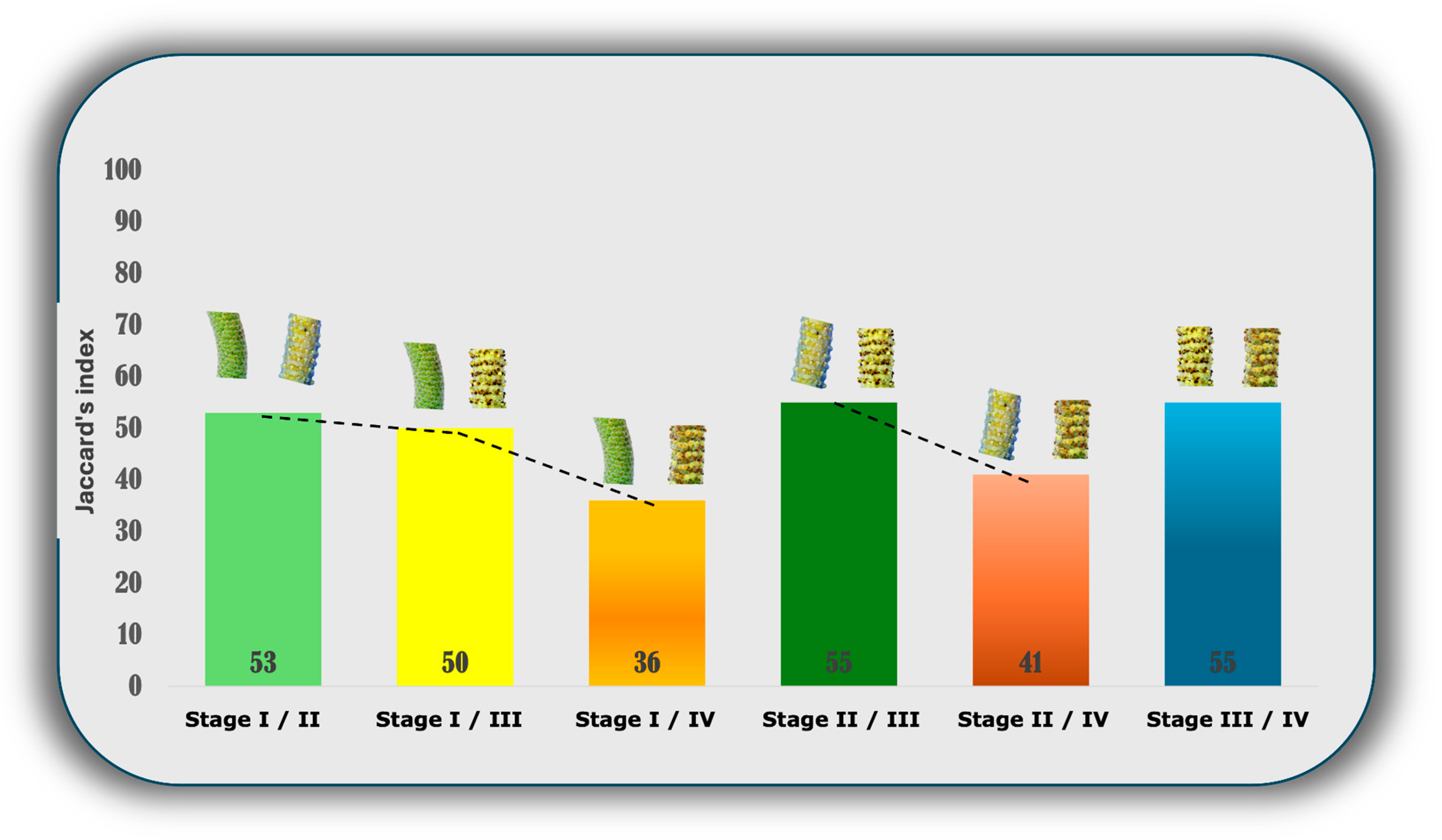
3.3. Similarity between the Chemical Profiles of the Reproductive Organs of P. mollicomum Using the Jaccard Index
3.4. Ramos and Moreira Index: A Redox Evaluation
4. Conclusions
5. Materials and Methods
5.1. Study Area
5.2. Collection of Microclimatic Data
5.3. Evaluation of Pollinators Visit Frequency
5.4. Reproductive and Vegetative Phenological Study
5.5. Essential Oil Obtention and Analysis
5.6. Chemodiversity Indices Calculation
5.7. Ramos and Moreira Index (R&M)
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsahar, E.; Friedman, J.; Izhaki, I. Impact on fruit removal and seed predation of a secondary metabolite, emodin, in Rhamnus alaternus fruit pulp. Oikos 2002, 99, 290–299. [Google Scholar] [CrossRef]
- Irwin, R.E.; Adler, L.S.; Brody, A.K. The dual role of floral traits: Pollinator attraction and plant defense. Ecology 2004, 85, 1503–1511. [Google Scholar] [CrossRef]
- Cipollini, M.L.; Paulk, E.; Mink, K.; Vaughn, K.; Fischer, T. Defense Tradeoffs in Fleshy Fruits: Effects of Resource Variation on Growth, Reproduction, and Fruit Secondary Chemistry in Solanum carolinense. J. Chem. Ecol. 2004, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- Cazetta, E.; Schaefer, H.M.; Galetti, M. Does attraction to frugivores or defense against pathogens shape fruit pulp composition? Oecologia 2007, 155, 277–286. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: Predictions and case study. Funct. Ecol. 2009, 23, 901–912. [Google Scholar] [CrossRef]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia verticillata: Linking glandular trichomes, volatiles and pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef]
- Nazem, V.; Sabzalian, M.R.; Saeidi, G.; Rahimmalek, M. Essential oil yield and composition and secondary metabolites in self- and open-pollinated populations of mint (Mentha spp.). Ind. Crops Prod. 2019, 130, 332–340. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Schneider, G.F.; Dybzinski, R.; Nelson, A.S.; Gelambi, M.; Jos, E.; Beckman, N.G. Fruits, frugivores, and the evolution of phytochemical diversity. Oikos 2022, 2022, e08332. [Google Scholar] [CrossRef]
- De Brito-Machado, D.; Ramos, Y.J.; Defaveri, A.C.A.E.; Queiroz, G.A.; Guimarães, E.F.; Moreira, D.L. Volatile Chemical Variation of Essential Oils and Their Correlation with Insects, Phenology, Ontogeny and Microclimate: Piper mollicomum Kunth, a Case of Study. Plants 2022, 11, 3535. [Google Scholar] [CrossRef]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The reemergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Costa-Oliveira, C.; Gouvêa-Silva, J.G.; Brito-Machado, D.; Felisberto, J.R.S.; Queiroz, G.A.; Guimarães, E.F.; Ramos, Y.J.; Moreira, D.L. Chemical Diversity and Redox Values Change as a Function of Temporal Variations of the Essential Oil of a Tropical Forest Shrub. Diversity 2023, 15, 715. [Google Scholar] [CrossRef]
- Theodoridis, S.; Drakou, E.G.; Hickler, T.; Thines, M.; Nogues-Bravo, D. Evaluating natural medicinal resources and their exposure to global change. Lancet Planet Health 2023, 7, e155–e163. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.J.; Gouvêa-Silva, J.G.; de Brito, M.D.; Felisberto, J.S.; Pereira, R.C.; Sadgrove, N.J.; de Lima Moreira, D. Chemophenetic and Chemodiversity Approaches: New Insights on Modern Study of Plant Secondary Metabolite Diversity at Different Spatiotemporal and Organizational Scales. Rev. Bras. Farmacogn. 2023, 33, 49–72. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Studies of nectar-constitution and pollinator-plant coevolution. In Coevolution of Animals and Plants: Symposium V, Proceedings of the First International Congress of Systematic and Evolutionary Biology, Boulder, CO, USA, 4–12 August 1973; Gilbert, L.E., Raven, P.H., Eds.; University of Texas Press: New York, NY, USA, 1975; pp. 100–140. [Google Scholar]
- Detzel, A.; Wink, M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar] [CrossRef]
- Mačukanović-Jocić, M.; Zora, D.S.; Mića, M.; Gojko, J. Flower morphophysiology of selected Lamiaceae species in relation to pollinator attraction. J. Apic. Res. 2011, 50, 89–101. [Google Scholar] [CrossRef]
- Milet-Pinheiro, P.; Daniela, M.A.F.; Stefan, D.; Airton, T.C.; Carlos, E.P.; Manfred, A.; Clemens, S. Pollination biology in the dioecious orchid Catasetum uncatum: How does floral scent influence the behaviour of pollinators? Phytochemistry 2015, 116, 149–161. [Google Scholar] [CrossRef]
- Tölke, E.D.; Capelli, N.d.V.; Pastori, T.; Alencar, A.C.; Cole, T.C.H.; Demarco, D. Diversity of Floral Glands and Their Secretions in Pollinator Attraction. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. Rev. 1997, 2, 25–34. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Isman, M.B. Antifeedant and toxic activity of Trichilia americana extract against the larvae of Spodoptera litura. Entomol. Exp. Appl. 2001, 98, 9–16. [Google Scholar] [CrossRef]
- Barros, F.; Zambarda, E.; Heinzmann, B. Variabilidade sazonal e biossíntese de terpenoides presentes no óleo essencial de Lippia alba (Mill.) n.e. brown (Verbenaceae). Quim. Nova 2009, 32, 861–867. [Google Scholar] [CrossRef]
- Togashi, K.; Goto, M.; Rim, H.; Hattori, S.; Ozawa, R.; Arimura, G. Mint companion plants attract the predatory mite Phytoseiulus persimilis. Sci. Rep. 2019, 9, 1704. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.J.; Costa-Oliveira, C.D.; Candido-Fonseca, I.; Queiroz, G.A.D.; Guimarães, E.F.; Defaveri, A.C.A.; Sadgrove, N.J.; Moreira, D.L. Advanced Chemophenetic Analysis of Essential Oil from Leaves of Piper gaudichaudianum Kunth (Piperaceae) Using a New Reduction-Oxidation Index to Explore Seasonal and Circadian Rhythms. Plants 2021, 10, 2116. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.B.; Ramos, Y.J.; Queiroz, G.A.; Defaveri, A.C.A.; Gobatto, A.A.; Moreira, D.L. Study of volatile chemical constituents and insect-plant interaction in Piper mollicomum Kunth (Piperaceae) from Tijuca Forest, Rio de Janeiro—RJ, Brazil. Rev. Virtual Quím. 2021, 13, 1216–1225. [Google Scholar] [CrossRef]
- Queiroz, G.A.; Guimarães, E.F. Piper L. (Piperaceae) Do Leste Metropolitano, RJ, Brasil/Piper L. (Piperaceae) from Eastern Metropolitan, RJ, Brazil. Braz. J. Dev. 2020, 6, 93597–93634. [Google Scholar] [CrossRef]
- Flora do Brasil. Jardim Botânico do Rio de Janeiro. 2020. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 7 April 2022).
- Ramos, Y.J.; Machado, D.d.B.; de Queiroz, G.A.; Guimarães, E.F.; Defaveri, A.C.A.; Moreira, D.L. Chemical composition of the essential oils of circadian rhythm and of different vegetative parts from Piper mollicomum Kunth—A medicinal plant from Brazil. Biochem. Syst. Ecol. 2020, 92, 104116. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Ito, A.T.; Fernandes, C.M.; Young, M.C.M.; Kato, M.J. Secondary metabolites isolated from Piper chimonantifolium and their antifungal activity. Nat. Prod. Res. 2011, 26, 770–773. [Google Scholar] [CrossRef]
- Potrich, F.B.; Baggio, C.H.; Freitas, C.S.; Mayer, B.; Santos, A.C.; Twardowschy, A.; Guedes, A.; Marques, M.C.A. Ação de extratos de plantas medicinais sobre a motilidade do trato gastrointestinal. Rev. Bras. Plantas Med. 2014, 16, 750–754. [Google Scholar] [CrossRef]
- Stefnia, P.S.; Simone, S.V.; Nathalia, F.C.; Andrea, S.C.; Keila, S.C.L.; Valber, S.F.; Antonio, L.S.L. Chemical composition and antinociceptive activity of the essential oil of Piper mollicomum and Piper rivinoides. J. Med. Plants Res. 2014, 8, 788–793. [Google Scholar] [CrossRef]
- Messias, M.C.T.B.; Menegatto, M.F.; Prado, A.C.C.; Santos, B.R.; Guimarães, M.F.M. Popular use of medicinal plants and the socioeconomic profile of the users: A study in the urban area of Ouro Preto, Minas Gerais, Brazil. Rev. Bras. Plantas Med. 2015, 17, 76–104. [Google Scholar] [CrossRef]
- Thies, W.; Kalko, E.K.V. Phenology of neotropical pepper plants (Piperaceae) and their association with their main dispersers, two short-tailed fruit bats, Carollia perspicillata and C. castanea (Phyllostomidae). Oikos 2004, 104, 362–376. [Google Scholar] [CrossRef]
- Maynard, L.D.; Ananda, A.; Sides, M.F.; Burk, H.; Whitehead, S.R. Dietary resource overlap among three species of frugivorous bat in Costa Rica. J. Trop. Ecol. 2019, 35, 165–172. [Google Scholar] [CrossRef]
- Vargas, R.D.L.; Vieira, M.F. Sex expression, breeding system and pollinators of Piper caldense (piperaceae) in the brazilian atlantic forest. Acta Biol. Colomb. 2017, 22, 370–376. [Google Scholar]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Meena, R.K.; Jangra, S.; Wadhwa, Z.; Leela-Wati, M. Role of Plant Volatiles in Defense and Communication. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 300–313. [Google Scholar] [CrossRef]
- Zheng, L.; Jingrui, L.I.; Yan, D.; Wen, Z.; Hongtong, B.; Shu, L.; Su, W.; Hui, L.; Lei, S. Gene co-expression modulating terpene metabolism is associated with plant anti-herbivore defence during initial flowering stages. Authorea 2020. [Google Scholar] [CrossRef]
- Fujita, Y.; Koeduka, T.; Aida, M.; Suzuki, H.; Iijima, Y.; Matsui, K. Biosynthesis of volatile terpenes that accumulate in the secretory cavities of young leaves of Japanese pepper (Zanthoxylum piperitum): Isolation and functional characterization of monoterpene and sesquiterpene synthase genes. Plant Biotechnol. 2017, 34, 17–28. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.; Park, K.-C. Methyl Salicylate, a Soybean Aphid-Induced Plant Volatile Attractive to the Predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Picard, I.; Hollingsworth, R.G.; Salmieri, S.; Lacroix, M. Repellency of Essential Oils to Frankliniella occidentalis (Thysanoptera: Thripidae) as Affected by Type of Oil and Polymer Release. J. Econ. Entomol. 2012, 105, 1238–1247. [Google Scholar] [CrossRef]
- Smith, G.H.; Roberts, J.M.; Pope, T.W. Terpene based biopesticides as potential alternatives to synthetic insecticides for control of aphid pests on protected ornamentals. Crop Prot. 2018, 110, 125–130. [Google Scholar] [CrossRef]
- Reisenman, C.E.; Riffell, J.A.; Bernays, E.A.; Hildebrand, J.G. Antagonistic effects of floral scent in an insect–plant interaction. Proc. R. Soc. B Biol. Sci. 2010, 277, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Glinwood, R.; Blande, J.D. Deciphering Chemical Language of Plant Communication: Synthesis and Future Research Directions. Signal. Commun. Plants 2016, 1, 319–326. [Google Scholar]
- Ma, Q.; Ma, R.; Su, P.; Jin, B.; Guo, J.; Tang, J.; Chen, T.; Zeng, W.; Lai, C.; Ling, F.; et al. Elucidation of the essential oil biosynthetic pathways in Cinnamomum burmannii through identification of six terpene synthases. Plant Sci. 2022, 317, 111203. [Google Scholar] [CrossRef]
- Bergström, G.; Tengö, J. Linalool in mandibular gland secretion of Colletes bees (Hymenoptera: Apoidea). J. Chem. Ecol. 1978, 4, 437–449. [Google Scholar] [CrossRef]
- Wang, Q.; Hillwig, M.L.; Okada, K.; Yamazaki, K.; Wu, Y.; Swaminathan, S.; Yamane, H.; Peters, R.J. Characterization of CYP76M5–8 Indicates Metabolic Plasticity within a Plant Biosynthetic Gene Cluster. J. Biol. Chem. 2012, 287, 6159–6168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Q.; Hillwig, M.L.; Peters, R.J. Picking sides: Distinct roles for CYP76M6 and CYP76M8 in rice oryzalexin biosynthesis. Biochem. J. 2013, 454, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Boachon, B.; Junker, R.R.; Miesch, L.; Bassard, J.-E.; Höfer, R.; Caillieaudeaux, R.; Seidel, D.E.; Lesot, A.; Heinrich, C.; Ginglinger, J.F.; et al. CYP76C1 (Cytochrome P450)-Mediated Linalool Metabolism and the Formation of Volatile and Soluble Linalool Oxides in Arabidopsis Flowers: A Strategy for Defense against Floral Antagonists. Plant Cell 2015, 27, 2972–2990. [Google Scholar] [CrossRef]
- Shilpashree, H.B.; Sudharshan, S.J.; Shasany, A.K.; Nagegowda, D.A. Molecular characterization of three CYP450 genes reveals their role in withanolides formation and defense in Withania somnifera, the Indian Ginseng. Sci. Rep. 2022, 12, 1602. [Google Scholar] [CrossRef]
- Miehe-Steier, A.; Roscher, C.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Light and Nutrient Dependent Responses in Secondary Metabolites of Plantago lanceolata Offspring Are Due to Phenotypic Plasticity in Experimental Grasslands. PLoS ONE 2015, 10, e0136073. [Google Scholar] [CrossRef]
- Nafea, H.M.; Kawaz, A.M.N.A. Synthesis, Characterization, Antimicrobial, DNA Cleavage and Fluorescent Activity of Metal ion (II) Coordinate with 2H-Chromene Azo Novel Ligand. 2020. Available online: https://ijrps.com/home/article/view/1038 (accessed on 19 April 2022).
- Moraes, M.M.; Kato, M.J. Biosynthesis of Pellucidin A in Peperomia pellucida (L.) HBK. Front. Plant Sci. 2021, 12, 641717. [Google Scholar] [CrossRef] [PubMed]
- Gaia, A.M.; Yamaguchi, L.F.; Guerrero-Perilla, C.; Kato, M.J. Ontogenetic Changes in the Chemical Profiles of Piper Species. Plants 2021, 10, 1085. [Google Scholar] [CrossRef]
- Bianconi, G.V.; Mikich, S.B.; Teixeira, S.D.; Maia, B.H.L.N.S. Attraction of Fruit-Eating Bats with Essential Oils of Fruits: A Potential Tool for Forest Restoration. Biotropica 2007, 39, 136–140. [Google Scholar] [CrossRef]
- Bianconi, G.V.; Suckow, U.M.S.; Cruz-Neto, A.P.; Mikich, S.B. Use of Fruit Essential Oils to Assist Forest Regeneration by Bats. Restor. Ecol. 2010, 20, 211–217. [Google Scholar] [CrossRef]
- Wiggins, N.L.; McArthur, C.; McLean, S.; Boyle, R. Effects of Two Plant Secondary Metabolites, Cineole and Gallic Acid, on Nightly Feeding Patterns of the Common Brushtail Possum. J. Chem. Ecol. 2003, 29, 1447–1464. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.J.; Wallis, I.R.; Mclean, S.; Sorensen, J.S.; Foley, W.J. Conflicting demands on detoxification pathways influence how common brushtail possums choose their diets. Ecology 2006, 87, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Yearsley, J.M.; Villalba, J.J.; Gordon, I.J.; Kyriazakis, I.; Speakman, J.R.; Tolkamp, B.J.; Illius, A.W.; Duncan, A.J. A Theory of Associating Food Types with Their Postingestive Consequences. Am. Nat. 2006, 167, 705–716. [Google Scholar] [CrossRef]
- Dirk, S.; Maik, K. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar]
- De Macêdo, D.; Souza, M.M.A.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Dos Santos, A.T.L.; da Cruz, R.P.; da Costa, J.G.M.; Rodrigues, F.F.G.; Quintans-Junior, L.J.; da Silva, A.J.R.G.; et al. Effect of seasonality on chemical profile and antifungal activity of essential oil isolated from leaves Psidium salutare (Kunth). PeerJ–Life Environ. 2018, 6, e5476. [Google Scholar] [CrossRef]
- Sikron-Persi, N.; Granot, G.; Batushansky, A.; Toubiana, D.; Grafi, G.; Fait, A. Mass spectrometry-based metabolite profiling reveals functional seasonal shifts in the metabolome of Zygophyllum dumosum Boiss and its relation to environmental conditions. Planta 2023, 258, 10. [Google Scholar] [CrossRef]
- Gottlieb, O.R. The role of oxygen in phytochemical evolution towards diversity. Phytochemistry 1989, 28, 2545–2558. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Kaplan, M.A.C. Phytochemical Evolution: The Redox Theory. Nat. Prod. Lett. 1993, 2, 171–176. [Google Scholar] [CrossRef]
- Gil, M.; Bottini, R.; Berli, F.; Pontin, M.; Silva, M.F.; Piccoli, P. Volatile organic compounds characterized from grapevine (Vitis vinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry 2013, 96, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Schrader, W.; Geiger, J.; Klockow, D.; Korte, E.H. Degradation of α-Pinene on Tenax during Sample Storage: Effects of Daylight Radiation and Temperature. Environ. Sci. Technol. 2001, 35, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.A.; Arruda, R.; Massuda, K.F.; Cardoso-Gustavson, P.; Guimarães, E.F.; Trigo, J.R. Volatiles released by damaged leaves of Piper mollicomum (Piperaceae) act as cues for predaceous wasps: Evidence using plasticine dummies as herbivore model. Arthropod-Plant Interact. 2019, 13, 593–601. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Abdelkrim, H. Allelopathic Effect of Eucalyptus citriodora Essential Oil and Its Potential Use as Bioherbicide. Chem. Biodivers. 2018, 15, e1800202. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Presto, A.A.; Huff-Hartz, K.E.; Donahue, N.M. Secondary Organic Aerosol Production from Terpene Ozonolysis. Effect of UV Radiation. Environ. Sci. Technol. 2005, 39, 7036–7045. [Google Scholar] [CrossRef]
- Babar, Z.B.; Park, J.H.; Lim, H.J. Influence of NH3 on secondary organic aerosols from the ozonolysis and photooxidation of α-pinene in a flow reactor. Atmos. Environ. 2017, 164, 71–84. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Ramya, M.; Jang, S.; An, H.-R.; Lee, S.-Y.; Park, P.-M.; Park, P.H. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef] [PubMed]
- Valentin-Silva, A.; Coelho, V.P.M.; Ventrella, M.C.; Vieira, M.F. Timing of pollen release and stigma receptivity period of Piper vicosanum: New insights into sexual reproduction of the genus. Am. J. Bot. 2015, 102, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.H. Fecundity, fruiting pattern, and seed dispersal in Piper amalago (Piperaceae), a bat-dispersed tropical shrub. Oecologia 1981, 51, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.K. Seasonality of Fruit Characters and Seed Removal by Birds. Oikos 1986, 46, 303–310. [Google Scholar] [CrossRef]
- Rodríguez, A.; Alquézar, B.; Peña, L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol. 2012, 197, 36–48. [Google Scholar] [CrossRef]
- Rocha, V.J.; Barbosa, G.P.; Rossi, H.R.S.; Sekiama, M.L. Chiropteran richness and diversity (Chiroptera; Mammalia) in Permanent Preservation Areas from UFSCar-Araras campus (SP). Ciência Tecnol. Ambiente 2018, 8, 21–29. [Google Scholar] [CrossRef][Green Version]
- Maynard, L.D.; Slinn, H.L.; Glassmire, A.E.; Matarrita-Carranza, B.; Dodson, C.D.; Nguyen, T.T.; Whitehead, S.R. Secondary metabolites in a neotropical shrub: Spatiotemporal allocation and role in fruit defense and dispersal. Ecology 2020, 101, e03192. [Google Scholar] [CrossRef]
- Silber, A.; Goldberg, T.; Shapira, O.; Hochberg, U. Nitrogen up take and Macronutrients Distribution in Mango (Mangifera indica L. Cv. Keitt) Trees. Plant Physiol. Biochem 2022, 181, 23–32. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Ebadollahi, A. Estragole-rich essential oil of summer savory (Satureja hortensis L.) as an eco-friendly alternative to the synthetic insecticides in management of two stored-products insect pests. Acta Agric. Slov. 2020, 115, 307–314. [Google Scholar] [CrossRef]
- Amaral, W.; Deschamps, C.; Biasi, A.L.; Bizzo, R.H.; Machado, M.P.; Silva, L.E. Yield and chemical composition of the essential oil of species of the Asteraceae family from Atlantic Forest, South of Brazil. J. Essent. Oil Res. 2018, 30, 278–284. [Google Scholar] [CrossRef]
- Schiestl, F.P. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015, 206, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.J. Identificação de Possíveis Quimiotipos de Piper aduncum L. e Piper mollicomum Kunth (Piperaceae) com Base no Estudo dos Componentes Químicos de Óleos Essenciais. Master’s Thesis, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil, 2018; 246p. Available online: https://www.bdtd.uerj.br:8443/handle/1/17559 (accessed on 29 April 2024).
- Kawano, T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003, 21, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Koppen, W.; Geiger, G.C. Das geographisca System der Klimate, Handbuch der Klimatologie; Borntraeger: Berlin, Germany, 1936; pp. 1–44. [Google Scholar]
- Sakagami, S.F.; Laroca, S.; Moure, J.S. Wild bee biocoenotics in São José dos Pinhais (PR), South Brazil. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1967, 16, 253–291. [Google Scholar]
- Campos, G.P.A.; Barros, C.T.; Carneiro, L.T.; Santa-Martinez, E.; Oliveira, M.M.; Castro, C.C. Pollinator efficiency in openly grown eggplants: Can non-vibrating bees produce high-quality fruits? Arthropod-Plant Interact. 2022, 16, 159–170. [Google Scholar] [CrossRef]
- Polizel, A.L.; Nanka, S.; Conte, H. Insetos dípteras como polinizadores em Orchidaceae. Rev. Uningá 2015, 46, 11–15. [Google Scholar]
- Valentin-Silva, A.; Staggemeier, V.G.; Batalha, M.A.; Guimarães, E. What factors can influence the reproductive phenology of Neotropical Piper species (Piperaceae) in a semi-deciduous seasonal forest? Botany 2018, 96, 675–684. [Google Scholar] [CrossRef]
- Fournier, L.A. Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 1974, 24, 422–423. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Business: Carol Stream, IL, USA, 2009. [Google Scholar]
- Jaccard, P. Distribution de la flore alpine dans le bassin des dranses et dans quelques régions voisines. Bull. Société Vaudoise Sci. Nat. 1901, 37, 241–272. [Google Scholar]
- Sørensen, T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons; Biologiske Skrifter/Kongelige Danske Videnskabernes Selskab: Copenhagen, Denmark, 1948; pp. 1–34. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Cody, M.L. Towards a theory of continental species diversities. In Ecology and Evolution of Communities; Cody, M.L., MacArthur, R.H., Diamond, J.M., Eds.; Harvard University Press: Cambridge, MA, USA, 1975. [Google Scholar]
- Gouyon, P.H.; Vernet, P.H.; Guillerm, J.L.; Valdeyron, G. Polymorphisms and environment: The adaptive value of the oil polymorphisms in Thymus vulgaris L. Heredity 1986, 57, 59–66. [Google Scholar] [CrossRef]
- Iason, G.R.; Lennon, J.J.; Pakeman, R.J.; Thoss, V.; Beaton, J.K.; Sim, D.A.; Elston, D.A. Does chemical composition of individual scots pine trees determine the biodiversity of their associated ground vegetation? Ecol. Lett. 2005, 8, 364–369. [Google Scholar] [CrossRef]
- Salazar, D.; Jaramillo, A.; Marquis, R.J. The impact of plant chemical diversity on plant–herbivore interactions at the community level. Oecologia 2016, 181, 1199–1208. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, W.; Wu, W.; Bai, R.; Kuang, S.; Shi, B.; Li, D. Chemical composition and diversity of the essential oils of Juniperus rigida along the elevations in Helan and Changbai Mountains and correlation with the soil characteristics. Ind. Crops Prod. 2021, 159, 113032. [Google Scholar] [CrossRef]
- Hendrickson, J.B.; Cram, D.J.; Hammond, G.S. Organic Chemistry, 3rd ed.; McGraw-Hill: New York, NY, USA, 1970. [Google Scholar]
- Emerenciano, V.P.; Cabrol-Bass, D.; Ferreira, M.J.; Alvarenga, S.A.; Brant, A.J.; Scotti, M.T.; Barbosa, K.O. Chemical evolution in the asteraceae. The oxidation-reduction mechanism and production of secondary metabolites. Nat. Prod. Commun. 2006, 1, 495–507. [Google Scholar] [CrossRef]
- Peroni, N.; Hernández, M.I.M. Ecologia de Populações e Comunidades, 1st ed.; CCB/EAD/UFSC: Florianópolis, Brazil, 2011; ISBN 978-85-61485-39-9. [Google Scholar]
- Ricklefs, R.; Relyea, R. Economia da Natureza, 7th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2018; ISBN 9788527728768. [Google Scholar]
- Kevser, M.; Doğan, M. Islamic Financial Literacy and Its Determinants: A Field Study on Turkey. J. Transit. Stud. Rev. 2021, 28, 91–120. [Google Scholar]
- Ainsley, M.; Kate, P. Anxiety around Learning R in First Year Undergraduate Students: Mathematics versus Biomedical Sciences Students. J. Stat. Data Sci. Educ. 2024, 32, 47–53. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Brito Machado, D.; Felisberto, J.S.; Queiroz, G.A.d.; Guimarães, E.F.; Ramos, Y.J.; Moreira, D.d.L. From Leaves to Reproductive Organs: Chemodiversity and Chemophenetics of Essential Oils as Important Tools to Evaluate Piper mollicomum Kunth Chemical Ecology Relevance in the Neotropics. Plants 2024, 13, 2497. https://doi.org/10.3390/plants13172497
de Brito Machado D, Felisberto JS, Queiroz GAd, Guimarães EF, Ramos YJ, Moreira DdL. From Leaves to Reproductive Organs: Chemodiversity and Chemophenetics of Essential Oils as Important Tools to Evaluate Piper mollicomum Kunth Chemical Ecology Relevance in the Neotropics. Plants. 2024; 13(17):2497. https://doi.org/10.3390/plants13172497
Chicago/Turabian Stylede Brito Machado, Daniel, Jéssica Sales Felisberto, George Azevedo de Queiroz, Elsie Franklin Guimarães, Ygor Jessé Ramos, and Davyson de Lima Moreira. 2024. "From Leaves to Reproductive Organs: Chemodiversity and Chemophenetics of Essential Oils as Important Tools to Evaluate Piper mollicomum Kunth Chemical Ecology Relevance in the Neotropics" Plants 13, no. 17: 2497. https://doi.org/10.3390/plants13172497
APA Stylede Brito Machado, D., Felisberto, J. S., Queiroz, G. A. d., Guimarães, E. F., Ramos, Y. J., & Moreira, D. d. L. (2024). From Leaves to Reproductive Organs: Chemodiversity and Chemophenetics of Essential Oils as Important Tools to Evaluate Piper mollicomum Kunth Chemical Ecology Relevance in the Neotropics. Plants, 13(17), 2497. https://doi.org/10.3390/plants13172497






