Exploring the Frontier of Wheat Rust Resistance: Latest Approaches, Mechanisms, and Novel Insights
Abstract
:1. Introduction
2. Etiology and Epidemiology of Wheat Rust
2.1. Types of Wheat Rust Diseases: Leaf Rust, Stem Rust, and Stripe Rust
2.2. Global Distribution and Economic Impact
3. Advances in Genetic Resistance to Wheat Rusts
3.1. Traditional Breeding Techniques
3.2. Genomic Approaches and Genetic Engineering
3.3. Identifying Key Resistance Genes
4. Molecular Mechanisms of Wheat Rust Pathogenesis
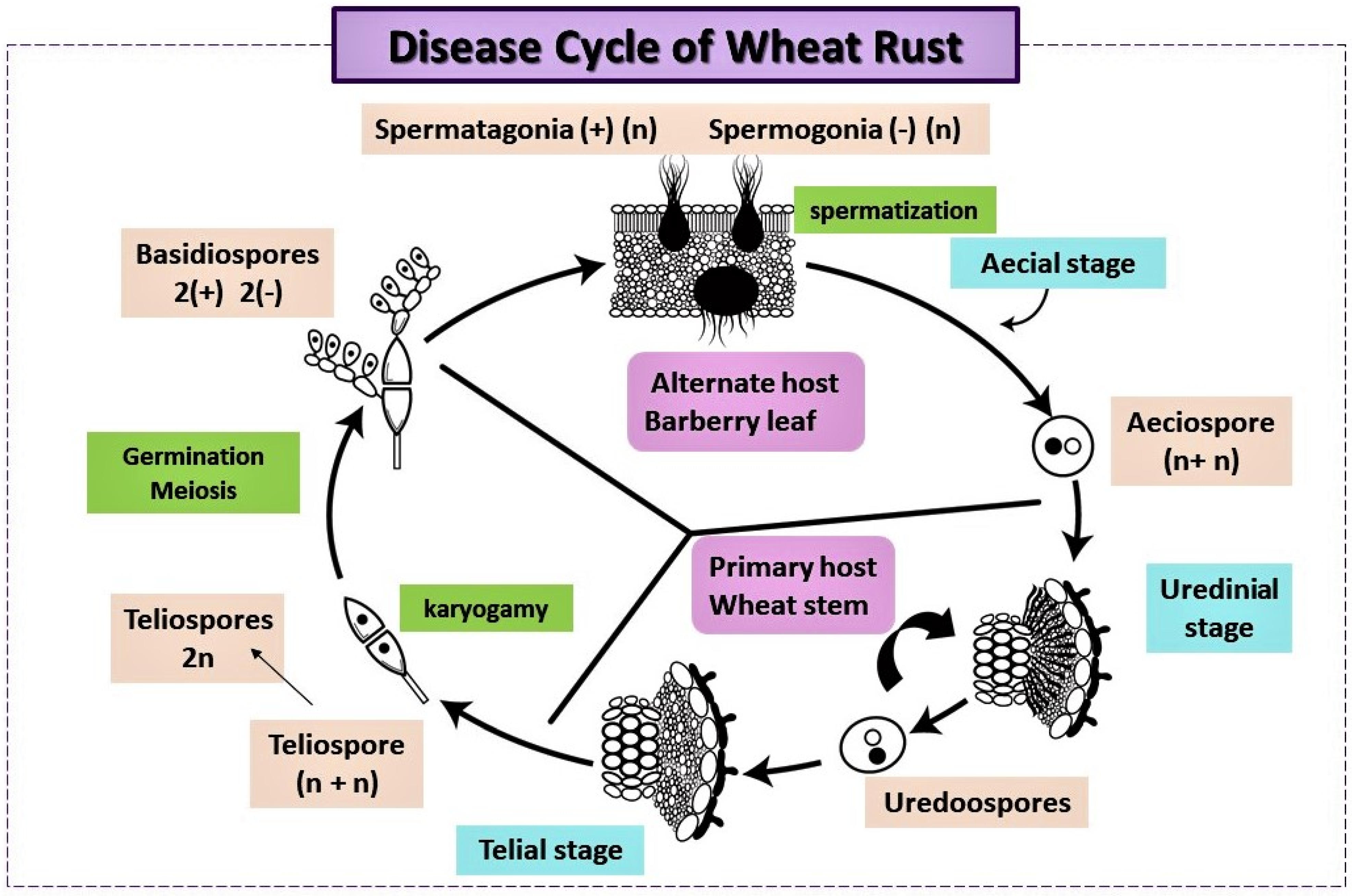
4.1. Pathogen Life Cycle and Infection Process
4.2. Host–Pathogen Interactions
4.3. Signal Transduction and Defense Mechanisms
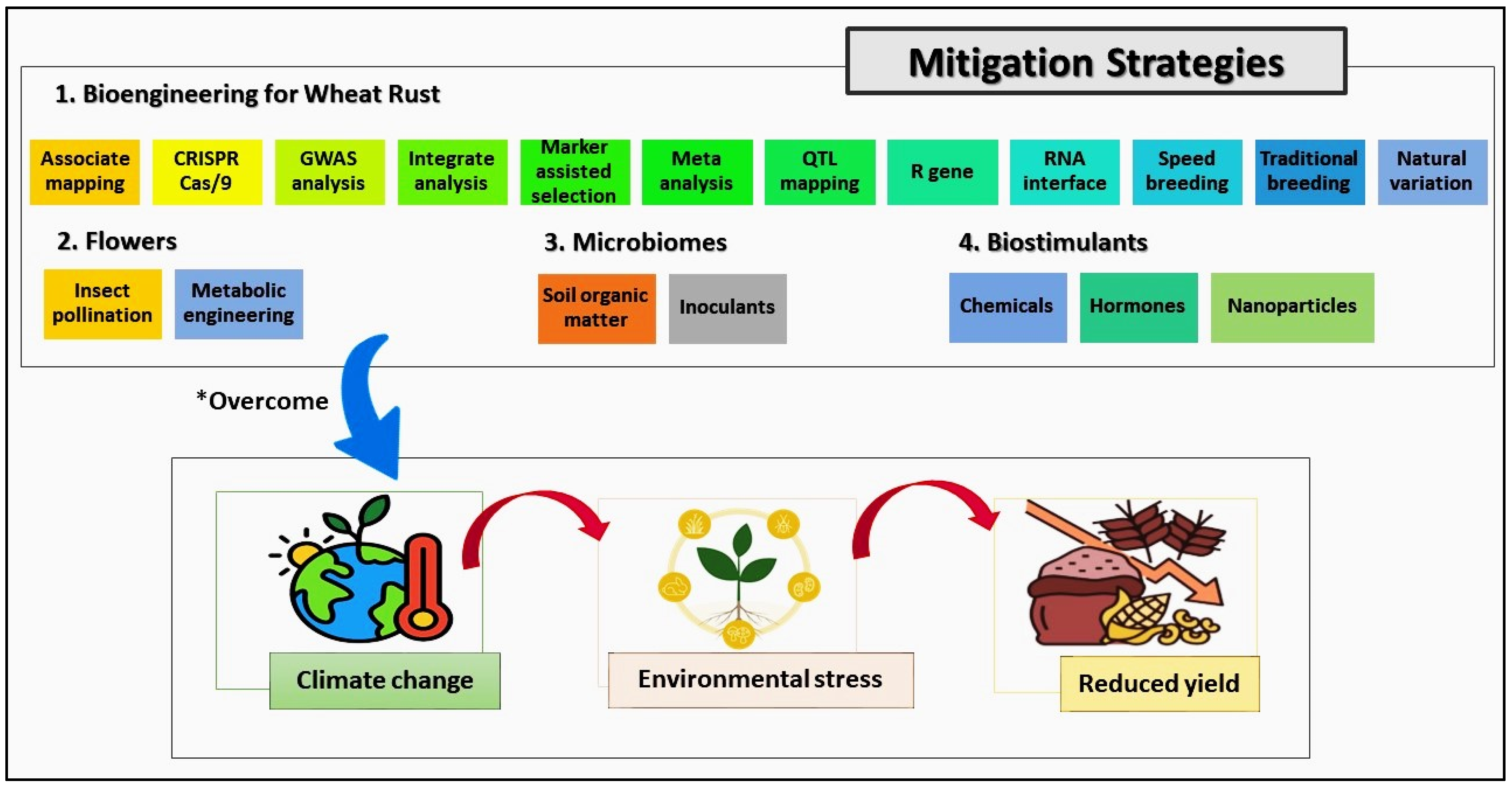
5. Innovative Management Strategies for Wheat Rust
5.1. Developments in Fungicides
5.2. Biological Control and Integrated Disease Management
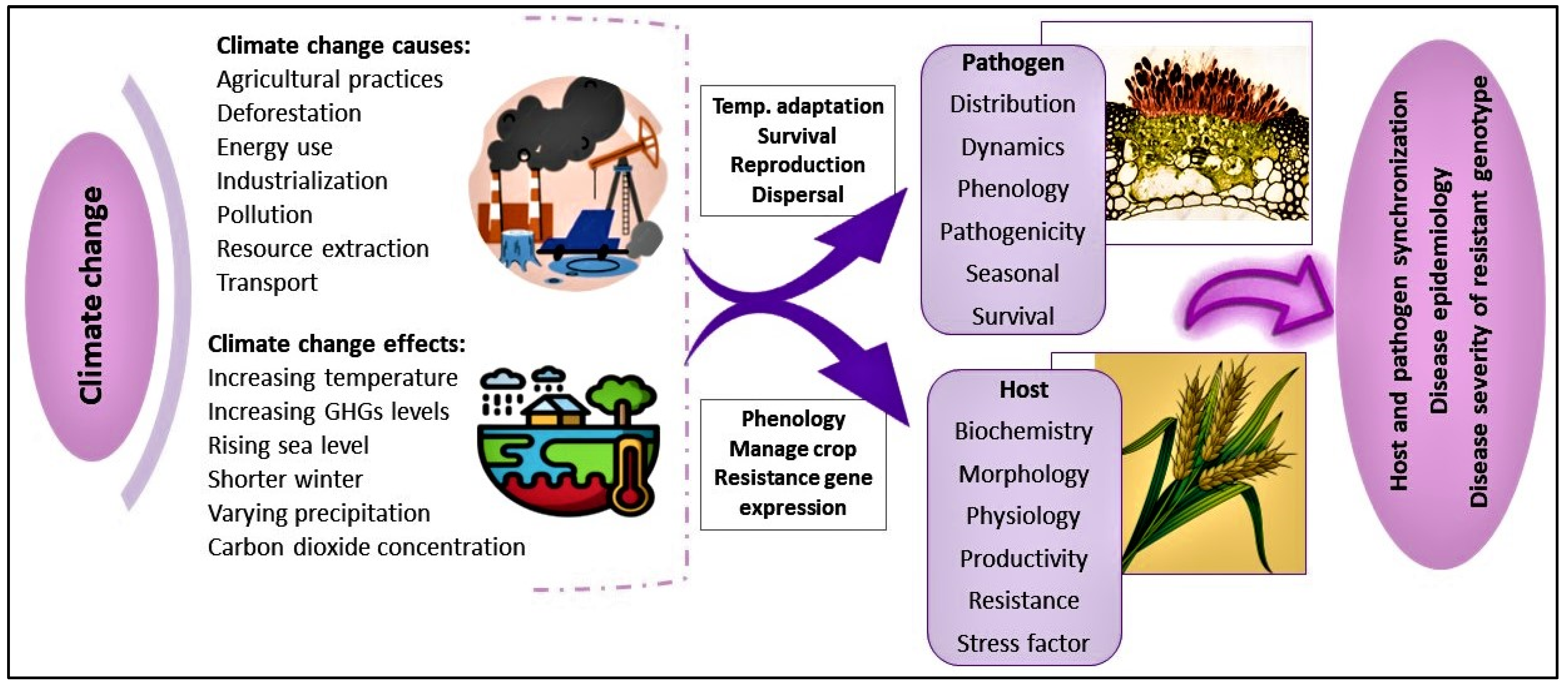
6. Impact of Climate Change on Wheat Rust
6.1. Changes in Wheat Rust Epidemiology
6.2. Adapting Wheat Cultivation to Climate Change
6.3. Developing Climate-Resilient Wheat Varieties
7. Future Directions and Research Gaps
7.1. Unexplored Areas in Wheat Rust Research
7.2. Potential for Technological Innovations
7.3. Policy and Research Recommendations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shafi, U.; Mumtaz, R.; Shafaq, Z.; Zaidi, S.M.H.; Kaifi, M.O.; Mahmood, Z.; Zaidi, S.A.R. Wheat rust disease detection techniques: A technical perspective. J. Plant Dis. Prot. 2022, 129, 489–504. [Google Scholar] [CrossRef]
- Dubin, H.; Brennan, J.P. Combating Stem and Leaf Rust of Wheat: Historical Perspective, Impacts, and Lessons Learned; International Food Policy Research Institute: Washington, DC, USA, 2009. [Google Scholar]
- Meyer, M.; Bacha, N.; Tesfaye, T.; Alemayehu, Y.; Abera, E.; Hundie, B.; Woldeab, G.; Girma, B.; Gemechu, A.; Negash, T. Wheat rust epidemics damage Ethiopian wheat production: A decade of field disease surveillance reveals national-scale trends in past outbreaks. PLoS ONE 2021, 16, e0245697. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Baranwal, D.K.; Harikrishna; Pal, D.; Bharti, H.; Joshi, P.; Thiyagarajan, B.; Gaikwad, K.B.; Bhardwaj, S.C.; Singh, G.P. Application of genomics tools in wheat breeding to attain durable rust resistance. Front. Plant Sci. 2020, 11, 567147. [Google Scholar] [CrossRef]
- Bouvet, L.; Holdgate, S.; James, L.; Thomas, J.; Mackay, I.J.; Cockram, J. The evolving battle between yellow rust and wheat: Implications for global food security. Theor. Appl. Genet. 2022, 135, 741–753. [Google Scholar] [CrossRef]
- Singh, J.; Chhabra, B.; Raza, A.; Yang, S.H.; Sandhu, K.S. Important wheat diseases in the US and their management in the 21st century. Front. Plant Sci. 2023, 13, 1010191. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhao, J.; Wu, J.; Zhan, G.; Han, D.; Kang, Z. Wheat stripe rust and integration of sustainable control strategies in China. Front. Agric. Sci. Eng 2022, 9, 37–51. [Google Scholar] [CrossRef]
- Kayim, M.; Nawaz, H.; Alsalmo, A. Fungal Diseases of Wheat. In Wheat; IntechOpen: London, UK, 2022. [Google Scholar]
- Mehmood, S.; Sajid, M.; Zhao, J.; Huang, L.; Kang, Z. Alternate hosts of Puccinia striiformis f. sp. tritici and their role. Pathogens 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Kolmer, J.A.; Fajolu, O. Virulence phenotypes of the wheat leaf rust pathogen, Puccinia triticina, in the United States from 2018 to 2020. Plant Dis. 2022, 106, 1723–1729. [Google Scholar] [CrossRef]
- Riar, A.K.; Chhuneja, P.; Keller, B.; Singh, K. Mechanism of leaf rust resistance in wheat wild relatives, Triticum monococcum L. and T. boeoticum L. Plant Genet. Resour. 2021, 19, 320–327. [Google Scholar] [CrossRef]
- Bellameche, F.; Jasim, M.A.; Mauch-Mani, B.; Mascher, F. Histopathological aspects of resistance in wheat to Puccinia triticina, induced by Pseudomonas protegens CHA0 and β-aminobutyric acid. Phytopathol. Mediterr. 2021, 60, 441–453. [Google Scholar] [CrossRef]
- Gultyaeva, E.; Shaydayuk, E.; Gannibal, P. Leaf rust resistance genes in wheat cultivars registered in Russia and their influence on adaptation processes in pathogen populations. Agriculture 2021, 11, 319. [Google Scholar] [CrossRef]
- Mapuranga, J.; Chang, J.; Zhao, J.; Liang, M.; Li, R.; Wu, Y.; Zhang, N.; Zhang, L.; Yang, W. The underexplored mechanisms of wheat resistance to leaf rust. Plants 2023, 12, 3996. [Google Scholar] [CrossRef] [PubMed]
- Panthi, U.; McCallum, B.; Kovalchuk, I.; Rampitsch, C.; Badea, A.; Yao, Z.; Bilichak, A. Foliar application of plant-derived peptides decreases the severity of leaf rust (Puccinia triticina) infection in bread wheat (Triticum aestivum L.). J. Genet. Eng. Biotechnol. 2024, 22, 100357. [Google Scholar] [CrossRef]
- Karelov, A.; Kozub, N.; Sozinova, O.; Pirko, Y.; Sozinov, I.; Yemets, A.; Blume, Y. Wheat genes associated with different types of resistance against stem rust (Puccinia graminis Pers.). Pathogens 2022, 11, 1157. [Google Scholar] [CrossRef]
- Tolossa, M.; Adugna, G.; Hundie, B. Spatial distribution and intensity of wheat stem rust (Puccinia graminis f. sp. tritici) in western and south-western Ethiopia. Am. J. Biol. Environ. Stat. 2022, 7, 70–80. [Google Scholar]
- Kosgey, Z.C.; Edae, E.A.; Dill-Macky, R.; Jin, Y.; Bulbula, W.D.; Gemechu, A.; Macharia, G.; Bhavani, S.; Randhawa, M.S.; Rouse, M.N. Mapping and validation of stem rust resistance loci in spring wheat line CI 14275. Front. Plant Sci. 2021, 11, 609659. [Google Scholar] [CrossRef]
- Upadhaya, A.; Upadhaya, S.G.; Brueggeman, R. Association mapping with a diverse population of Puccinia graminis f. sp. tritici identified avirulence loci interacting with the barley Rpg1 stem rust resistance gene. BMC Genom. 2024, 25, 751. [Google Scholar] [CrossRef]
- Chen, X. Pathogens which threaten food security: Puccinia striiformis, the wheat stripe rust pathogen. Food Secur. 2020, 12, 239–251. [Google Scholar] [CrossRef]
- Bai, Q.; Wan, A.; Wang, M.; See, D.R.; Chen, X. Molecular characterization of wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici) collections from nine countries. Int. J. Mol. Sci. 2021, 22, 9457. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Y.; Kang, Z.; Zhao, J. First report of a Puccinia striiformis f. sp. tritici race virulent to wheat stripe rust resistance gene Yr5 in China. Plant Dis. 2020, 104, 284. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, H.; Wang, H. Two new methods for severity assessment of wheat stripe rust caused by Puccinia striiformis f. sp. tritici. Front. Plant Sci. 2022, 13, 1002627. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E.M. Role of fungicide applications on the integrated management of wheat stripe rust. Front. Plant Sci. 2020, 11, 733. [Google Scholar] [CrossRef]
- Afzal, A.; Syed, S.; Nawaz, H.H.; Mustafa, R.; Aziz, M.; Khan, M.; Khan, A.; Javed, U.; Rehman, A.U.; Altaf, R. Unraveling the genetic and geographic diversity of Puccinia striiformis f. sp. tritici (Pst) populations for effective control of stripe rust in global wheat production. Pak. J. Agric. Res. 2024, 37, 88–101. [Google Scholar] [CrossRef]
- Belay, A.F.; Jenber, A.J. Integrated management of stripe rust (Puccinia striiformis f. sp. tritici) and its impact on the growth and yield of bread wheat (Triticum aestivum) in Ethiopia. Arch. Phytopathol. Plant Prot. 2024, 57, 112–141. [Google Scholar] [CrossRef]
- Bhavani, S.; Singh, P.; Qureshi, N.; He, X.; Biswal, A.K.; Juliana, P.; Dababat, A.; Mourad, A.M. Globally important wheat diseases: Status, challenges, breeding and genomic tools to enhance resistance durability. In Genomic Designing for Biotic Stress Resistant Cereal Crops; Springer: Cham, Switzerland, 2021; pp. 59–128. [Google Scholar]
- Aboukhaddour, R.; Fetch, T.; McCallum, B.D.; Harding, M.W.; Beres, B.L.; Graf, R.J. Wheat diseases on the prairies: A Canadian story. Plant Pathol. 2020, 69, 418–432. [Google Scholar] [CrossRef]
- Gessese, M.K. Description of wheat rusts and their virulence variations determined through annual pathotype surveys and controlled multi-pathotype tests. Adv. Agric. 2019, 2019, 2673706. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Lagudah, E.S.; Ayliffe, M.A.; Bhavani, S.; Rouse, M.N.; Pretorius, Z.A.; Szabo, L.J.; Huerta-Espino, J. Emergence and spread of new races of wheat stem rust fungus: Continued threat to food security and prospects of genetic control. Phytopathology 2015, 105, 872–884. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.; German, S.; McCallum, B.; Park, R.; Chen, W.Q.; Bhardwaj, S.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Srinivas, K.; Singh, V.K.; Srinivas, B.; Sameriya, K.K.; Prasad, L.; Singh, G. Determining the impact of stripe rust and leaf rust on grain yield and yield components’ losses in Indian wheat cultivars. Cereal Res. Commun. 2024, 52, 733–746. [Google Scholar] [CrossRef]
- Shahin, A.; Ashmawy, M.; El-Orabey, W.; Esmail, S. Yield losses in wheat caused by stripe rust (Puccinia striiformis) in Egypt. Am. J. Life Sci. 2020, 8, 127–134. [Google Scholar]
- Lidwell-Durnin, J.; Lapthorn, A. The threat to global food security from wheat rust: Ethical and historical issues in fighting crop diseases and preserving genetic diversity. Glob. Food Secur. 2020, 26, 100446. [Google Scholar] [CrossRef]
- Figueroa, M.; Dodds, P.N.; Henningsen, E.C. Evolution of virulence in rust fungi-multiple solutions to one problem. Curr. Opin. Plant Biol. 2020, 56, 20–27. [Google Scholar] [CrossRef]
- Olivera, P.D.; Bulbula, W.D.; Badebo, A.; Bockelman, H.E.; Edae, E.A.; Jin, Y. Field resistance to wheat stem rust in durum wheat accessions deposited at the USDA National Small Grains Collection. Crop Sci. 2021, 61, 2565–2578. [Google Scholar] [CrossRef]
- Shahin, A.; Mazrou, Y.S.; Omara, R.I.; Hermas, G.; Gad, M.; Mabrouk, O.I.; Abd-Elsalam, K.A.; Nehela, Y. Geographical correlation and genetic diversity of newly emerged races within the Ug99 lineage of stem rust pathogen, Puccinia graminis f. sp. tritici, in different wheat-producing areas. J. Fungi 2022, 8, 1041. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Ni, P.; Ni, Z.; Sun, Q.; Zong, Y. CRISPR-mediated acceleration of wheat improvement: Advances and perspectives. J. Genet. Genom. 2023, 50, 815–834. [Google Scholar] [CrossRef]
- Kumar, D.M.; Talekar, N.; Janeja, H.S.; Reddy, M.P.K. Breeding for rust resistance in wheat: A review. J. Surv. Fish. Sci. 2023, 10, 562–576. [Google Scholar]
- Ghimire, B.; Sapkota, S.; Bahri, B.A.; Martinez-Espinoza, A.D.; Buck, J.W.; Mergoum, M. Fusarium head blight and rust diseases in soft red winter wheat in the southeast United States: State of the art, challenges and future perspective for breeding. Front. Plant Sci. 2020, 11, 1080. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Yang, X.; Zhang, A.; Liu, D. Molecular markers and their applications in marker-assisted selection (MAS) in bread wheat (Triticum aestivum L.). Agriculture 2023, 13, 642. [Google Scholar] [CrossRef]
- Miedaner, T.; Boeven, A.L.G.-C.; Gaikpa, D.S.; Kistner, M.B.; Grote, C.P. Genomics-assisted breeding for quantitative disease resistances in small-grain cereals and maize. Int. J. Mol. Sci. 2020, 21, 9717. [Google Scholar] [CrossRef]
- Zhang, J.; Nirmala, J.; Chen, S.; Jost, M.; Steuernagel, B.; Karafiatova, M.; Hewitt, T.; Li, H.; Edae, E.; Sharma, K. Single amino acid change alters specificity of the multi-allelic wheat stem rust resistance locus SR9. Nat. Commun. 2023, 14, 7354. [Google Scholar] [CrossRef]
- Marchal, C.; Zhang, J.; Zhang, P.; Fenwick, P.; Steuernagel, B.; Adamski, N.M.; Boyd, L.; McIntosh, R.; Wulff, B.B.; Berry, S. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 2018, 4, 662–668. [Google Scholar] [CrossRef]
- Steuernagel, B.; Periyannan, S.K.; Hernández-Pinzón, I.; Witek, K.; Rouse, M.N.; Yu, G.; Hatta, A.; Ayliffe, M.; Bariana, H.; Jones, J.D. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 2016, 34, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hewitt, T.C.; Boshoff, W.H.; Dundas, I.; Upadhyaya, N.; Li, J.; Patpour, M.; Chandramohan, S.; Pretorius, Z.A.; Hovmøller, M. A recombined Sr26 and Sr61 disease resistance gene stack in wheat encodes unrelated NLR genes. Nat. Commun. 2021, 12, 3378. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Steuernagel, B.; Ghosh, S.; Herren, G.; Hurni, S.; Adamski, N.; Vrána, J.; Kubaláková, M.; Krattinger, S.G.; Wicker, T. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Matny, O.; Gourdoupis, S.; Rayapuram, N.; Aljedaani, F.R.; Wang, Y.L.; Nürnberger, T.; Johnson, R.; Crean, E.E.; Saur, I.M.-L. The wheat stem rust resistance gene Sr43 encodes an unusual protein kinase. Nat. Genet. 2023, 55, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Matny, O.; Champouret, N.; Steuernagel, B.; Moscou, M.J.; Hernández-Pinzón, I.; Green, P.; Hayta, S.; Smedley, M.; Harwood, W. Aegilops sharonensis genome-assisted identification of stem rust resistance gene Sr62. Nat. Commun. 2022, 13, 1607. [Google Scholar] [CrossRef]
- Ni, F.; Zheng, Y.; Liu, X.; Yu, Y.; Zhang, G.; Epstein, L.; Mao, X.; Wu, J.; Yuan, C.; Lv, B. Sequencing trait-associated mutations to clone wheat rust-resistance gene YrNAM. Nat. Commun. 2023, 14, 4353. [Google Scholar] [CrossRef]
- Li, H.; Hua, L.; Zhao, S.; Hao, M.; Song, R.; Pang, S.; Liu, Y.; Chen, H.; Zhang, W.; Shen, T.; et al. Cloning of the wheat leaf rust resistance gene Lr47 introgressed from Aegilops speltoides. Nat. Commun. 2023, 14, 6072. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing future crops: Genomics-assisted breeding comes of age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef]
- Wulff, B.B.; Krattinger, S.G. The long road to engineering durable disease resistance in wheat. Curr. Opin. Biotechnol. 2022, 73, 270–275. [Google Scholar] [CrossRef]
- Jost, M.; Outram, M.A.; Dibley, K.; Zhang, J.; Luo, M.; Ayliffe, M. Plant and pathogen genomics: Essential approaches for stem rust resistance gene stacks in wheat. Front. Plant Sci. 2023, 14, 1223504. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, S.; Xu, J.; Yan, L.; Ma, Y.; Xia, L. Pyramiding favorable alleles in an elite wheat variety in one generation by CRISPR-Cas9-mediated multiplex gene editing. Mol. Plant 2021, 14, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.-L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, X.; Zhao, Q.; Liu, Z.; Li, X.; Ren, Y.; Tang, J.; Fang, J.; Xu, Q.; Bu, Q. The E3 ligase DROUGHT HYPERSENSITIVE negatively regulates cuticular wax biosynthesis by promoting the degradation of transcription factor ROC4 in rice. Plant Cell 2018, 30, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, X.; Xu, W.; Hu, T.; Ma, J.; Zhang, Y.; Hou, J.; Hao, C.; Zhang, X.; Li, T. TaGW2L, a GW2-like RING finger E3 ligase, positively regulates heading date in common wheat (Triticum aestivum L.). Crop J. 2022, 10, 972–979. [Google Scholar] [CrossRef]
- Haber, Z.; Sharma, D.; Selvaraj, K.V.; Sade, N. Is CRISPR/Cas9-based multi-trait enhancement of wheat forthcoming? Plant Sci. 2024, 341, 112021. [Google Scholar] [CrossRef]
- Dijkerman, A. Genome Editing in Bread Wheat Using CRISPR/Cas9. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2022. [Google Scholar]
- Wu, S.; Kyaw, H.; Tong, Z.; Yang, Y.; Wang, Z.; Zhang, L.; Deng, L.; Zhang, Z.; Xiao, B.; Quick, W.P. A simple and efficient CRISPR/Cas9 system permits ultra-multiplex genome editing in plants. Crop J. 2024, 12, 569–582. [Google Scholar] [CrossRef]
- Zou, J.; Hu, D.; Mason, A.S.; Shen, X.; Wang, X.; Wang, N.; Grandke, F.; Wang, M.; Chang, S.; Snowdon, R.J. Genetic changes in a novel breeding population of Brassica napus synthesized from hundreds of crosses between B. rapa and B. carinata. Plant Biotechnol. J. 2018, 16, 507–519. [Google Scholar] [CrossRef]
- Li, J.; Dundas, I.; Dong, C.; Li, G.; Trethowan, R.; Yang, Z.; Hoxha, S.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef]
- Kumar, K.; Jan, I.; Saripalli, G.; Sharma, P.; Mir, R.R.; Balyan, H.; Gupta, P. An update on resistance genes and their use in the development of leaf rust resistant cultivars in wheat. Front. Genet. 2022, 13, 816057. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Dodds, P.; Lagudah, E. How target-sequence enrichment and sequencing (TEnSeq) pipelines have catalyzed resistance gene cloning in the wheat-rust pathosystem. Front. Plant Sci. 2020, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.C.; Singh, G.P.; Gangwar, O.P.; Prasad, P.; Kumar, S. Status of wheat rust research and progress in rust management-Indian context. Agronomy 2019, 9, 892. [Google Scholar] [CrossRef]
- Dinh, H.X.; Singh, D.; Periyannan, S.; Park, R.F.; Pourkheirandish, M. Molecular genetics of leaf rust resistance in wheat and barley. Theor. Appl. Genet. 2020, 133, 2035–2050. [Google Scholar] [CrossRef]
- Prasad, P.; Jain, N.; Chaudhary, J.; Thakur, R.K.; Savadi, S.; Bhardwaj, S.C.; Gangwar, O.P.; Lata, C.; Adhikari, S.; Kumar, S.; et al. Candidate effectors for leaf rust resistance gene Lr28 identified through transcriptome and in-silico analysis. Front Microbiol. 2023, 14, 1143703. [Google Scholar] [CrossRef] [PubMed]
- McCallum, B.D.; Hiebert, C.W. Interactions between Lr67 or Lr34 and other leaf rust resistance genes in wheat (Triticum aestivum). Front. Plant Sci. 2022, 13, 871970. [Google Scholar] [CrossRef]
- Bokore, F.E.; Knox, R.E.; Hiebert, C.W.; Cuthbert, R.D.; DePauw, R.M.; Meyer, B.; N’Diaye, A.; Pozniak, C.J.; McCallum, B.D. A combination of leaf rust resistance genes, including Lr34 and Lr46, is the key to the durable resistance of the Canadian wheat cultivar, Carberry. Front. Plant Sci. 2022, 12, 775383. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhao, C.; Liu, D.; Jambuthenne, D.T.; Sun, M.; Dinglasan, E.; Periyannan, S.K.; Hickey, L.T.; Hayes, B.J. Genome-wide atlas of rust resistance loci in wheat. Theor Appl Genet. 2024, 137, 179. [Google Scholar] [CrossRef]
- Luo, J.; Rouse, M.N.; Hua, L.; Li, H.; Li, B.; Li, T.; Zhang, W.; Gao, C.; Wang, Y.; Dubcovsky, J. Identification and characterization of Sr22b, a new allele of the wheat stem rust resistance gene Sr22 effective against the Ug99 race group. Plant Biotechnol. J. 2022, 20, 554–563. [Google Scholar] [CrossRef]
- Omara, R.I.; Shahin, A.A.; Ahmed, S.M.; Mostafa, Y.S.; Alamri, S.A.; Hashem, M.; Elsharkawy, M.M. Wheat resistance to stripe and leaf rusts conferred by introgression of slow rusting resistance genes. J. Fungi 2021, 7, 622. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, Q.; Han, D.; Wu, J.; Wu, L.; Tong, J.; Xu, X.; Yan, J.; Zhang, Y.; Xu, K. Molecular characterization and validation of adult-plant stripe rust resistance gene Yr86 in Chinese wheat cultivar Zhongmai 895. Theor. Appl. Genet. 2023, 136, 142. [Google Scholar] [CrossRef]
- Ye, B.; Singh, R.P.; Yuan, C.; Liu, D.; Randhawa, M.S.; Huerta-Espino, J.; Bhavani, S.; Lagudah, E.; Lan, C. Three co-located resistance genes confer resistance to leaf rust and stripe rust in wheat variety Borlaug 100. Crop J. 2022, 10, 490–497. [Google Scholar] [CrossRef]
- Bariana, H.; Kant, L.; Qureshi, N.; Forrest, K.; Miah, H.; Bansal, U. Identification and characterisation of stripe rust resistance genes Yr66 and Yr67 in wheat cultivar VL Gehun 892. Agronomy 2022, 12, 318. [Google Scholar] [CrossRef]
- Anupriya, C.; Shradha, N.; Prasun, B.; Abha, A.; Pankaj, S.; Abdin, M.Z.; Neeraj, S. Genomic and molecular perspectives of host-pathogen interaction and resistance strategies against white rust in oilseed mustard. Curr. Genom. 2020, 21, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Shilpa; Thakur, R.K.; Prasad, P.; Bhardwaj, S.; Gangwar, O.; Kumar, S. Epigenetics of wheat–rust interaction: An update. Planta 2022, 255, 50. [Google Scholar] [CrossRef]
- Kanyuka, K.; Rudd, J.J. Cell surface immune receptors: The guardians of the plant’s extracellular spaces. Curr. Opin. Plant Biol. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, N. Defense signaling in plants against micro-creatures: Do or die. Indian Phytopathol. 2020, 73, 605–613. [Google Scholar] [CrossRef]
- Wang, Y.; Abrouk, M.; Gourdoupis, S.; Koo, D.-H.; Karafiátová, M.; Molnár, I.; Holušová, K.; Doležel, J.; Athiyannan, N.; Cavalet-Giorsa, E. An unusual tandem kinase fusion protein confers leaf rust resistance in wheat. Nat. Genet. 2023, 55, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Malzahn, A.A.; Sretenovic, S.; Qi, Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants 2019, 5, 778–794. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Tang, Y.; Zhang, S.; Xu, M.; Liu, M.; Zhang, J.; Loake, G.J.; Jiang, J. The biological roles of Puccinia striiformis f. sp. tritici effectors during infection of wheat. Biomolecules 2023, 13, 889. [Google Scholar] [CrossRef]
- Nykiel, M.; Gietler, M.; Fidler, J.; Prabucka, B.; Rybarczyk-Płońska, A.; Graska, J.; Boguszewska-Mańkowska, D.; Muszyńska, E.; Morkunas, I.; Labudda, M. Signal transduction in cereal plants struggling with environmental stresses: From perception to response. Plants 2022, 11, 1009. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Aggarwal, S.K.; Kaul, N.; Jasrotia, P.; Gupta, A.; Singh, G.P. Resistance inducers and their role in reinforcing wheat defense system against fungal pathogens. J. Cereal Res. 2021, 13, 229–254. [Google Scholar] [CrossRef]
- Liu, W.; Frick, M.; Huel, R.; Nykiforuk, C.L.; Wang, X.; Gaudet, D.A.; Eudes, F.; Conner, R.L.; Kuzyk, A.; Chen, Q. The stripe rust resistance gene Yr10 encodes an evolutionary-conserved and unique CC–NBS–LRR sequence in wheat. Mol. Plant 2014, 7, 1740–1755. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Jordan, D.R.; Mace, E.S.; Raghavan, C.; Luo, M.-C.; Keller, B.; Lagudah, E.S. Recent emergence of the wheat Lr34 multi-pathogen resistance: Insights from haplotype analysis in wheat, rice, sorghum and Aegilops tauschii. Theor. Appl. Genet. 2013, 126, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Annan, E.N.; Huang, L. Molecular mechanisms of the co-evolution of wheat and rust pathogens. Plants 2023, 12, 1809. [Google Scholar] [CrossRef]
- Thind, T. Changing trends in discovery of new fungicides: A perspective. Indian Phytopathol. 2021, 74, 875–883. [Google Scholar] [CrossRef]
- Ul Haq, I.; Ijaz, S. History and recent trends in plant disease control: An overview. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches; Springer: Cham, Switzerland, 2020; Volume 13. [Google Scholar]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Wanyera, R.; Wamalwa, M.; Odemba, M.; Wanga, H.; Kinyanjui, P.; Onyango, V.; Owuoche, J. Management of wheat rusts at different growth stages using Nativo 300 SC (trifloxystrobin 100g/L+ tebuconazole 200g/L) fungicide. Aust. J. Crop Sci. 2016, 10, 1273–1280. [Google Scholar] [CrossRef]
- Bajoriya, D.K.; Mishra, K.K.; Yadav, V.K. Evaluation of newer fungicides for the management of wheat stem rust caused by (Puccinia graminis f. sp. tritici). J. Cereal Res. 2023, 15, 277–283. [Google Scholar] [CrossRef]
- Abdissa, T.; Gutu, K.; Zeleke, T.; Bekele, B. Evaluation of different fungicides against wheat rusts in West and Southwest Shewa zones, Ethiopia. Afr. J. Plant Sci. 2024, 18, 41–47. [Google Scholar]
- Fei, H.; Tianjie, L.; Wei, Z.; Shengming, L.; Jianqiang, X. Control efficacy of the combination of pyraclostrobin and epoxiconazole against wheat leaf rust and its safety evaluation on wheat plants. Chin. J. Pestic. Sci. 2023, 25, 97–103. [Google Scholar]
- da Cruz, T.P.; da Rocha, M.R.; da Silva, S.M.F.; Moraes, W.B.; Moraes, S.d.P.C.B.; Gazolla, P.A.R.; de Oliveira, M.B.; de Queiroz, V.T.; Teixeira, R.R.; de Oliveira, O.V. Fungicidal activity and molecular docking of glycerol-derived triazole compounds for controlling coffee leaf rust. Plant Pathol. 2024. [Google Scholar] [CrossRef]
- Basandrai, A.K.; Mehta, A.; Rathee, V.; Basandrai, D.; Sharma, B. Efficacy of fungicides in managing yellow rust of wheat. J. Cereal Res. 2020, 12, 103–108. [Google Scholar] [CrossRef]
- Gupta, H.K.; Singh, S.; Awasthi, R.K.; Kumar, A. Integrated disease management in agriculture: A comprehensive approach to crop health. Emerg. Trends Entomol. 2023, 135. Available online: https://www.researchgate.net/profile/Sunny-Maanju/publication/381398466_Emerging_Trends_in_Entomology/links/666b169b85a4ee7261c0c2f8/Emerging-Trends-in-Entomology.pdf#page=143 (accessed on 29 August 2024).
- Maulenbay, A.; Rsaliyev, A. Fungal disease tolerance with a focus on wheat: A review. J. Fungi 2024, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Eltaher, S.; Mourad, A.M.; Baenziger, P.S.; Wegulo, S.; Belamkar, V.; Sallam, A. Identification and validation of high LD hotspot genomic regions harboring stem rust resistant genes on 1B, 2A (Sr38), and 7B chromosomes in wheat. Front. Genet. 2021, 12, 749675. [Google Scholar] [CrossRef]
- Ren, X.; Wang, C.; Ren, Z.; Wang, J.; Zhang, P.; Zhao, S.; Li, M.; Yuan, M.; Yu, X.; Li, Z. Genetics of resistance to leaf rust in wheat: An overview in a genome-wide level. Sustainability 2023, 15, 3247. [Google Scholar] [CrossRef]
- Nazarov, T.; Liu, Y.; Chen, X.; See, D.R. Molecular mechanisms of the stripe rust interaction with resistant and susceptible wheat genotypes. Int. J. Mol. Sci. 2024, 25, 2930. [Google Scholar] [CrossRef]
- Muthe, S.T.; Kulwal, P.L.; Gadekar, D.A.; Jadhav, A.S. Molecular marker based detection of leaf rust resistance gene Lr34 in Indian bread wheat (Triticum aestivum L.). Australas. Plant Pathol. 2016, 45, 369–376. [Google Scholar] [CrossRef]
- Savadi, S.; Prasad, P.; Kashyap, P.; Bhardwaj, S. Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant Pathol. 2018, 67, 771–791. [Google Scholar] [CrossRef]
- Aradottir, G.I.; Crespo-Herrera, L. Host plant resistance in wheat to barley yellow dwarf viruses and their aphid vectors: A review. Curr. Opin. Insect Sci. 2021, 45, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Li, J.; Yan, L.; Wang, N.; Xia, L. Present and future prospects for wheat improvement through genome editing and advanced technologies. Plant Commun. 2021, 2, 100211. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 1–26. [Google Scholar] [CrossRef]
- Reynolds, M.; Chapman, S.; Crespo-Herrera, L.; Molero, G.; Mondal, S.; Pequeno, D.N.; Pinto, F.; Pinera-Chavez, F.J.; Poland, J.; Rivera-Amado, C. Breeder friendly phenotyping. Plant Sci. 2020, 295, 110396. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Espinosa, K.C.; Fernández-González, M.; Almaguer, M.; Guada, G.; Rodríguez-Rajo, F.J. Puccinia spore concentrations in relation to weather factors and phenological development of a wheat crop in northwestern Spain. Agriculture 2023, 13, 1637. [Google Scholar] [CrossRef]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Muhammad, M.; Alqahtani, F.M.; Hashem, M.; Salih, H.; Zhang, D. Climate change reshaping plant-fungal interaction. Environ. Res. 2023, 238, 117282. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmar, S.; Sohail, M.A.; Kamran, M.; Ali, M.; Saleem, M.H.; Rizwan, M.; Ahmed, A.M.; Mora-Poblete, F.; do Amaral Júnior, A.T. Research advances and applications of biosensing technology for the diagnosis of pathogens in sustainable agriculture. Environ. Sci. Pollut. Res. 2021, 28, 9002–9019. [Google Scholar] [CrossRef]
- Wani, S.H.; Khan, H.; Riaz, A.; Joshi, D.C.; Hussain, W.; Rana, M.; Kumar, A.; Athiyannan, N.; Singh, D.; Ali, N. Genetic diversity for developing climate-resilient wheats to achieve food security goals. Adv. Agron. 2022, 171, 255–303. [Google Scholar]
- Xia, C.; Qiu, A.; Wang, M.; Liu, T.; Chen, W.; Chen, X. Current status and future perspectives of genomics research in the rust fungi. Int. J. Mol. Sci. 2022, 23, 9629. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Arif, A.R.; Yasin, M.H.; Zubair, M.M.; Salman, M.; Mustafa, M.G.; Khalid, S.; Bashir, U.; Usman, M. Worldwide wheat diseases their current status and mode of resistance: A review. Asian J. Biotechnol. Genet. Eng. 2024, 7, 139–149. [Google Scholar]
- Wang, R.; Shakir, A.M.; Geng, M.; Tian, J. Dissection of QTLs underlying the genetic basis of drought resistance in wheat: A Meta-Analysis. Theor. Appl. Genet. 2024. [Google Scholar] [CrossRef]
- Gilligan, C.A. Developing predictive models and early warning systems for invading pathogens: Wheat rusts. Annu. Rev. Phytopathol. 2024, 62. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Bhardwaj, S.C.; Thakur, R.K.; Adhikari, S.; Gangwar, O.P.; Lata, C.; Kumar, S. Prospects of climate change effects on crop diseases with particular reference to wheat. J. Cereal 2021, 13, 118–135. [Google Scholar]
- Bhandawat, A.; Sharma, V.; Rishi, V.; Roy, J.K. Biolistic delivery of programmable nuclease (CRISPR/Cas9) in bread wheat. In Biolistic DNA Delivery in Plants. Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2124, pp. 309–329. [Google Scholar]
- Wang, S.; Gao, C.; Sun, Q.; Liu, Q.; Wang, C.; Guo, F.; Ma, Z. Genetic characterization of Puccinia striiformis f. sp. tritici populations from different wheat cultivars using simple sequence repeats. J. Fungi 2022, 8, 705. [Google Scholar] [CrossRef]
- Adamchuk, V.; Allred, B.; Viscarra Rossel, R. Proximal soil sensing: Global perspective. Fast Times News Near Surf. Geophys. Sci. 2012, 17, 13–16. [Google Scholar]
- Jiang, J.; Zhao, J.; Duan, W.; Tian, S.; Wang, X.; Zhuang, H.; Fu, J.; Kang, Z. TaAMT2; 3a, a wheat AMT2-type ammonium transporter, facilitates the infection of stripe rust fungus on wheat. BMC Plant Biol. 2019, 19, 239. [Google Scholar] [CrossRef]
- Hussain, N. Development of a Smart Variable Rate Sprayer Using Deep Convolutional Neural Networks for Site-Specific Application of Agrochemicals. Master’s Thesis, University of Prince Edward Island, Charlottetown, PE, Canada, 2020. Available online: https://islandscholar.ca/islandora/object/ir%3A23560/datastream/PDF/view (accessed on 29 August 2024).

| Wheat Rusts | Current Importance | Historical Importance | ||||||
|---|---|---|---|---|---|---|---|---|
| Major | Minor | Local | Rare | Major | Minor | Local | Rare | |
| Leaf Rust | North Africa Central and Southeast Asia East Europe America | -- | East and South Africa East, South, and West Asia Australia New Zealand West Europe | -- | North Africa Central, South and Southeast Asia Europe America | -- | East and South Africa East, South, and West Asia Australia New Zealand | -- |
| Stem Rust | East Africa | Central, South, and Southeast Asia Europe North America | North and South Africa East and West Asia Australia New Zealand South America | -- | Africa East, South, and West Asia Australia New Zealand Europe America | Central and Southeast Asia | -- | -- |
| Stripe Rust | East Africa East and West Asia West Europe | -- | North and South Africa Central and South Asia Australia New Zealand East Europe America | Southeast Asia | East Africa East and West Asia West Europe | -- | North Africa Central and South Asia East Europe America | South Africa Southeast Asia Australia New Zealand |
| Disease | Resistance Genes | References |
|---|---|---|
| Leaf Rust | Lr1, Lr9/Lr58, Lr10, Lr13, Lr14a, Lr21, Lr22a, Lr34, Lr42, Lr47, Lr67 | [51,68,69,70] |
| Stem Rust | Sr9, Sr13, Sr21, Sr33, Sr35, Sr50, Sr55, Sr57, Sr60, Sr62, Sr22, Sr26, Sr27, Sr45, Sr61, Sr43, Sr46, SrTA1662 | [48,49,71,72] |
| Stripe Rust | Yr5/YrSP, Yr7, Yr27, Yr15, Yr18, Yr36, Yr46, Yr28, YrU1, Yr10/YrNAM | [63,73,74,75,76] |
| Generic Name | Chemical Name | Refs. |
|---|---|---|
| Triadimefon | (RS)-1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl) butan-2-one | [38] |
| Tebuconazole | (RS)-1-(4-chlorophenyl)-4,4-dimethyl-3-(1,2,4-triazol-1-ylmethyl) pentan-3-ol | [94] |
| Propiconazole | 1-[[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl] methyl]-1H-1,2,4-triazole | [95] |
| Azoxystrobin | methyl (E)-2-{2-[6-(2-cyanophenoxy) pyrimidin-4-yloxy] phenyl}-3-methoxyacrylate | [96] |
| Epoxiconazole | (2RS,3SR)-1-{(2RS)-2-[4-(2,4-dichlorophenyl) phenyl]-1,3-dioxolan-2-yl] methyl}-1H-1,2,4-triazole | [97] |
| Cyproconazole | 2-(4-chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl) pentan-2-ol | [96] |
| Flutriafol | (RS)-2,4-difluoro-α-(1H-1,2,4-triazol-1-ylmethyl) benzhydryl alcohol | [98] |
| Difenoconazole | (E)-1-[2-[2-chloro-4-(4-chlorophenoxy) phenyl]-4-methyl-1,3-dioxolan-2-ylmethyl]-1H-1,2,4-triazole | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.u.; Qiao, L.; Shen, T.; Hua, L.; Li, H.; Ahmad, Z.; Chen, S. Exploring the Frontier of Wheat Rust Resistance: Latest Approaches, Mechanisms, and Novel Insights. Plants 2024, 13, 2502. https://doi.org/10.3390/plants13172502
Rehman Su, Qiao L, Shen T, Hua L, Li H, Ahmad Z, Chen S. Exploring the Frontier of Wheat Rust Resistance: Latest Approaches, Mechanisms, and Novel Insights. Plants. 2024; 13(17):2502. https://doi.org/10.3390/plants13172502
Chicago/Turabian StyleRehman, Shams ur, Liang Qiao, Tao Shen, Lei Hua, Hongna Li, Zishan Ahmad, and Shisheng Chen. 2024. "Exploring the Frontier of Wheat Rust Resistance: Latest Approaches, Mechanisms, and Novel Insights" Plants 13, no. 17: 2502. https://doi.org/10.3390/plants13172502






