Effects of Different Straw Return Modes on Soil Carbon, Nitrogen, and Greenhouse Gas Emissions in the Semiarid Maize Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Field Experimental Design
2.3. Sampling and Analysis Methods
2.3.1. Soil Samples
2.3.2. Plant Samples
2.3.3. Determination of GHG Emissions
2.4. Data Analysis
3. Results
3.1. Soil Dissolved Organic Carbon (DOC)
3.2. Soil Nitrogen
3.2.1. Soil Total Nitrogen (TN)
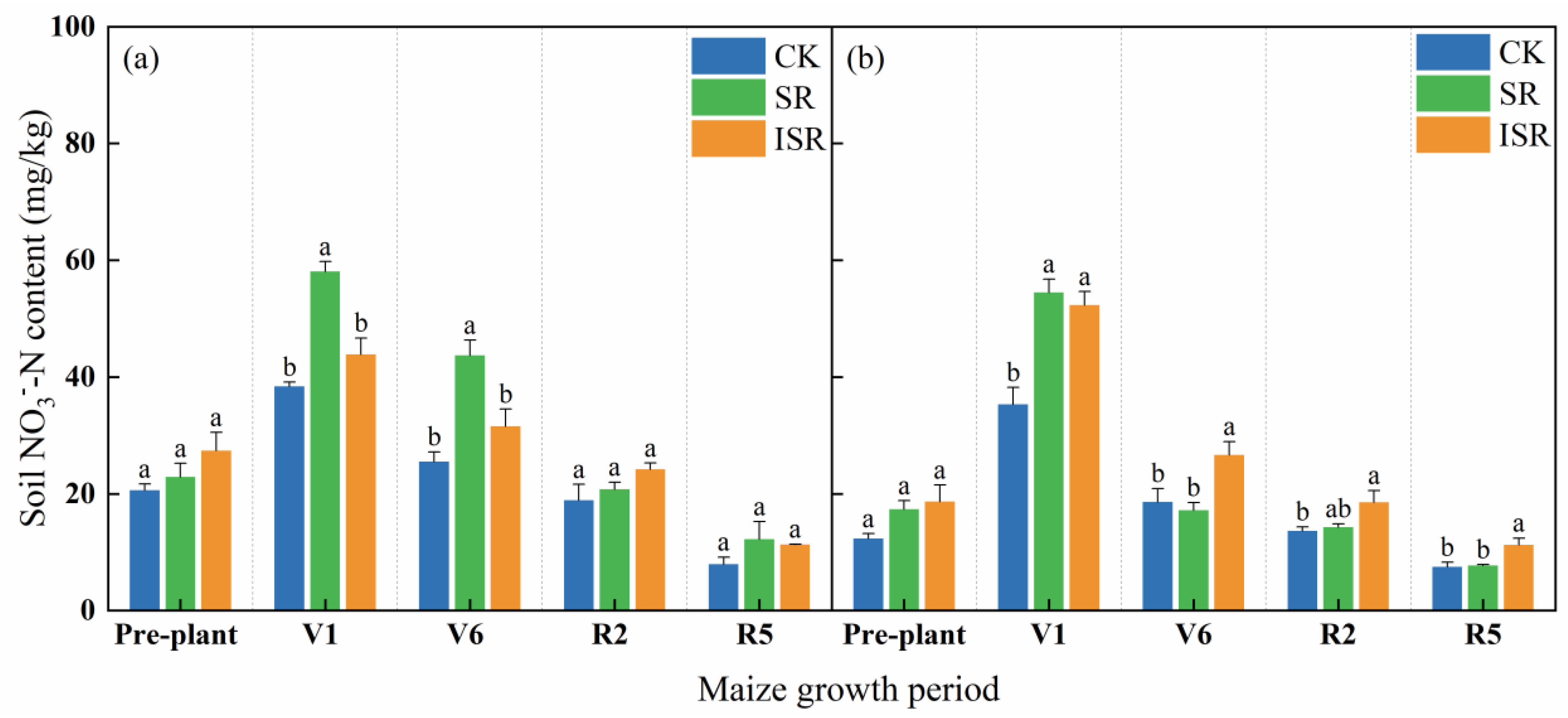
3.2.2. Soil Nitrate Nitrogen (NO3−-N)
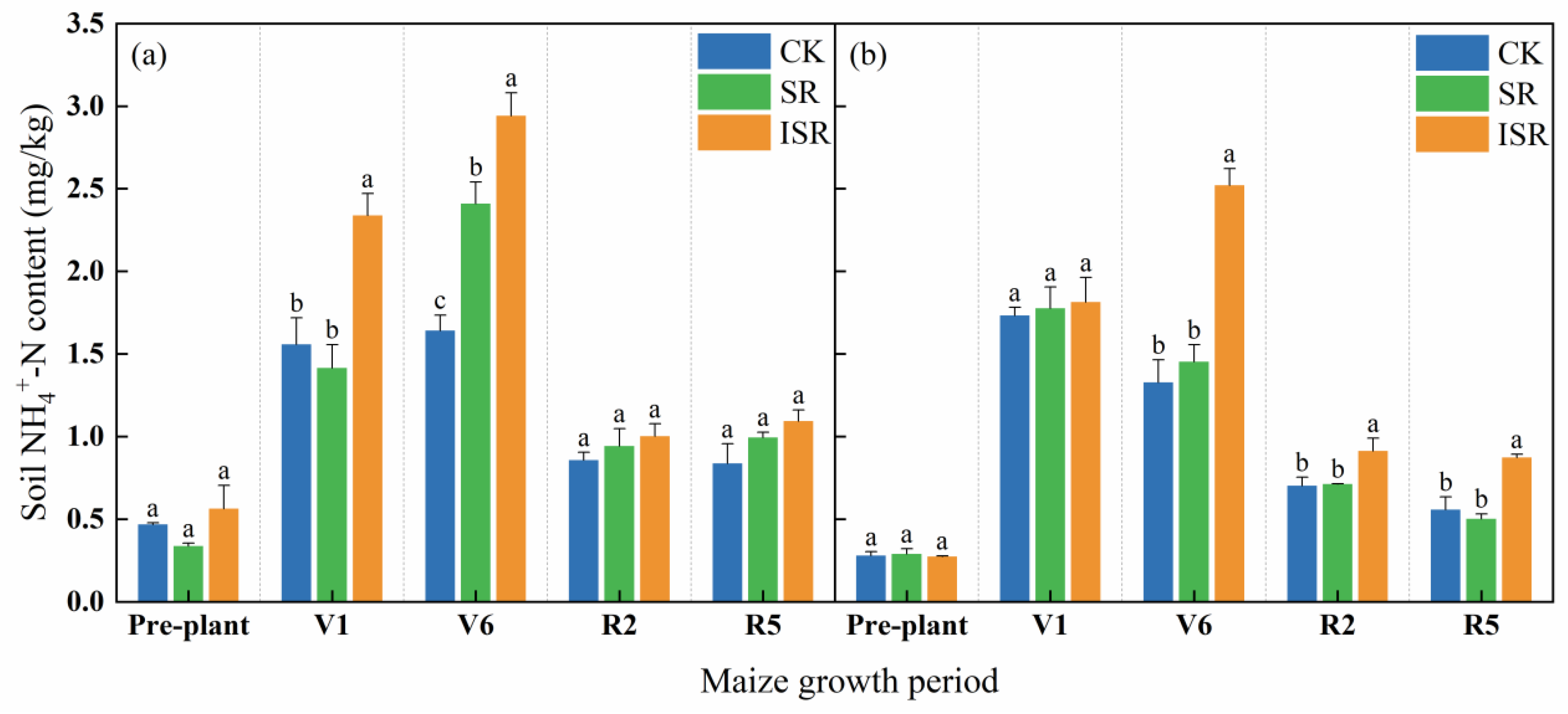
3.2.3. Soil Ammonium Nitrogen (NH4+-N)
3.2.4. Soil Dissolved Organic Nitrogen (DON)
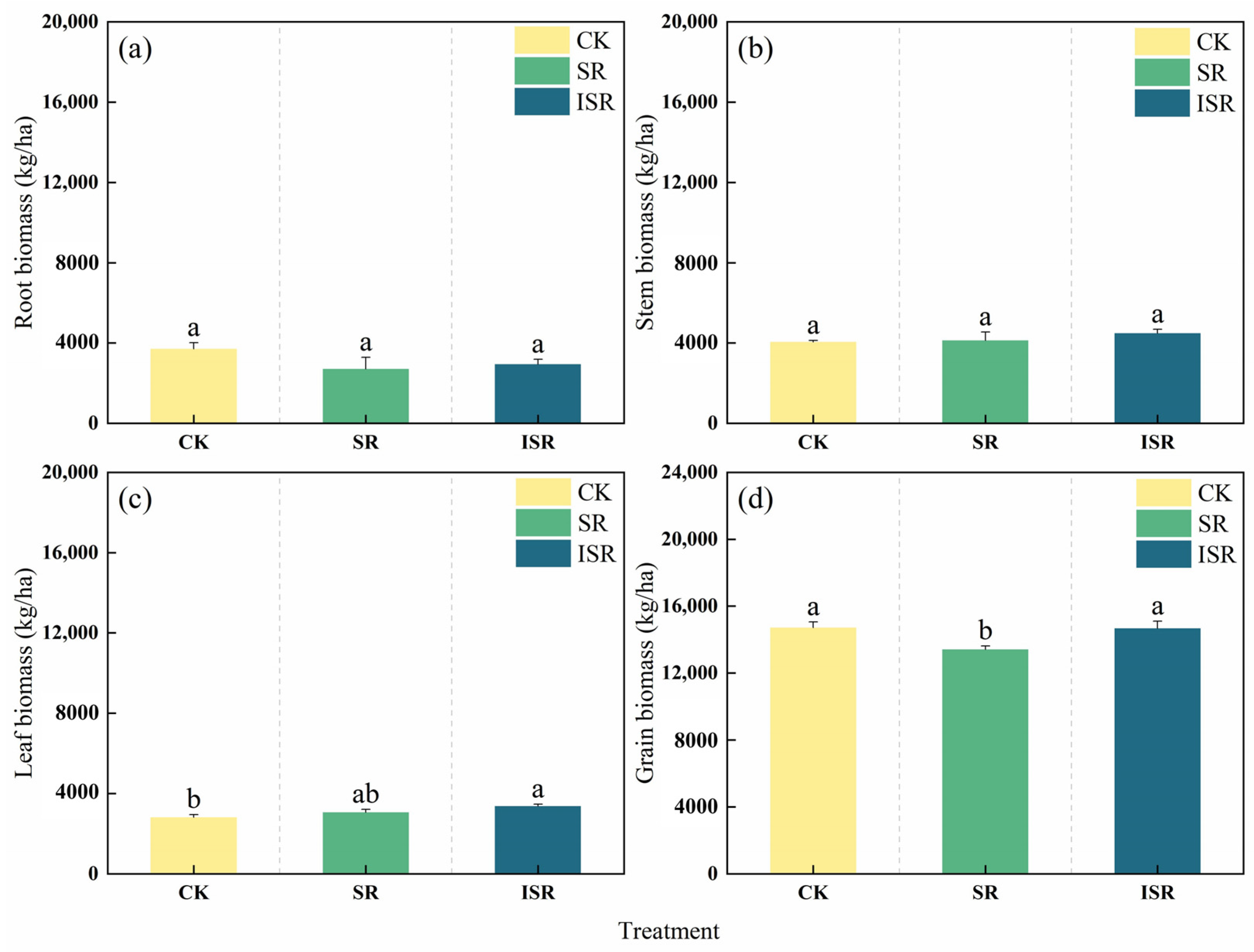
3.3. Biomass of Different Parts of Maize
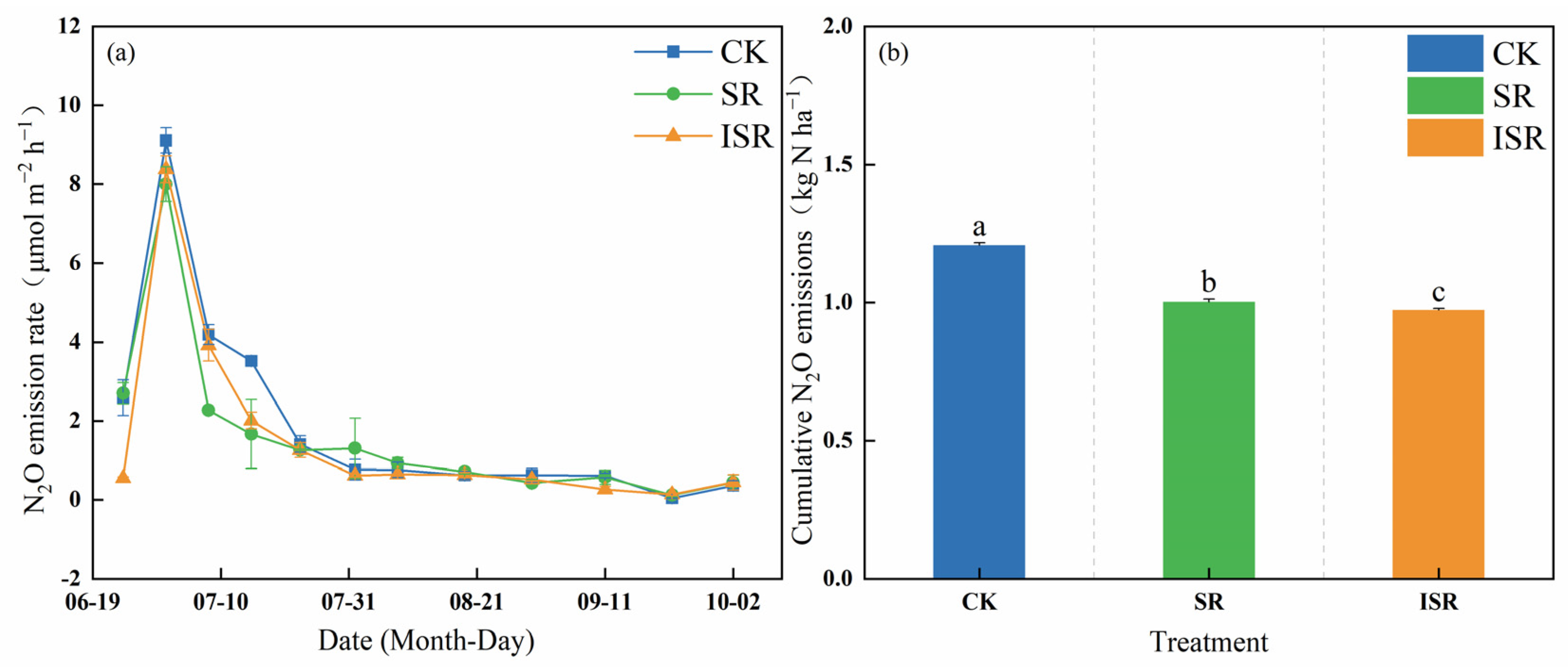
3.4. Soil GHG Emissions
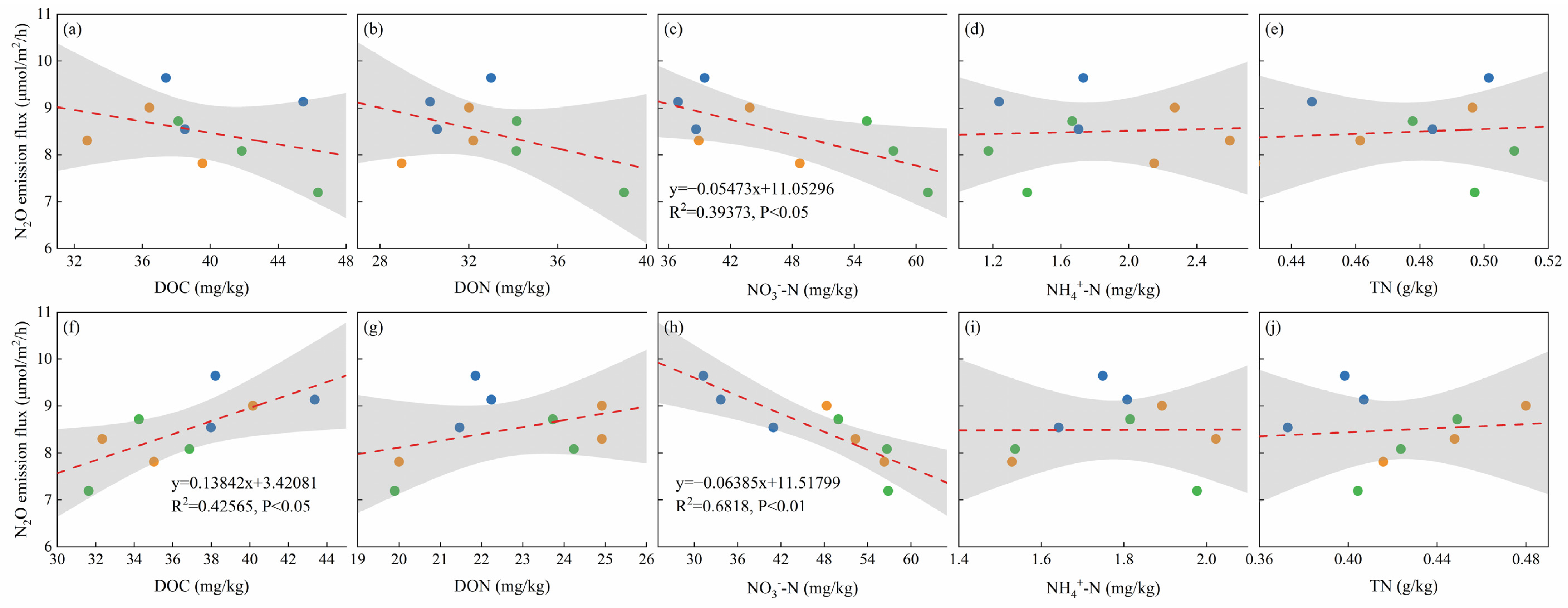
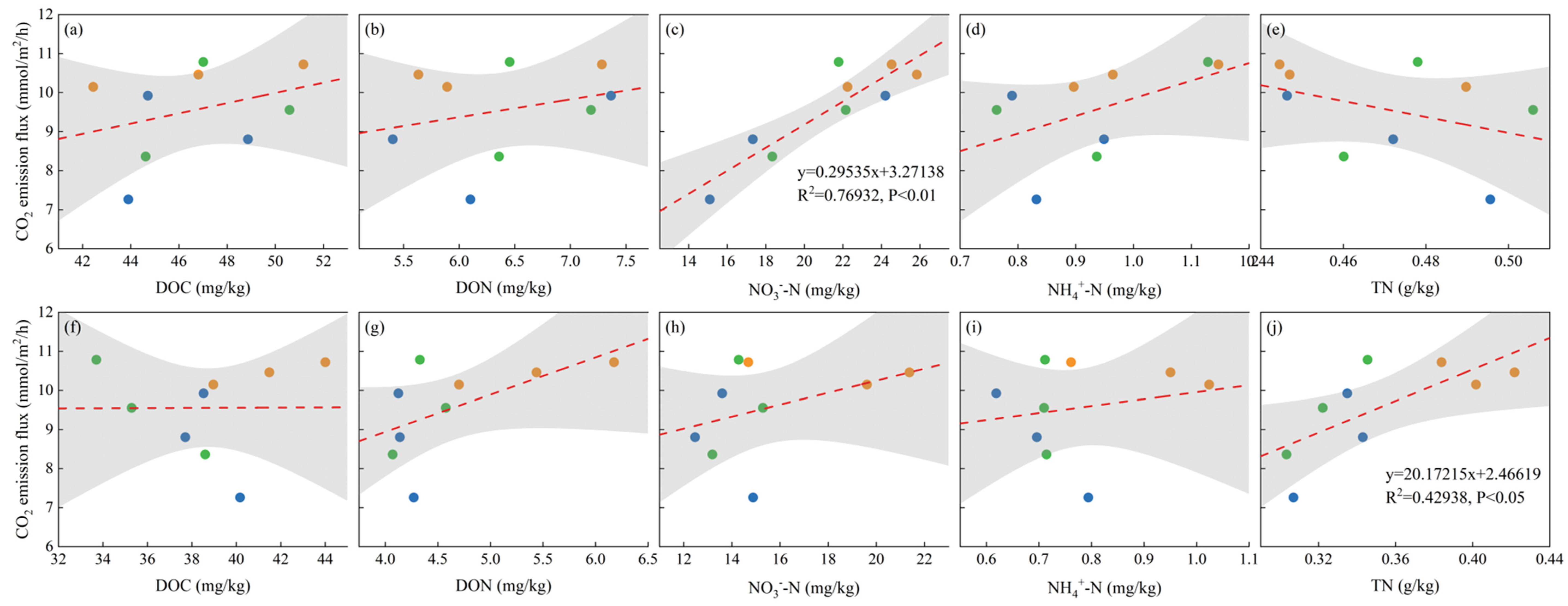
3.5. Correlations between GHG Emissions, Maize Biomass, and Soil Chemical Parameters
4. Discussion
4.1. Effects of Different Straw Return Modes on Soil Dissolved Organic Carbon
4.2. Effects of Different Straw Return Modes on Soil Nitrogen
4.3. Effect of Different Straw Return Modes on Yield
4.4. Effect of Different Straw Return Modes on GHG Emissions
4.5. Correlation Analysis of Soil Carbon and Nitrogen Properties with GHG Emissions and Maize Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, J.; Yu, P.; Xu, X. Straw utilization in China—Status and recommendations. Sustainability 2019, 11, 1762. [Google Scholar] [CrossRef]
- Bai, W.; Yan, L.; Zhang, L.; Ye, L. Practice, pathways, and performance for resource utilization of crop straw: A case study of Xinyang City in China. Environ. Sci. Pollut. Res. 2023, 30, 10812–10829. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L.; He, Z.C.; Shen, A.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef]
- Zhang, M.; Song, D.; Pu, X.; Dang, P.; Qin, X.; Siddique, K.H. Effect of different straw returning measures on resource use efficiency and spring maize yield under a plastic film mulch system. Eur. J. Agron. 2022, 134, 126461. [Google Scholar] [CrossRef]
- Huang, T.; Yang, N.; Lu, C.; Qin, X.; Siddique, K.H. Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Tillage Res. 2021, 214, 105171. [Google Scholar] [CrossRef]
- Du, C.; Li, L.; Effah, Z. Effects of straw mulching and reduced tillage on crop production and environment: A review. Water-Sui 2022, 14, 2471. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Zhang, J. Effect of Straw Returning via Deep Burial Coupled with Application of Fertilizer as Primer on Soil Nutrients and Winter Wheat Yield. Acta Pedol. Sin. 2016, 53, 438–449. [Google Scholar]
- Li, J.; Ye, X.; Zhang, Y.; Chen, J.; Yu, N.; Zou, H. Maize straw deep-burying promotes soil bacteria community abundance and improves soil fertility. J. Soil Sci. Plant Nutr. 2021, 21, 1397–1407. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavail 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Lu, Q.; Xiao, Y.; Wu, P. Emerging technologies of employing algae and microorganisms to promote the return-to-field of crop straws: A mini-review. Front. Bioeng. Biotech. 2023, 11, 1152778. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Li, D.; Xu, C.; Wu, M.; Liu, M.; Li, P.; Li, G.; Zhang, T.; Li, Z. Variation of soil dissolved organic carbon under long-term different fertilizations and its correlation with maize yields. J. Soil Sediment. 2020, 20, 2761–2770. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Xia, X. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, S.; Wang, Y.; Liu, S.; Sun, H. Greenhouse gas emissions of rice straw return varies with return depth and soil type in paddy systems of Northeast China. Arch. Agron. Soil Sci. 2021, 67, 1591–1602. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, L.; Liu, L.; Sheng, F.; Cao, C.; Li, C. Effects of long-term no tillage and straw return on greenhouse gas emissions and crop yields from a rice-wheat system in central China. Agric. Ecosyst. Environ. 2021, 322, 107650. [Google Scholar] [CrossRef]
- Song, J.; Song, J.; Xu, W.; Gao, G.; Bai, J.; Zhang, Z.; Yu, Q.; Hao, J.; Yang, G.; Ren, G. Straw return with fertilizer improves soil CO2 emissions by mitigating microbial nitrogen limitation during the winter wheat season. Catena 2024, 241, 108050. [Google Scholar] [CrossRef]
- Lin, J.; Xu, Z.; Xue, Y.; Sun, R.; Yang, R.; Cao, X.; Li, H.; Shao, Q.; Lou, Y.; Wang, H. N2O emissions from soils under short-term straw return in a wheat-corn rotation system are associated with changes in the abundance of functional microbes. Agric. Ecosyst. Environ. 2023, 341, 108217. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, G.; Zheng, X.; Wang, R.; Liu, C.; Butterbach-Bahl, K. Straw return reduces yield-scaled N2O plus NO emissions from annual winter wheat-based cropping systems in the North China Plain. Sci. Total Environ. 2017, 590–591, 174–185. [Google Scholar] [CrossRef]
- FAO. World Soil Resources Report 60: FAO-UNESCO Soil Map of the World Revised Legend; UN Food and Agriculture Organization: Rome, Italy, 1988. [Google Scholar]
- Wu, G.; Ling, J.; Xu, Y.; Zhao, D.; Liu, Z.; Wen, Y.; Zhou, S. Effects of soil warming and straw return on soil organic matter and greenhouse gas fluxes in winter wheat seasons in the North China Plain. J. Clean. Prod. 2022, 356, 131810. [Google Scholar] [CrossRef]
- Bremner, J.M. Inorganic forms of nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1179–1237. [Google Scholar]
- Zou, J.; Osborne, B. Spatially related sampling uncertainty in the assessment of labile soil carbon and nitrogen in an Irish forest plantation. Appl. Sci. 2021, 11, 2139. [Google Scholar] [CrossRef]
- Doyle, A.; Weintraub, M.N.; Schimel, J.P. Persulfate digestion and simultaneous colorimetric analysis of carbon and nitrogen in soil extracts. Soil Sci. Soc. Am. J. 2004, 68, 669–676. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Mohammed, S.; Mirzaei, M.; Pappné Törő, Á.; Anari, M.G.; Moghiseh, E.; Asadi, H.; Szabó, S.; Kakuszi Széles, A.; Harsányi, E. Soil carbon dioxide emissions from maize (Zea mays L.) fields as influenced by tillage management and climate. Irrig. Drain. 2022, 71, 228–240. [Google Scholar] [CrossRef]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Wan, Z.; Zuo, Y.; He, L.; Li, D.; Yuan, F.; Wang, N.; Liu, J.; Song, Y. Soil dissolved organic carbon in terrestrial ecosystems: Global budget, spatial distribution and controls. Glob. Ecol. Biogeogr. 2020, 29, 2159–2175. [Google Scholar] [CrossRef]
- Hao, X.; Han, X.; Wang, S.; Li, L. Dynamics and composition of soil organic carbon in response to 15 years of straw return in a Mollisol. Soil Tillage Res. 2022, 215, 105221. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, T.; Ding, H.; Li, C.; Yu, M.; Liu, J.; Cao, C. The effects of straw returning and nitrogen fertilizer application on soil labile organic carbon fractions and carbon pool management index in a rice–wheat rotation system. Pedobiologia 2023, 101, 150913. [Google Scholar] [CrossRef]
- Yan, M.; Pan, G.; Lavallee, J.M.; Conant, R.T. Rethinking sources of nitrogen to cereal crops. Glob. Chang. Biol. 2020, 26, 191–199. [Google Scholar] [CrossRef]
- Li, R.; Mian, Y.; Hou, X.; Li, P.; Wang, X. Effects of nitrogen application on decomposition and nutrient release of returning straw, soil fertility, and maize yield. Acta Agron. Sin. 2023, 49, 2012–2022. [Google Scholar]
- Liu, Y.X.; Pan, Y.Q.; Yang, L.; Ahmad, S.; Zhou, X.B. Stover return and nitrogen application affect soil organic carbon and nitrogen in a double-season maize field. Plant Biol. 2022, 24, 387–395. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Zhou, Y.; Zhang, J.; Nadeem, M.Y.; Miao, C.; You, J.; Li, W.; Jiang, Y.; Ding, Y. Long-term straw returning improved soil nitrogen sequestration by accelerating the accumulation of amino acid nitrogen. Agric. Ecosyst. Environ. 2024, 362, 108846. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Gao, J.; Chen, L.; Ning, H.; Hu, Z.; Lu, J.; Tan, X.; Zeng, Y.; Pan, X. Long-term straw return with reducing chemical fertilizers application improves soil nitrogen mineralization in a double rice-cropping system. Agronomy 2022, 12, 1767. [Google Scholar] [CrossRef]
- Wan, X.; Zhao, L.; Wang, Z.; Che, L.; Xu, Y.; Zhou, Y.; Shi, C.; Li, L.; Liu, Y. Effects of Straw Return and Nitrogen Application Rates on Soil Ammonia Volatilization and Yield of Winter Wheat. Agronomy 2024, 14, 1469. [Google Scholar] [CrossRef]
- Davies, B.; Coulter, J.A.; Pagliari, P.H. Timing and rate of nitrogen fertilization influence maize yield and nitrogen use efficiency. PLoS ONE 2020, 15, e233674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, M.; Zhan, M.; Liu, T.; Yuan, J. Straw return-enhanced soil carbon and nitrogen fractions and nitrogen use efficiency in a maize–rice rotation system. Exp. Agr. 2024, 60, e5. [Google Scholar] [CrossRef]
- Lemke, R.L.; VandenBygaart, A.J.; Campbell, C.A.; Lafond, G.P.; Grant, B. Crop residue removal and fertilizer N: Effects on soil organic carbon in a long-term crop rotation experiment on a Udic Boroll. Agric. Ecosyst. Environ. 2010, 135, 42–51. [Google Scholar] [CrossRef]
- Fernandez, J.A.; DeBruin, J.; Messina, C.D.; Ciampitti, I.A. Late-season nitrogen fertilization on maize yield: A meta-analysis. Field Crop Res. 2020, 247, 107586. [Google Scholar] [CrossRef]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Gao, J.; Shi, J.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Grain yield and root characteristics of summer maize (Zea mays L.) under shade stress conditions. J. Agron. Crop Sci. 2017, 203, 562–573. [Google Scholar] [CrossRef]
- Islam, M.U.; Guo, Z.; Jiang, F.; Peng, X. Does straw return increase crop yield in the wheat-maize cropping system in China? A meta-analysis. Field Crop Res. 2022, 279, 108447. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, S.; Zhao, Y.; Shi, W.; Zhang, C.; Liu, F.; Long, Q.; Dong, S. Effects of Straw and Nitrogen Fertilizer on Emission and Absorption of CH4, CO2, and N2O in Alluvial Soil. Chin. J. Soil Sci. 2019, 50, 157–164. [Google Scholar]
- Wu, G.; Chen, X.; Ling, J.; Li, F.; Li, F.; Peixoto, L.; Wen, Y.; Zhou, S. Effects of soil warming and increased precipitation on greenhouse gas fluxes in spring maize seasons in the North China Plain. Sci. Total Environ. 2020, 734, 139269. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C: N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Li, S.; Guo, L.; Cao, C.; Li, C. Effects of straw returning levels on carbon footprint and net ecosystem economic benefits from rice-wheat rotation in central China. Environ. Sci. Pollut. R. 2021, 28, 5742–5754. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J. Management strategies to mitigate N2O emissions in agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; In press. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotox Environ. Safe 2018, 159, 293–300. [Google Scholar] [CrossRef]
- Lal, R. Soil organic matter content and crop yield. J. Soil Water Conserv. 2020, 75, 27A–32A. [Google Scholar] [CrossRef]
- Liang, Y.; Al-Kaisi, M.; Yuan, J.; Liu, J.; Zhang, H.; Wang, L.; Cai, H.; Ren, J. Effect of chemical fertilizer and straw-derived organic amendments on continuous maize yield, soil carbon sequestration and soil quality in a Chinese Mollisol. Agric. Ecosyst. Environ. 2021, 314, 107403. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, F.; Miao, Y.; Sun, Q.; Li, F.; Chen, X.; Li, J.; Ye, Y.; Yang, Z.; Zhang, Q. Soil nitrate-N levels required for high yield maize production in the North China Plain. Nutr. Cycl. Agroecosys 2008, 82, 187–196. [Google Scholar] [CrossRef]
- Samater, A.H.; Van Cleemput, O.; Ertebo, T. Influence of the presence of nitrite and nitrate in soil on maize biomass production, nitrogen immobilization and nitrogen recovery. Biol. Fert. Soils 1998, 27, 211–218. [Google Scholar] [CrossRef]
- Rummel, P.S.; Well, R.; Pfeiffer, B.; Dittert, K.; Floßmann, S.; Pausch, J. Nitrate uptake and carbon exudation–do plant roots stimulate or inhibit denitrification? Plant Soil 2021, 459, 217–233. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. The effects of nitrogen enrichment on soil CO2 fluxes depending on temperature and soil properties. Glob. Ecol. Biogeogr. 2016, 25, 475–488. [Google Scholar] [CrossRef]
- Dai, X.; Liu, Y.; Sun, J.; Zhao, Z.; Zuo, Y.; Geng, L.; Zhang, J. Combined effects of straw return and reduced nitrogen fertilizer application on greenhouse gas emissions and nitrogen use efficiency in a coastal saline paddy field. Chin. J. Appl. Environ. Biol. 2023, 29, 994–1005. [Google Scholar]
- Tang, J.; Wang, J.; Li, Z.; Wang, S.; Qu, Y. Effects of Irrigation Regime and Nitrogen Fertilizer Management on CH4, N2O and CO2 Emissions from Saline–Alkaline Paddy Fields in Northeast China. Sustainability 2018, 10, 475. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: A review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Zhang, Y.; Zhang, L.; Ma, Y.; Ju, X.; Ji, Y. Effect of the Availability of Nitrate Nitrogen and Carbon Source on N2O and CO2 Emission from Soil. J. Agric. Resour. Environ. 2016, 33, 170–175. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, L.; Yang, Z.; Li, W.; Zhao, Y.; Xia, J.; Dong, W.; Chen, B. Effects of Different Straw Return Modes on Soil Carbon, Nitrogen, and Greenhouse Gas Emissions in the Semiarid Maize Field. Plants 2024, 13, 2503. https://doi.org/10.3390/plants13172503
Hua L, Yang Z, Li W, Zhao Y, Xia J, Dong W, Chen B. Effects of Different Straw Return Modes on Soil Carbon, Nitrogen, and Greenhouse Gas Emissions in the Semiarid Maize Field. Plants. 2024; 13(17):2503. https://doi.org/10.3390/plants13172503
Chicago/Turabian StyleHua, Lu, Zhenxing Yang, Wenqian Li, Yidong Zhao, Jie Xia, Wenyi Dong, and Baoqing Chen. 2024. "Effects of Different Straw Return Modes on Soil Carbon, Nitrogen, and Greenhouse Gas Emissions in the Semiarid Maize Field" Plants 13, no. 17: 2503. https://doi.org/10.3390/plants13172503
APA StyleHua, L., Yang, Z., Li, W., Zhao, Y., Xia, J., Dong, W., & Chen, B. (2024). Effects of Different Straw Return Modes on Soil Carbon, Nitrogen, and Greenhouse Gas Emissions in the Semiarid Maize Field. Plants, 13(17), 2503. https://doi.org/10.3390/plants13172503





