Meta-Analysis of the Impact of Far-Red Light on Vegetable Crop Growth and Quality
Abstract
:1. Introduction
2. Result
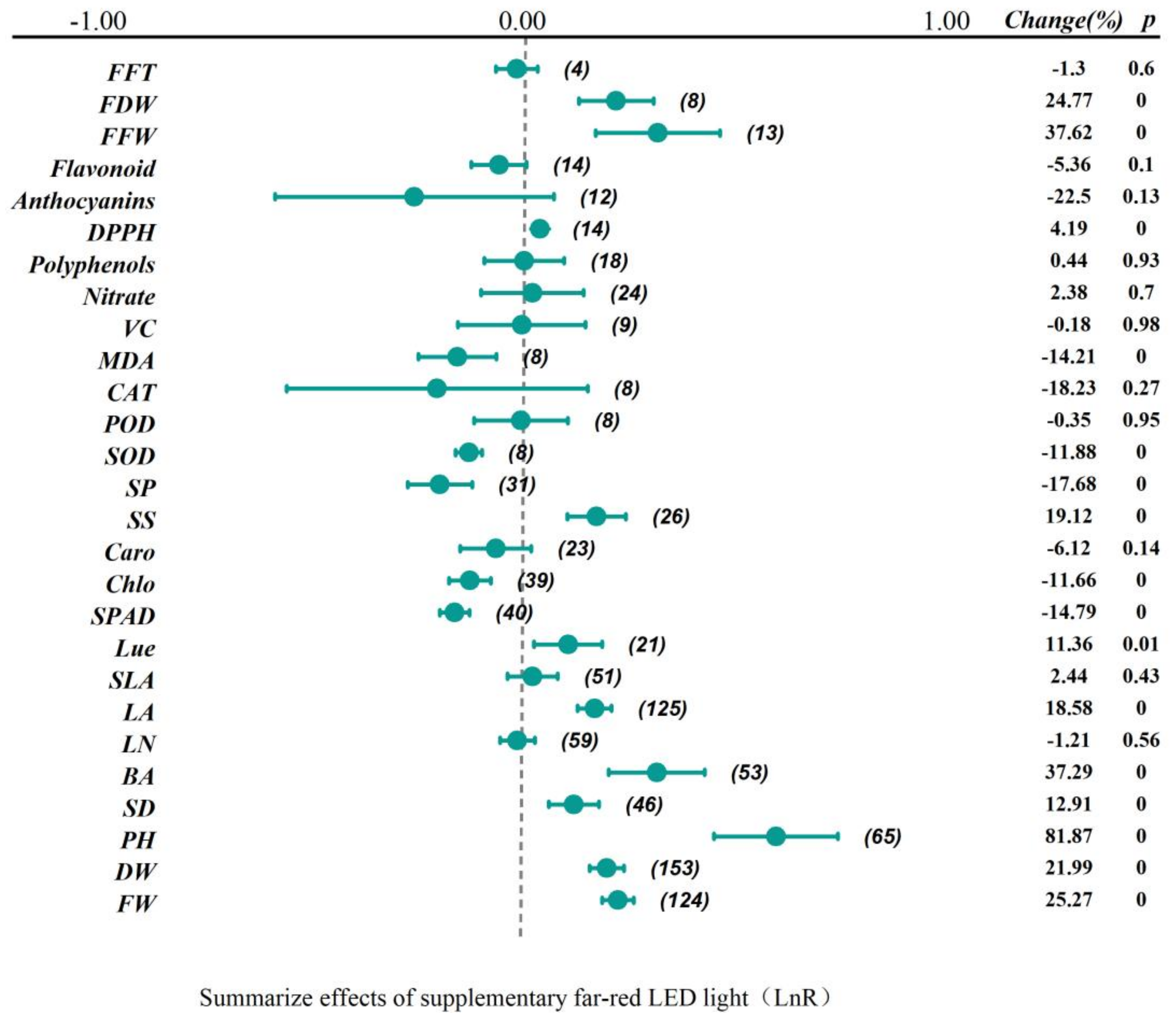
2.1. Overall Summary Effects
2.2. Subgroup Analysis
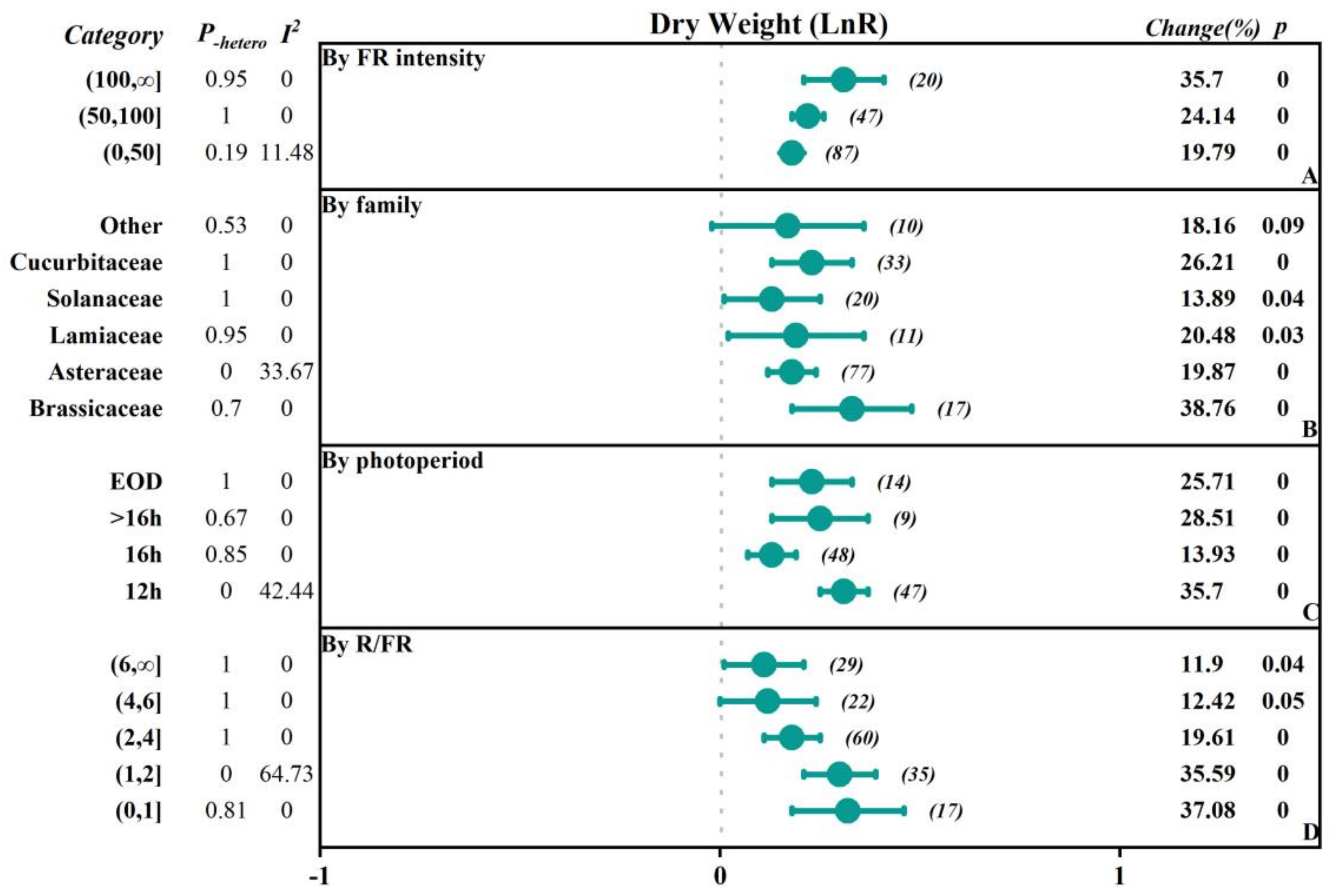
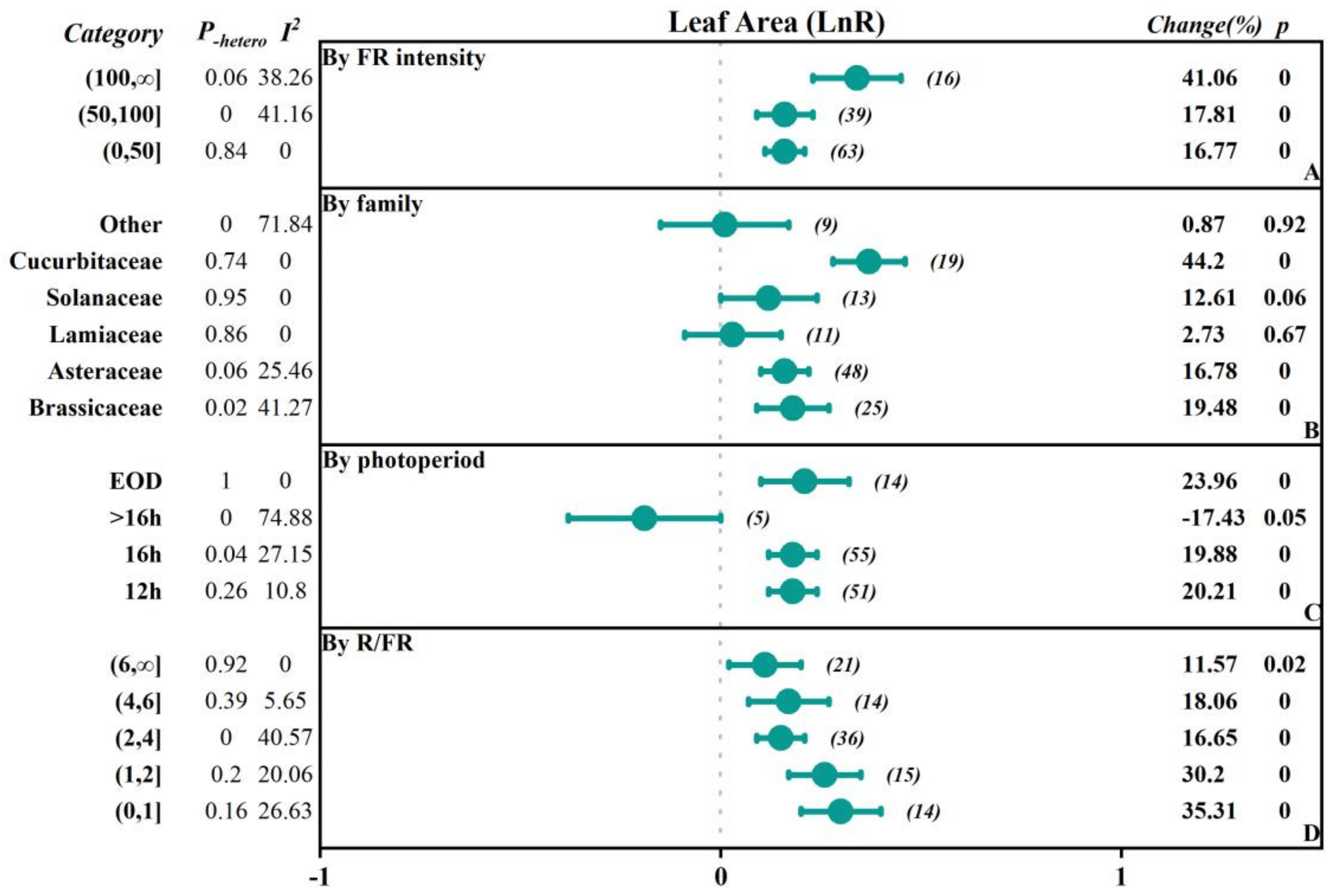
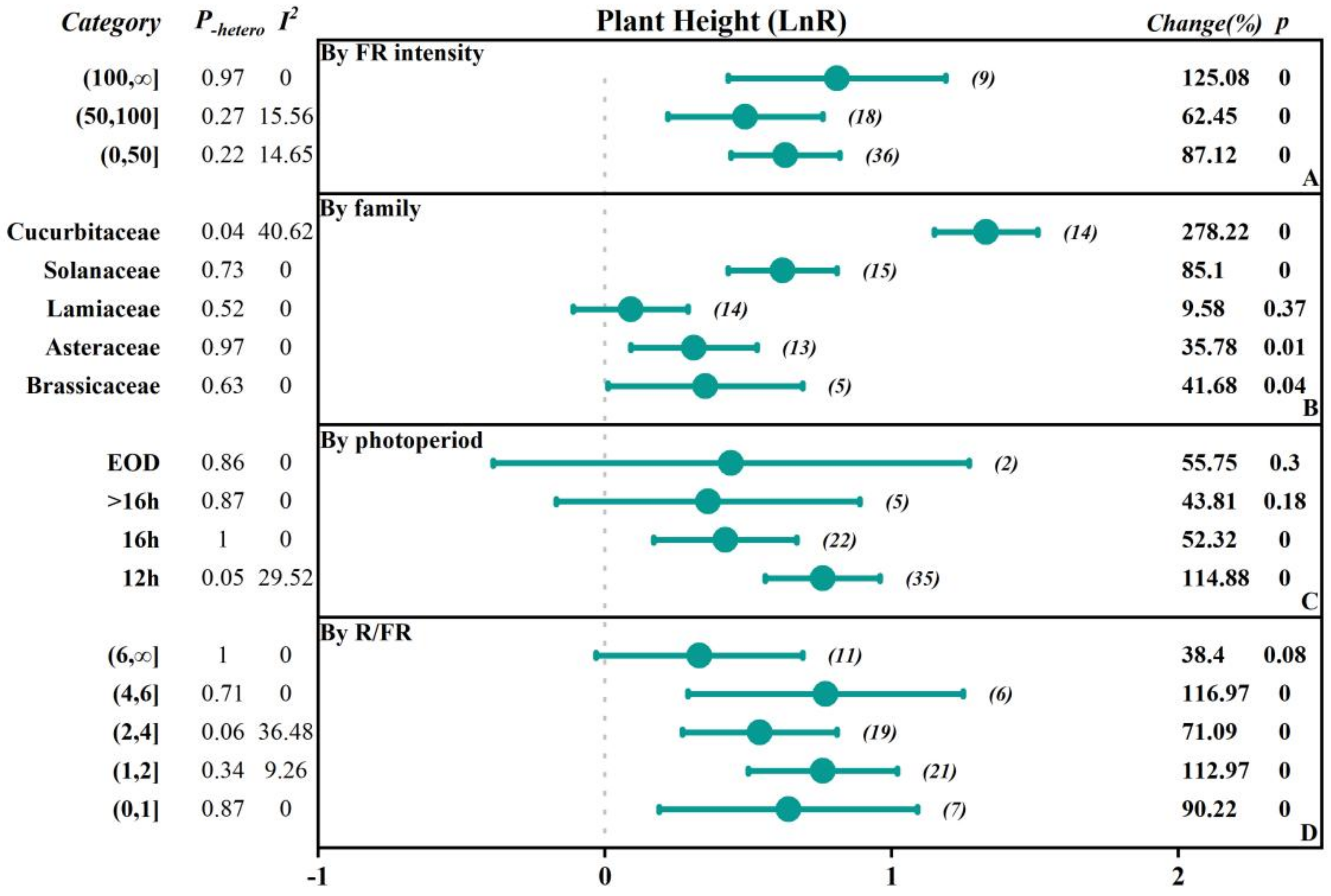
2.2.1. The Influence of FR Intensity
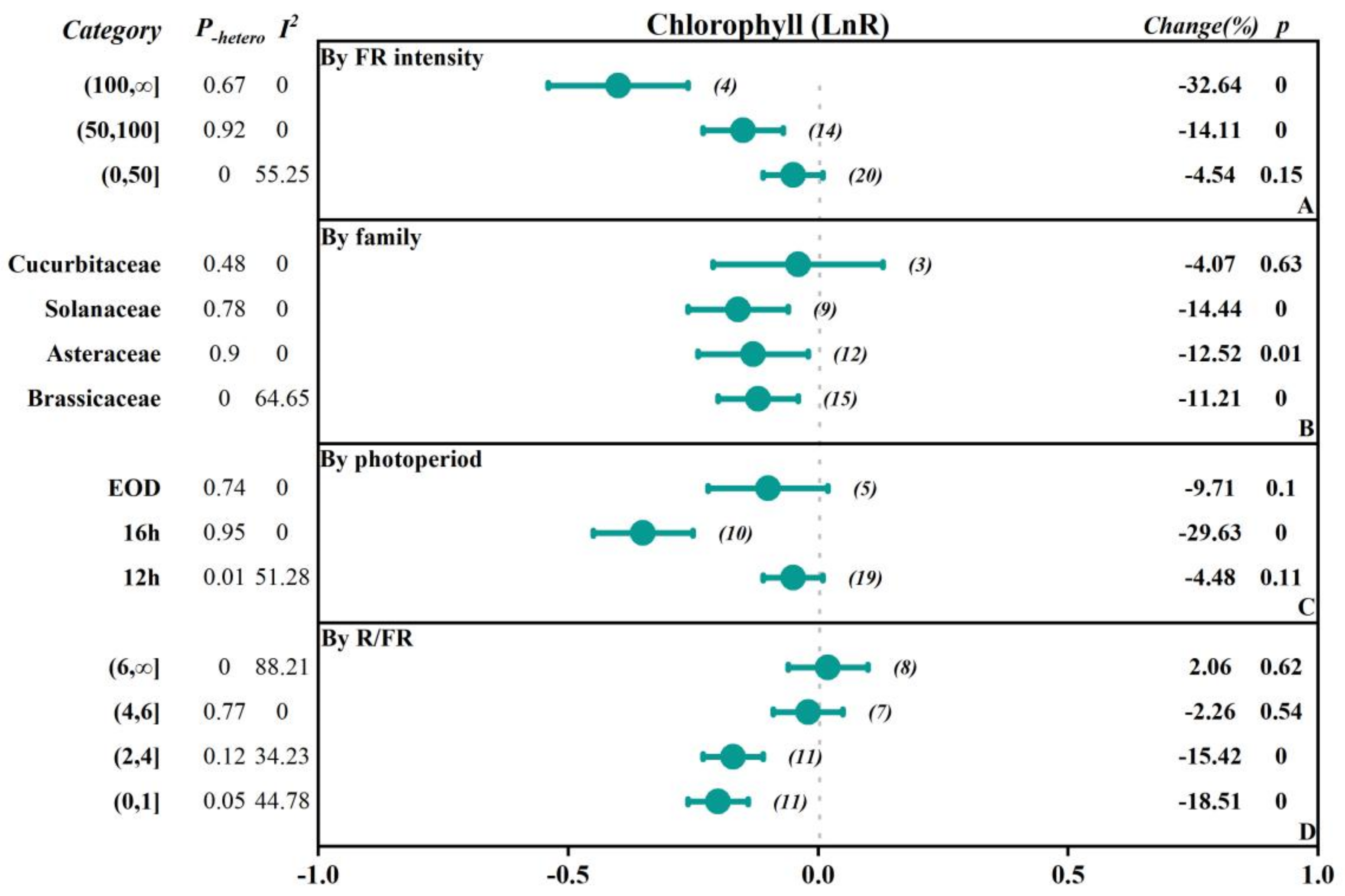
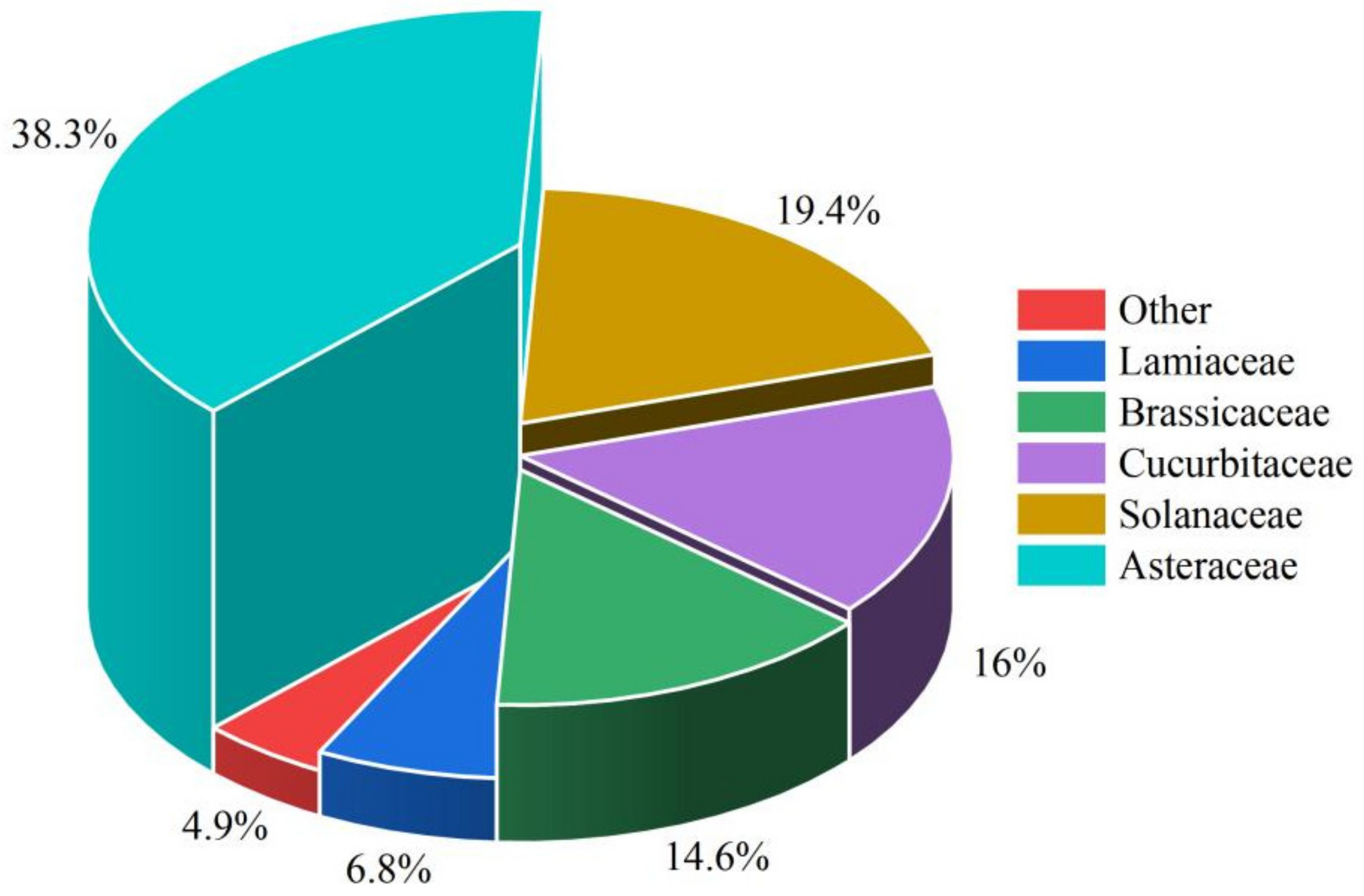
2.2.2. The Influence of Vegetable Family
2.2.3. The Influence of the Photoperiod with FR Supplementation
2.2.4. The Influence of the Red to FR Ratio (R/FR)
2.3. Heterogeneity Analysis
2.4. Publication Bias
3. Discussion
3.1. Research Statement and Summary Analysis
3.2. The Accuracy of Data
3.3. Limitations and Recommendations
4. Materials and Methods
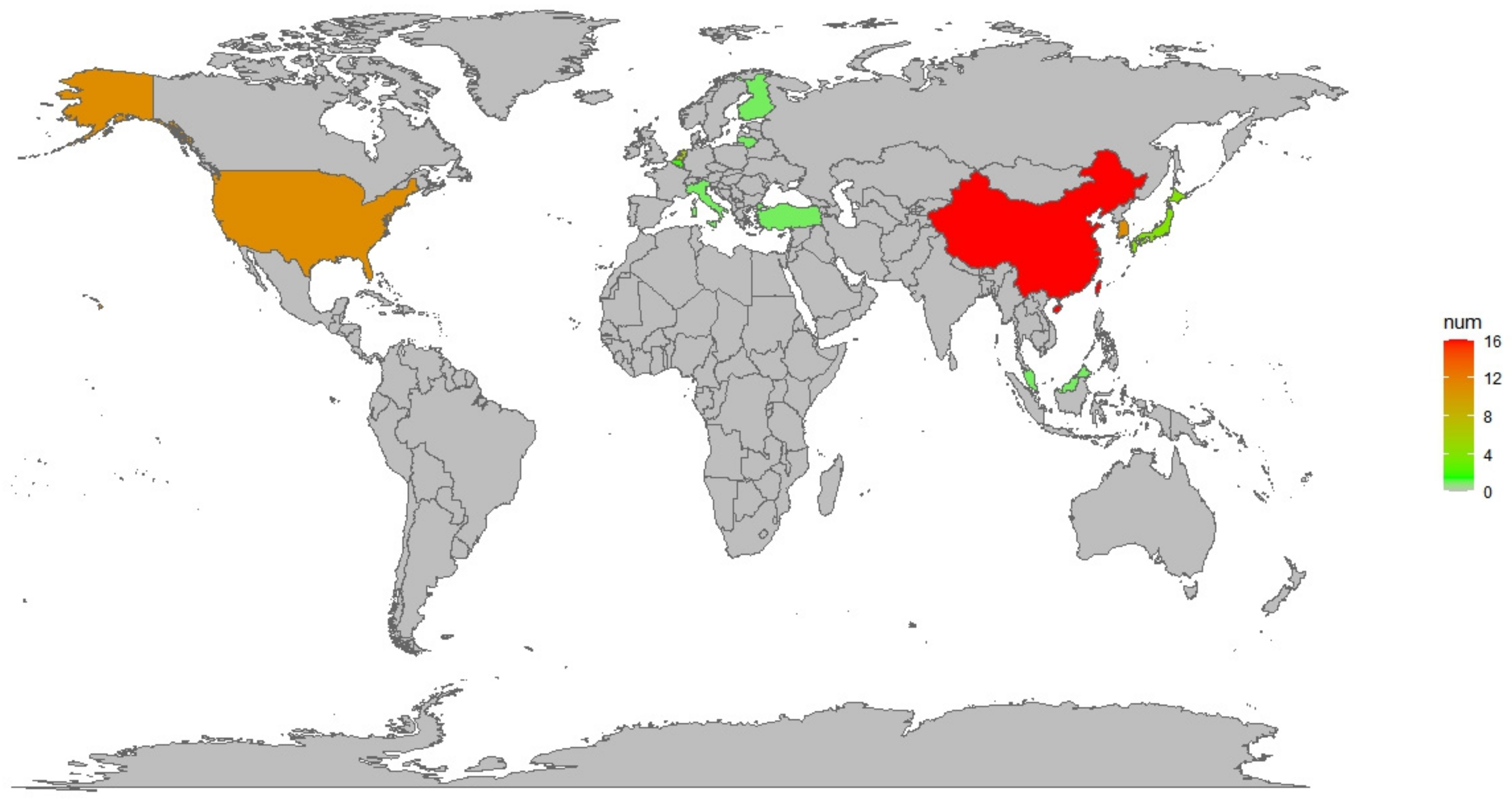
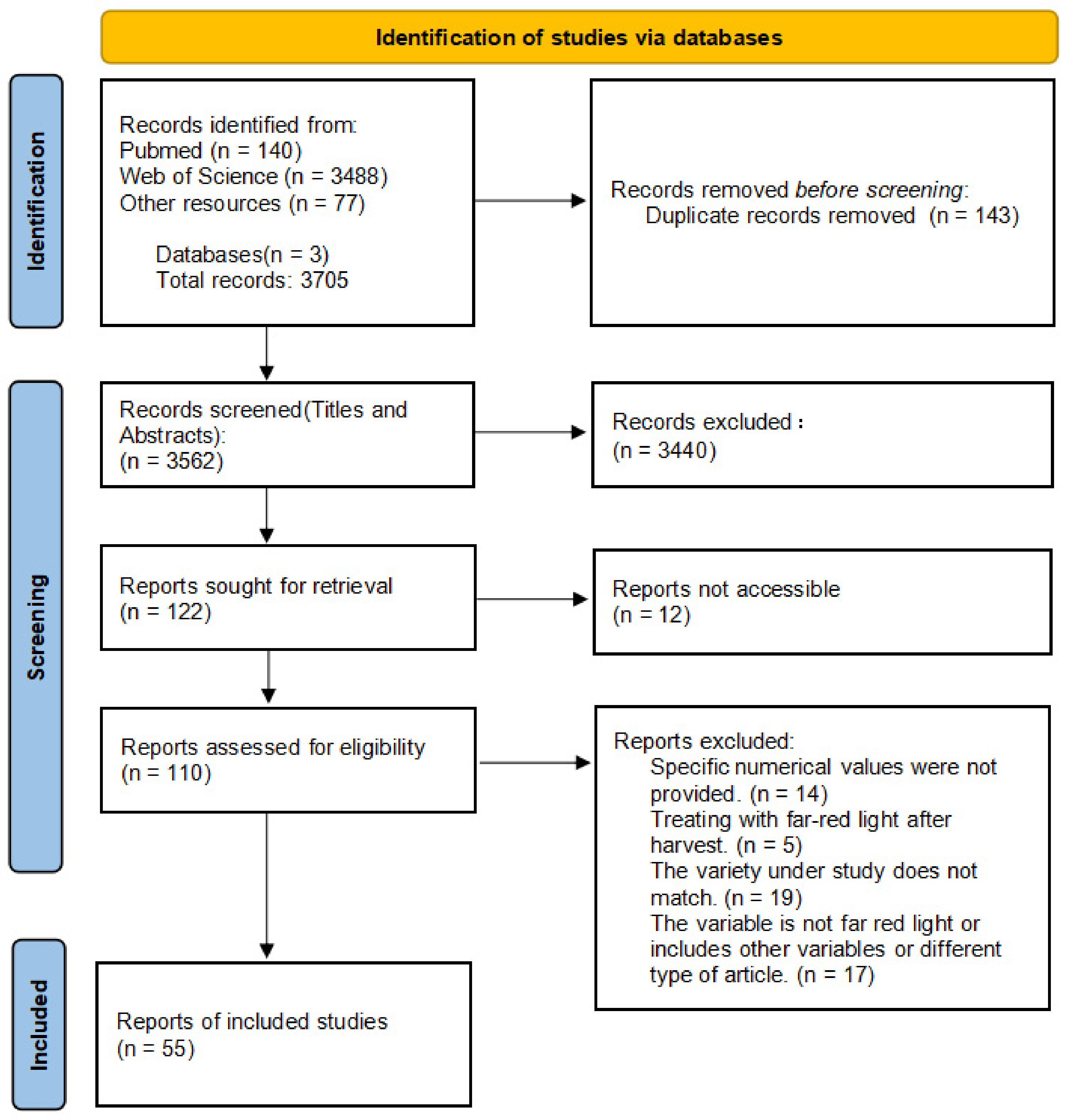
4.1. Data Collection
4.2. Categorical Variable
4.3. Meta Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and Physiological Properties of Indoor Cultivated Lettuce in Response to Additional Far-Red Light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Legendre, R.; Van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant Responses to Red and Far-Red Lights, Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Ali Raza, M.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-Red Light: A Regulator of Plant Morphology and Photosynthetic Capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Moon, Y.H.; Yang, M.; Woo, U.J.; Sim, H.S.; Lee, T.Y.; Shin, H.R.; Jo, J.S.; Kim, S.K. Evaluation of Growth and Photosynthetic Rate of Cucumber Seedlings Affected by Far-Red Light Using a Semi-Open Chamber and Imaging System. Horticulturae 2023, 9, 98. [Google Scholar] [CrossRef]
- Meng, L.; Song, J.; Ni, D.; Kamaruddin, M.A. Effects of Far-Red Light on Growth, Endogenous Hormones, Antioxidant Capacity and Quality of Lettuce. Food Prod. Process Nutr. 2024, 6, 25. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and Shade-Avoidance Responses in Plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Hermann, G.; Rentsch, S.; Strehlow, D. First Steps in the Phytochrome Phototransformation: A Comparative Femtosecond Study on the Forward (Pr→Pfr) and Back Reaction (Pfr→Pr). Biochemistry 2001, 40, 181–186. [Google Scholar] [CrossRef]
- Klose, C.; Nagy, F.; Schäfer, E. Thermal Reversion of Plant Phytochromes. Mol. Plant 2020, 13, 386–397. [Google Scholar] [CrossRef]
- Legris, M. Molecular Mechanisms Underlying Phytochrome-Controlled Morphogenesis in Plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef]
- Van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell 2018, 30, 101–116. [Google Scholar] [CrossRef]
- Van de Velde, E.; Steppe, K.; Van Labeke, M.-C. Leaf Morphology, Optical Characteristics and Phytochemical Traits of Butterhead Lettuce Affected by Increasing the Far-Red Photon Flux. Front. Plant Sci. 2023, 14, 1129335. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Shinto, Y.; Endo, R.; Kitaya, Y. Far-Red Light Interacts with Wind-Induced Stress in Cucumber Seedlings. Sci. Hortic. 2022, 295, 110805. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, R.; Gao, M.; He, R.; Li, Y.; Liu, H. Growth of Tomato and Cucumber Seedlings under Different Light Environments and Their Development after Transplanting. Front. Plant Sci. 2023, 14, 1164768. [Google Scholar] [CrossRef]
- Chen, S.; Marcelis, L.F.M.; Heuvelink, E. Far-Red Radiation Increases Flower and Fruit Abortion in Sweet Pepper (Capsicum annuum L.). Sci. Hortic. 2022, 305, 111386. [Google Scholar] [CrossRef]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Adding UVA and Far-Red Light to White LED Affects Growth, Morphology, and Phytochemicals of Indoor-Grown Microgreens. Sustainability 2022, 14, 8552. [Google Scholar] [CrossRef]
- Weeplian, T.; Yen, T.-B.; Ho, Y.-S. Growth, Development, and Chemical Constituents of Edible Ice Plant (Mesembryanthemum crystallinum L.) Produced under Combinations of Light-Emitting Diode Lights. Horts 2018, 53, 865–874. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce Growth, Morphology and Critical Leaf Trait Responses to Far-Red Light during Cultivation Are Low Fluence and Obey the Reciprocity Law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Bae, J.-H.; Park, S.-Y.; Oh, M.-M. Supplemental Irradiation with Far-Red Light-Emitting Diodes Improves Growth and Phenolic Contents in Crepidiastrum Denticulatum in a Plant Factory with Artificial Lighting. Hortic. Environ. Biotechnol. 2017, 58, 357–366. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Gao, M.; He, R.; Liu, X.; Su, W.; Liu, H. Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra bailey). Int. J. Mol. Sci. 2023, 24, 5563. [Google Scholar] [CrossRef] [PubMed]
- Gurevitch, J.; Koricheva, J.; Nakagawa, S.; Stewart, G. Meta-Analysis and the Science of Research Synthesis. Nature 2018, 555, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Shang, X.; Shao, L.; Wang, Y.; Zhu, X.; Fang, W.; Ma, Y. Meta-Analysis of the Effect of Expression of MYB Transcription Factor Genes on Abiotic Stress. PeerJ 2021, 9, e11268. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, A.; Cheng, Z.-M. Effects of Light Emitting Diode Lights on Plant Growth, Development and Traits a Meta-Analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Appolloni, E.; Orsini, F.; Pennisi, G.; Gabarrell Durany, X.; Paucek, I.; Gianquinto, G. Supplemental LED Lighting Effectively Enhances the Yield and Quality of Greenhouse Truss Tomato Production: Results of a Meta-Analysis. Front. Plant Sci. 2021, 12, 596927. [Google Scholar] [CrossRef]
- Badmus, U.O.; Ač, A.; Klem, K.; Urban, O.; Jansen, M.A.K. A Meta-Analysis of the Effects of UV Radiation on the Plant Carotenoid Pool. Plant Physiol. Biochem. 2022, 183, 36–45. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef]
- Liu, X.; Shi, R.; Gao, M.; He, R.; Li, Y.; Liu, H. Effects of LED Light Quality on the Growth of Pepper (Capsicum spp.) Seedlings and the Development after Transplanting. Agronomy 2022, 12, 2269. [Google Scholar] [CrossRef]
- Demir, K.; Sarıkamış, G.; Çakırer Seyrek, G. Effect of LED Lights on the Growth, Nutritional Quality and Glucosinolate Content of Broccoli, Cabbage and Radish Microgreens. Food Chem. 2023, 401, 134088. [Google Scholar] [CrossRef]
- Li, Y.; Shi, R.; Jiang, H.; Wu, L.; Zhang, Y.; Song, S.; Su, W.; Liu, H. End-Of-Day LED Lightings Influence the Leaf Color, Growth and Phytochemicals in Two Cultivars of Lettuce. Agronomy 2020, 10, 1475. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The Relationship between Leaf Area Growth and Biomass Accumulation in Arabidopsis Thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Verheijen, F.G.A.; Van Der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical Tests, P Values, Confidence Intervals, and Power: A Guide to Misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yang, Q.; Li, T. Overhead Supplemental Far-Red Light Stimulates Tomato Growth under Intra-Canopy Lighting with LEDs. J. Integr. Agric. 2019, 18, 62–69. [Google Scholar] [CrossRef]
- Percival, A.C.; Craver, J.K. End-of-Day Far-Red Lighting with a Low Daily Light Integral Increases Stem Length but Does Not Promote Early Leaf Expansion for Petunia x Hybrida Seedlings. Hortscience 2023, 58, 1010–1017. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, Nutritional Quality, and Energy Use Efficiency in Two Lettuce Cultivars as Influenced by White plus Red versus Red plus Blue LEDs. Int. J. Agric. Biol. Eng. 2020, 13, 33–40. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Ou, S.; Gao, M.; Zhang, Y.; Song, S.; Liu, H. Regulation of Growth and Main Health-Promoting Compounds of Chinese Kale Baby-Leaf by UV-A and FR Light. Front. Plant Sci. 2021, 12, 799376. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- Lin, L. Bias Caused by Sampling Error in Meta-Analysis with Small Sample Sizes. PLoS ONE 2018, 13, e0204056. [Google Scholar] [CrossRef]
- Tong, G.; Guo, G. Meta-Analysis in Sociological Research: Power and Heterogeneity. Sociol. Methods Res. 2022, 51, 566–604. [Google Scholar] [CrossRef]
- Koricheva, J.; Gurevitch, J. Uses and Misuses of Meta-Analysis in Plant Ecology. J. Ecol. 2014, 102, 828–844. [Google Scholar] [CrossRef]
- Klümper, W.; Qaim, M. A Meta-Analysis of the Impacts of Genetically Modified Crops. PLoS ONE 2014, 9, e111629. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Rillig, M.C. Arbuscular Mycorrhizal Contribution to Copper, Manganese and Iron Nutrient Concentrations in Crops—A Meta-Analysis. Soil. Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P.T. How Should Meta-regression Analyses Be Undertaken and Interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Higgins, J.P.T. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, T.; Yang, Y.; Li, P.; Qiu, L.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Meta-Analysis of the Effect of Overexpression of MYB Transcription Factors on the Regulatory Mechanisms of Anthocyanin Biosynthesis. Front. Plant Sci. 2021, 12, 781343. [Google Scholar] [CrossRef]
- Ma, Y.; Augé, R.M.; Dong, C.; Cheng, Z. Increased Salt Tolerance with Overexpression of Cation/Proton Antiporter 1 Genes: A Meta-analysis. Plant Biotechnol. J. 2017, 15, 162–173. [Google Scholar] [CrossRef]
- Rosenberg, M.S. MetaWin 3: Open-Source Software for Meta-Analysis. Front. Bioinform. 2024, 4, 1305969. [Google Scholar] [CrossRef]
- Cheung, M.W. Computing Effect Sizes for Meta-Analysis. Meta-Analysis: A Structural Equation Modeling Approach; John Wiley and Sons, Ltd.: Chichester, UK, 2015; pp. 48–80. [Google Scholar] [CrossRef]
- Snell, K.I.; Ensor, J.; Debray, T.P.; Moons, K.G.; Riley, R.D. Meta-Analysis of Prediction Model Performance across Multiple Studies: Which Scale Helps Ensure between-Study Normality for the C-Statistic and Calibration Measures? Stat. Methods Med. Res. 2018, 27, 3505–3522. [Google Scholar] [CrossRef]
- Bonett, D.G. Point-biserial Correlation: Interval Estimation, Hypothesis Testing, Meta-analysis, and Sample Size Determination. Br. J. Math. Statis 2020, 73, 113–144. [Google Scholar] [CrossRef]
- Petitti, D.B. Approaches to Heterogeneity in Meta-analysis. Stat. Med. 2001, 20, 3625–3633. [Google Scholar] [CrossRef] [PubMed]
- Hak, T.; Van Rhee, H.; Suurmond, R. How to Interpret Results of Meta-Analysis. SSRN J. 2016, 3241367. [Google Scholar] [CrossRef]
- Hoaglin, D.C. Misunderstandings about Q and ‘Cochran’s Q Test’ in Meta-Analysis. Stat. Med. 2016, 35, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Kang, H. Introduction to Systematic Review and Meta-Analysis. Korean J. Anesth. 2018, 71, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Alesini, D.; Palazzo, A.; Trenta, P.; Santoni, M.; De Marchis, L.; Cascinu, S.; Naso, G.; Cortesi, E. Targeted Therapies and Complete Responses in First Line Treatment of Metastatic Renal Cell Carcinoma. A Meta-Analysis of Published Trials. Cancer Treat. Rev. 2014, 40, 271–275. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Peters, J.L. Comparison of Two Methods to Detect Publication Bias in Meta-Analysis. JAMA 2006, 295, 676. [Google Scholar] [CrossRef]
- Nakagawa, S.; Santos, E.S.A. Methodological Issues and Advances in Biological Meta-Analysis. Evol. Ecol. 2012, 26, 1253–1274. [Google Scholar] [CrossRef]

| Subgroup Factors | No. of Studies | lnR (95%CI) | p | Heterogeneity | Change (%) | |
|---|---|---|---|---|---|---|
| P-hetero | I2 | |||||
| Total | 26 | 0.18 (0.11, 0.24) | 0.00 | 0.24 | 15.64 | 19.12 |
| R/FR | ||||||
| (0,1] | 8 | 0.21 (0.08, 0.34) | 0.00 | 0.98 | 0.00 | 23.07 |
| (1,2] | 2 | 0.13 (−0.13, 0.38) | 0.33 | 0.76 | 0.00 | 13.53 |
| (2,4] | 9 | 0.2 (0.07, 0.33) | 0.00 | 0.28 | 17.75 | 22.34 |
| (4,6] | 3 | −0.03 (−0.25, 0.19) | 0.78 | 0.14 | 48.74 | −3.11 |
| (6,∞] | 3 | 0.2 (−0.07, 0.46) | 0.14 | 0.15 | 47.56 | 21.59 |
| Photoperiod | ||||||
| 12 h | 10 | 0.21 (0.12, 0.29) | 0.00 | 0.69 | 0.00 | 22.79 |
| EOD | 10 | 0.12 (0.04, 0.21) | 0.00 | 0.06 | 44.99 | 13.10 |
| Family | ||||||
| Brassicaceae | 8 | 0.21 (0.08, 0.26) | 0.00 | 0.97 | 0.00 | 23.00 |
| Asteraceae | 17 | 0.17 (0.08, 0.33) | 0.01 | 0.05 | 38.85 | 17.99 |
| FR intensity | ||||||
| (0,50] | 6 | 0.06 (−0.1, 0.22) | 0.47 | 0.07 | 51.00 | 6.08 |
| (50,100] | 20 | 0.2 (0.13, 0.28) | 0.00 | 0.68 | 0.00 | 22.74 |
| Subgroup Factors | No. of Studies | lnR (95%CI) | p | Heterogeneity | Change (%) | |
|---|---|---|---|---|---|---|
| P-hetero | I2 | |||||
| Total | 124 | −0.19 (−0.27, −0.12) | 0.00 | 0.09 | 15.09 | −17.68 |
| R/FR | ||||||
| (0,1] | 8 | −0.05 (−0.19, 0.09) | 0.48 | 0.99 | 0.00 | −4.92 |
| (2,4] | 10 | −0.27 (−0.41, −0.14) | 0.00 | 0.03 | 52.65 | −23.70 |
| (4,6] | 8 | −0.36 (−0.51, −0.21) | 0.00 | 0.01 | 63.14 | −30.36 |
| (6,∞] | 4 | −0.04 (−0.24, 0.17) | 0.73 | 0.37 | 4.70 | −3.49 |
| Photoperiod | ||||||
| 12 h | 16 | −0.28 (−0.39, −0.17) | 0.00 | 0.00 | 60.20 | −24.63 |
| EOD | 9 | −0.11 (−0.25, 0.03) | 0.14 | 1.00 | 0.00 | −10.21 |
| Family | ||||||
| Brassicaceae | 9 | −0.08 (−0.17, 0.02) | 0.12 | 0.85 | 0.00 | −7.27 |
| Asteraceae | 14 | −0.09 (−0.17, 0) | 0.04 | 0.78 | 0.00 | −8.27 |
| Solanaceae | 5 | −0.45 (−0.59, −0.31) | 0.00 | 0.00 | 84.46 | −36.06 |
| Cucurbitaceae | 3 | −0.71 (−0.89, −0.52) | 0.00 | 0.06 | 65.19 | −50.66 |
| FR intensity | ||||||
| (0,50] | 15 | −0.31 (−0.41, −0.21) | 0.00 | 0.00 | 72.42 | −26.64 |
| (50,100] | 16 | −0.09 (−0.18, 0.01) | 0.07 | 1.00 | 0.00 | −8.21 |
| Summarize Effects Size | No. of Studies | ln R (95%CI) | p | Heterogeneity | |
|---|---|---|---|---|---|
| P-hetero | I2 | ||||
| Fresh Weight | 124 | 0.23 (0.19, 0.26) | 0.00 | 0.09 | 15.09 |
| Dry Weight | 168 | 0.2 (0.16, 0.24) | 0.00 | 0.20 | 8.28 |
| Plant Height | 65 | 0.6 (0.45, 0.74) | 0.00 | 0.47 | 0.09 |
| Stem Diameter | 46 | 0.12 (0.06, 0.18) | 0.00 | 0.34 | 6.81 |
| Hypocotyl Length | 53 | 0.32 (0.2, 0.43) | 0.00 | 0.24 | 11.58 |
| Leaf Number | 59 | −0.01 (−0.05, 0.03) | 0.56 | 0.86 | 0.00 |
| Leaf Area | 125 | 0.17 (0.13, 0.21) | 0.00 | 0.01 | 24.00 |
| Specific Leaf Area | 51 | 0.02 (−0.04, 0.08) | 0.43 | 0.70 | 0.00 |
| Light Use Efficiency | 21 | 0.11 (0.03, 0.19) | 0.01 | 0.85 | 0.00 |
| SPAD | 40 | −0.16 (−0.2, −0.12) | 0.00 | 0.04 | 30.75 |
| Chlorophyll | 39 | −0.12 (−0.17, −0.08) | 0.00 | 0.02 | 34.62 |
| Carotenoids | 23 | −0.06 (−0.15, 0.02) | 0.14 | 0.00 | 66.90 |
| Soluble Sugars | 26 | 0.18 (0.11, 0.24) | 0.00 | 0.24 | 15.64 |
| Soluble Protein | 31 | −0.19 (−0.27, −0.12) | 0.00 | 0.01 | 43.23 |
| SOD | 8 | −0.13 (−0.16, −0.1) | 0.00 | 0.15 | 34.31 |
| POD | 8 | 0 (−0.11, 0.11) | 0.95 | 0.21 | 27.96 |
| CAT | 8 | −0.2 (−0.56, 0.15) | 0.27 | 0.31 | 14.94 |
| MDA | 8 | −0.15 (−0.25, −0.06) | 0.00 | 0.49 | 0.00 |
| Vitamin C | 9 | 0 (−0.15, 0.15) | 0.98 | 0.49 | 0.00 |
| Nitrate | 24 | 0.02 (−0.1, 0.15) | 0.70 | 0.95 | 0.00 |
| Polyphenols | 18 | 0 (−0.09, 0.1) | 0.93 | 0.01 | 48.80 |
| DPPH | 14 | 0.04 (0.03, 0.06) | 0.00 | 0.00 | 95.01 |
| Anthocyanins | 12 | −0.25 (−0.58, 0.07) | 0.13 | 0.67 | 0.00 |
| Flavonoid | 14 | −0.06 (−0.12, 0.01) | 0.10 | 0.76 | 0.00 |
| Fruit Fresh Weight | 13 | 0.32 (0.17, 0.47) | 0.00 | 0.59 | 0.00 |
| Fruit Dry Weight | 8 | 0.22 (0.13, 0.31) | 0.00 | 0.38 | 5.99 |
| First Flowering Time | 4 | −0.01 (−0.06, 0.04) | 0.60 | 0.30 | 17.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Ju, J.; Hu, Y.; He, R.; Song, J.; Liu, H. Meta-Analysis of the Impact of Far-Red Light on Vegetable Crop Growth and Quality. Plants 2024, 13, 2508. https://doi.org/10.3390/plants13172508
Zhang M, Ju J, Hu Y, He R, Song J, Liu H. Meta-Analysis of the Impact of Far-Red Light on Vegetable Crop Growth and Quality. Plants. 2024; 13(17):2508. https://doi.org/10.3390/plants13172508
Chicago/Turabian StyleZhang, Minggui, Jun Ju, Youzhi Hu, Rui He, Jiali Song, and Houcheng Liu. 2024. "Meta-Analysis of the Impact of Far-Red Light on Vegetable Crop Growth and Quality" Plants 13, no. 17: 2508. https://doi.org/10.3390/plants13172508






