Genome-Wide Analysis of the Nramp Gene Family in Kenaf (Hibiscus cannabinus): Identification, Expression Analysis, and Response to Cadmium Stress
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of Nramp Genes in Kenaf
2.2. Secondary Structure Analysis of HcNramp Family Proteins
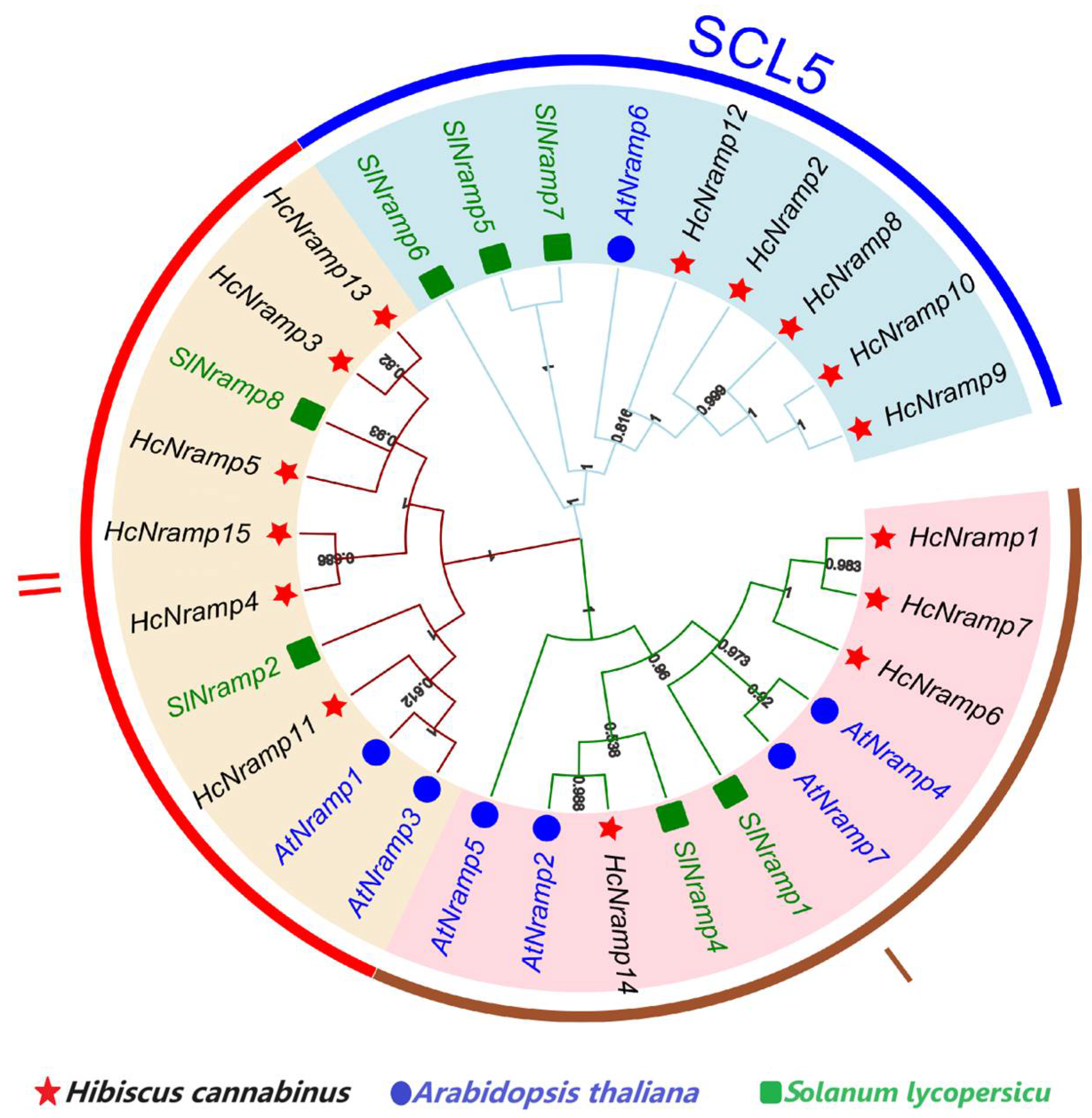
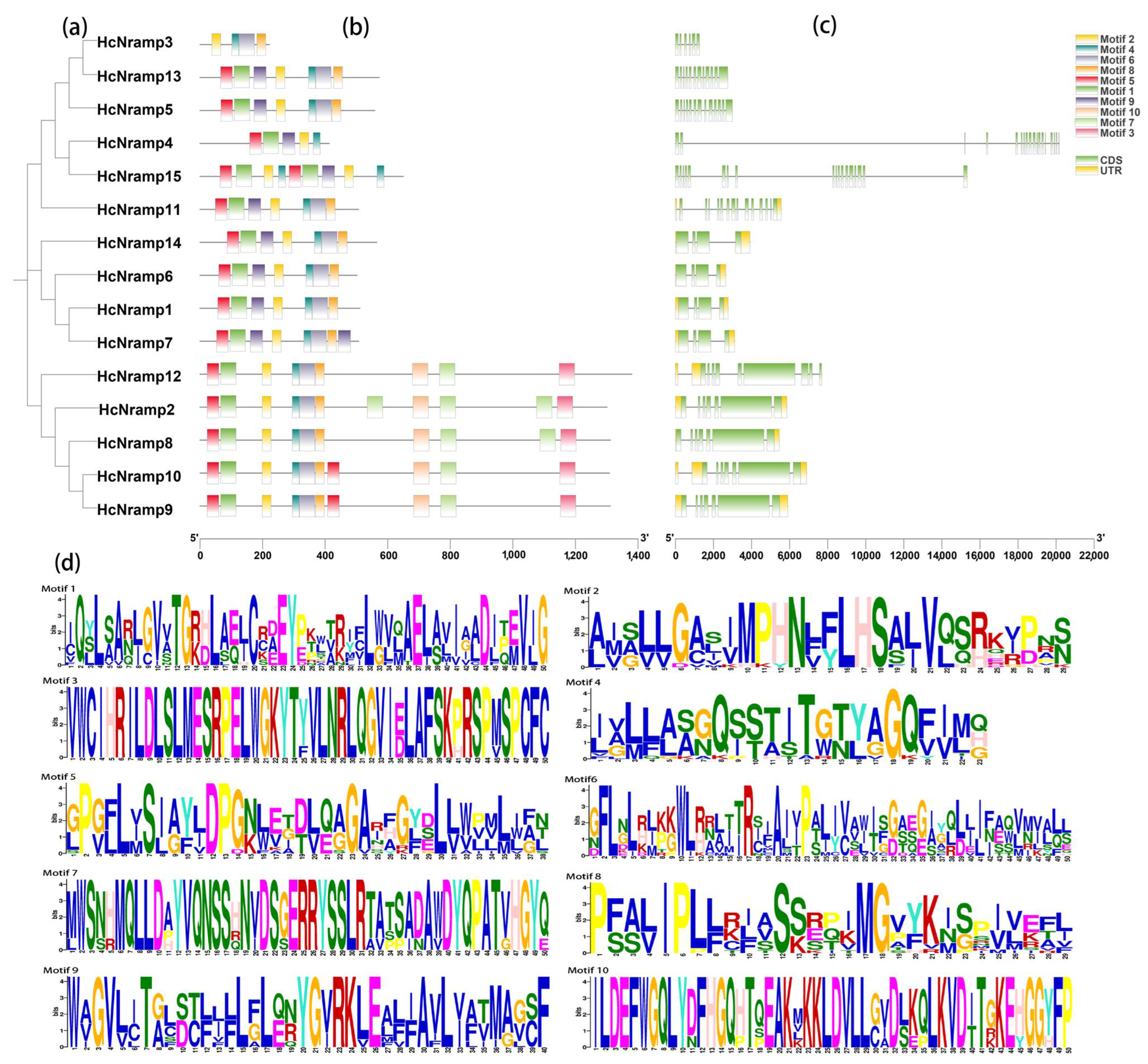
2.3. Classification, Phylogenetic Analysis, Gene Structure, and Conserved Motifs of the HcNramp Gene Family
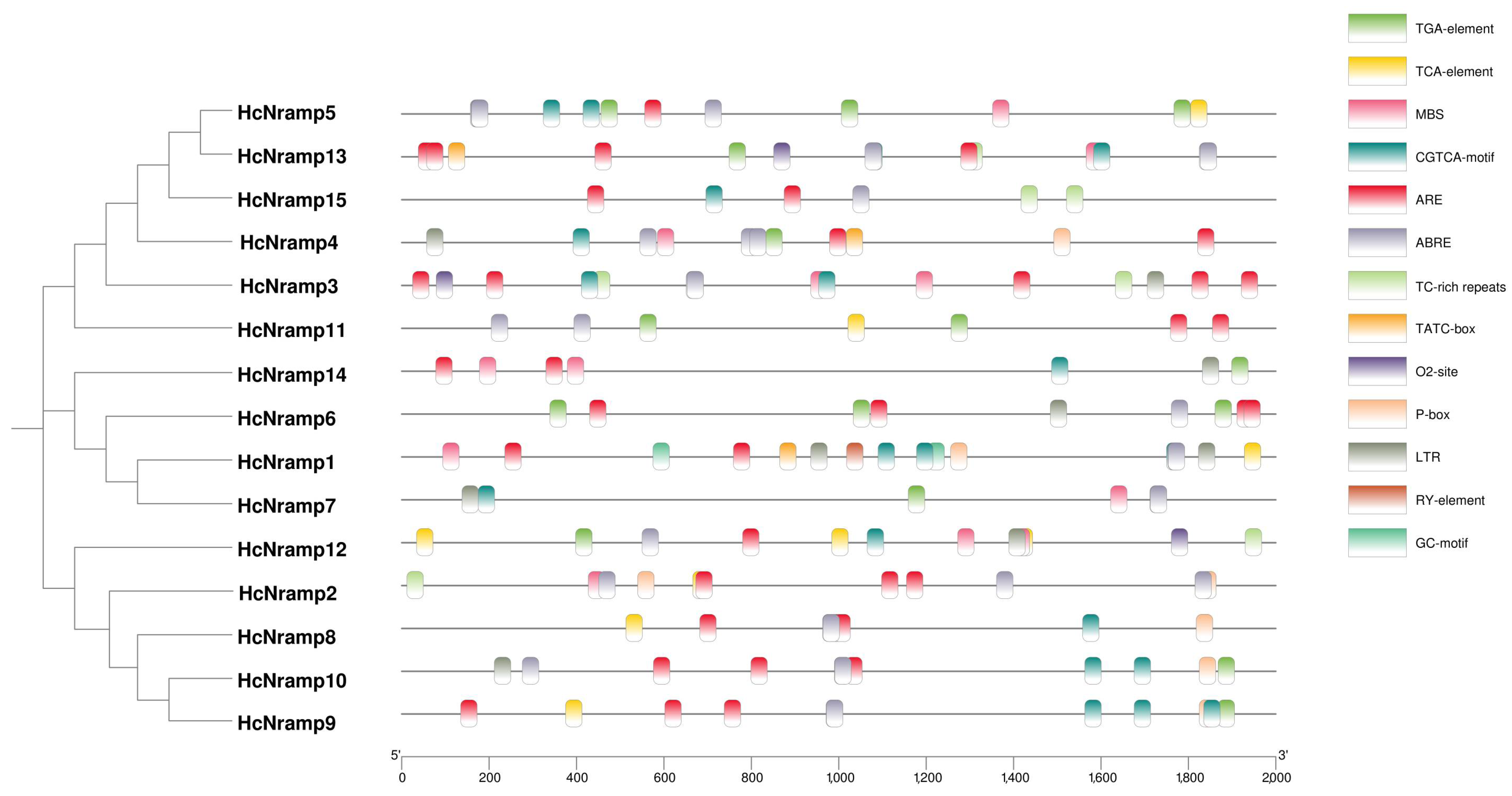
2.4. Analysis of Cis-Acting Elements of HcNramp Promoter
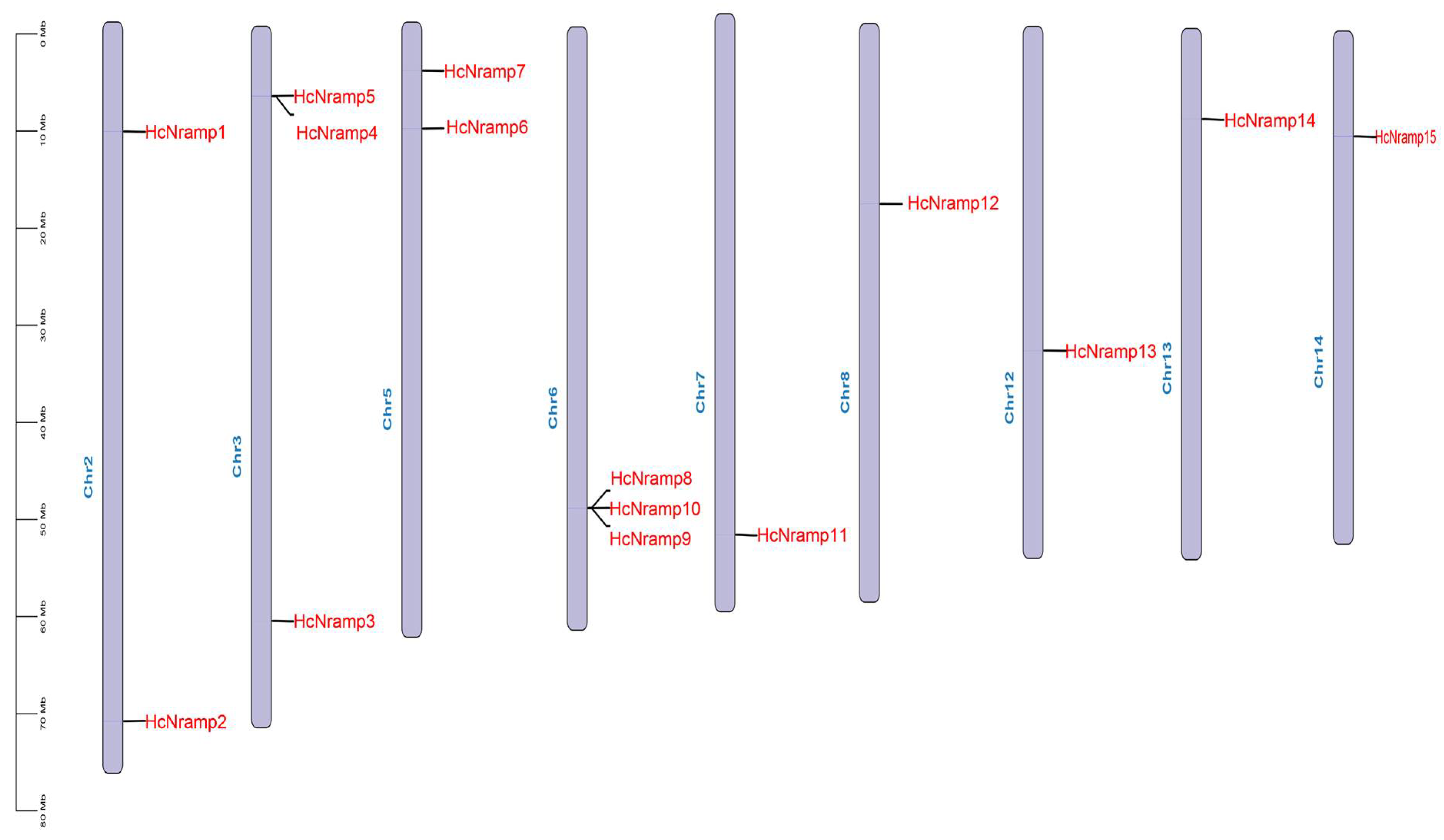
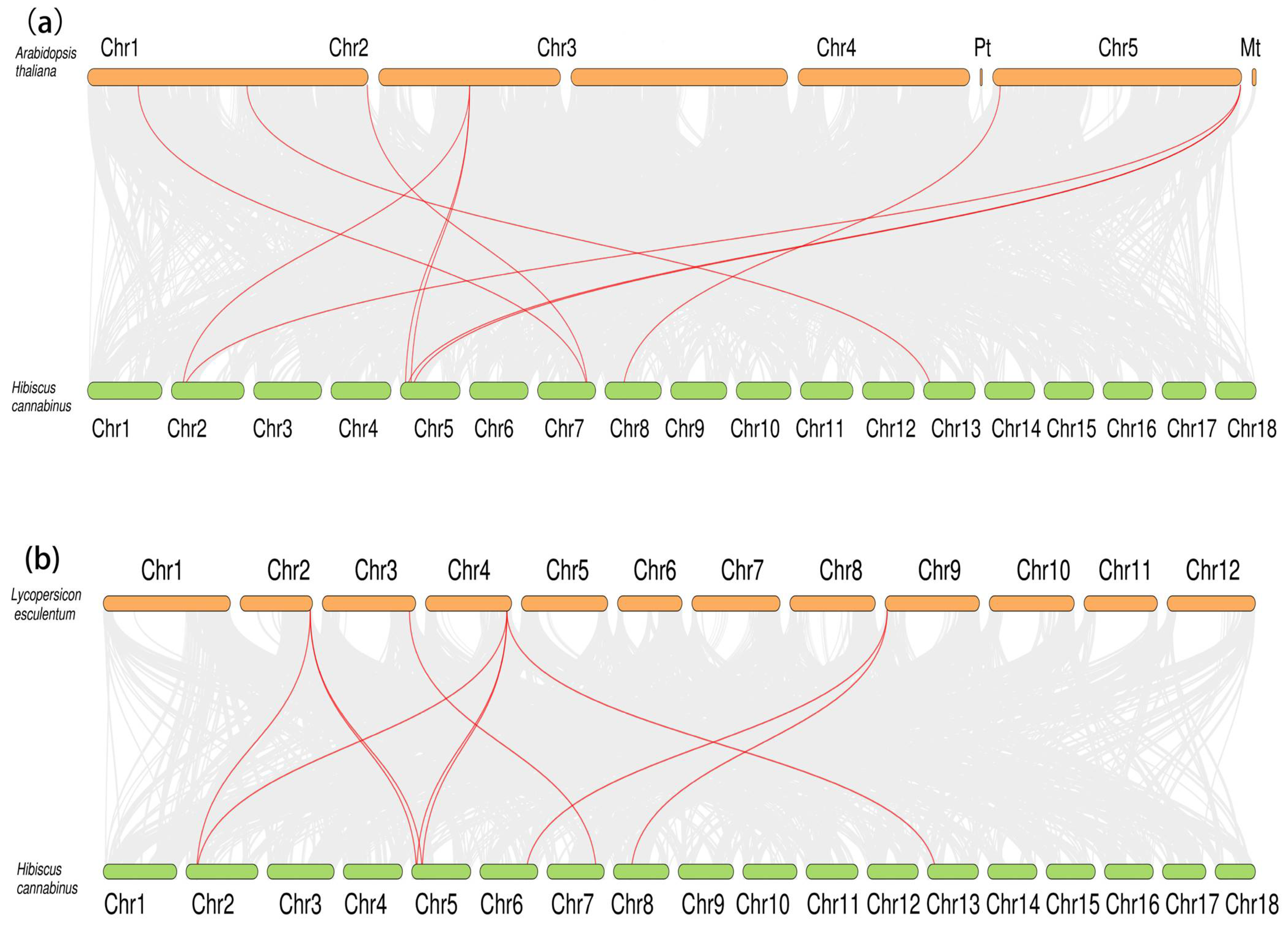
2.5. Chromosomal Localization and Collinearity Analysis of HcNramp Genes
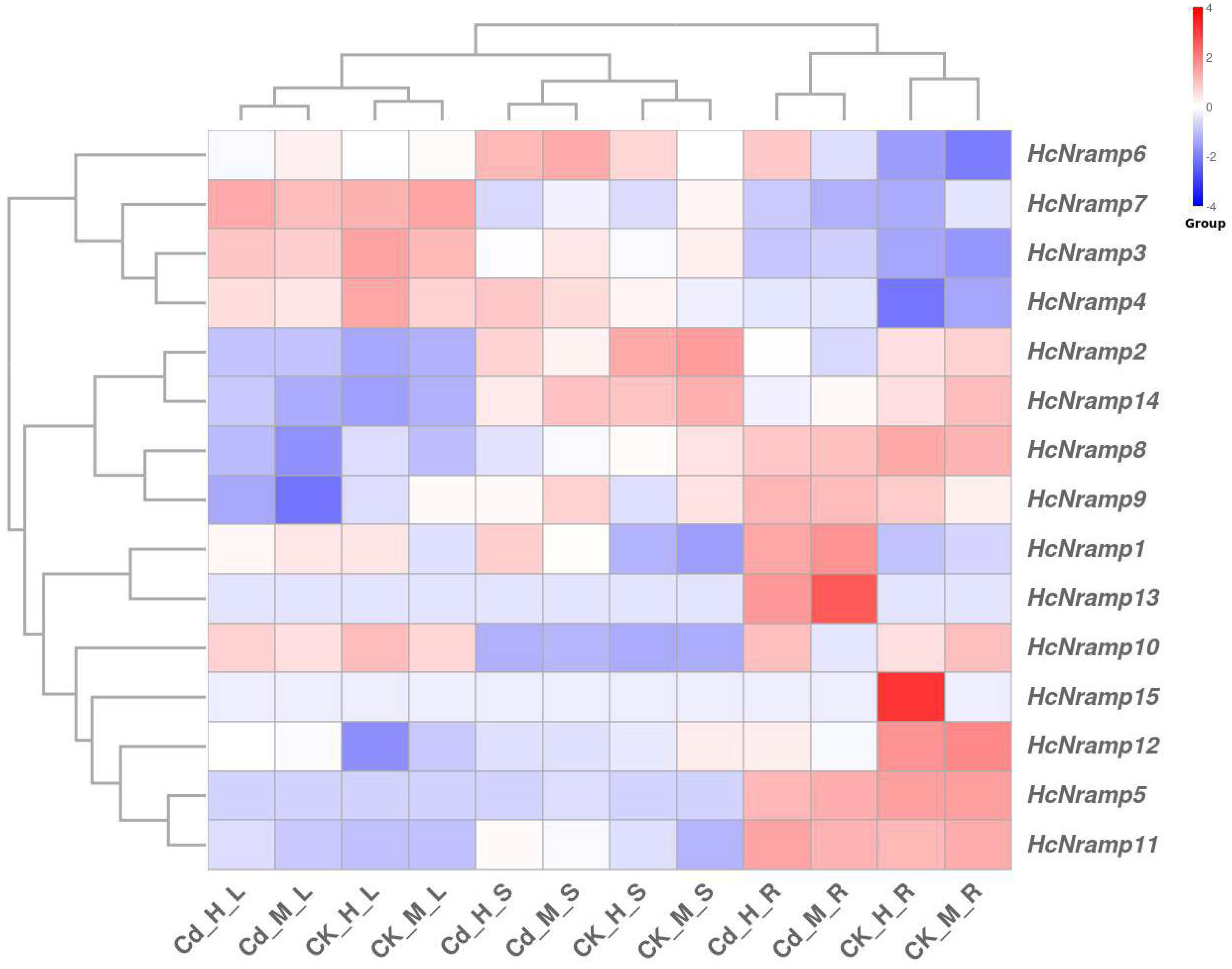
2.6. Tissue-Specific Expression Analysis of the HcNramp Genes
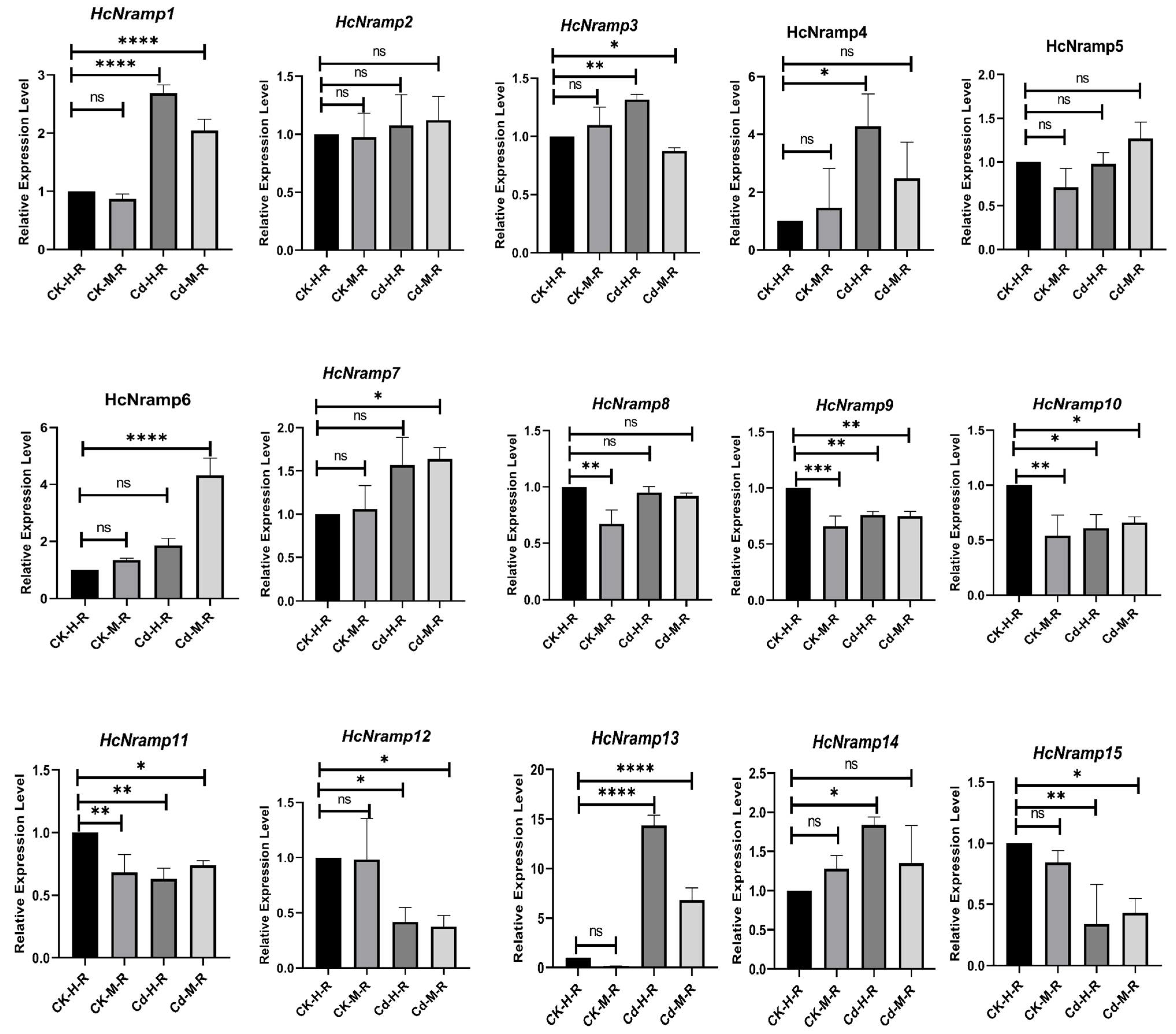
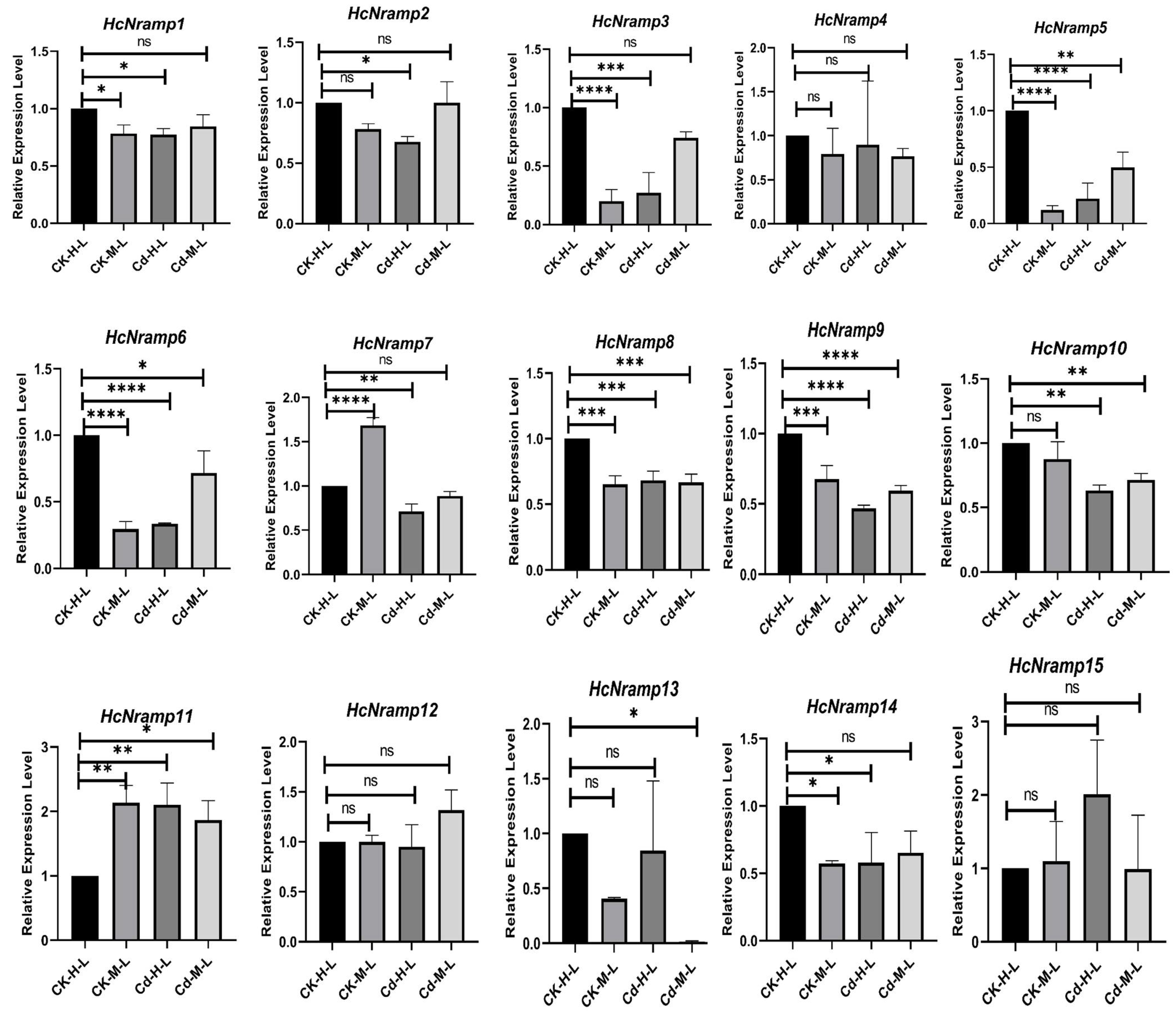
2.7. HcNramp Gene Expressions under Exposure to Different Treatments of Heavy Metals
3. Discussion
4. Materials and Methods
4.1. Identification and Sequence Analysis of Nramp Genes in Kenaf
4.2. Sequence Alignment, Phylogenetics, Gene Structure, and Conserved Motif Analysis of Nramp Genes
4.3. Cis Element Analysis of HcNramp Genes
4.4. Chromosome Localization and Collinearity Analyses of the Kenaf Nramp Gene Family
4.5. Plant Materials and Treatments
4.6. RNA Extraction and qRT-PCR Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Li, Z.; Luo, D.; Jia, R.; Lu, H.; Tang, M.; Hu, Y.; Yue, J.; Huang, Z. Comparative transcriptomic analysis reveals key genes and pathways in two different cadmium tolerance kenaf (Hibiscus cannabinus L.) cultivars. Chemosphere 2021, 263, 128211. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Duan, Y.; Liu, D.; Zong, Y.; Zhang, D.; Shi, X.; Hao, X.; Li, P. Physiological and transcriptome analysis of response of soybean (Glycine max) to cadmium stress under elevated CO2 concentration. J. Hazard. Mater. 2023, 448, 130950. [Google Scholar] [CrossRef]
- Meng, J.G.; Zhang, X.D.; Tan, S.K.; Zhao, K.X.; Yang, Z.M. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. BioMetals 2017, 30, 917–931. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yang, H.; Pian, R.; Wang, J.; Wu, A.-M. The Uptake, Transfer, and Detoxification of Cadmium in Plants and Its Exogenous Effects. Cells 2024, 13, 907. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.; Guan, J.; Wang, C.; Zhang, Z.; Shi, G. Genome-Wide Identification and Expression Analysis Reveals Roles of the NRAMP Gene Family in Iron/Cadmium Interactions in Peanut. Int. J. Mol. Sci. 2023, 24, 1713. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Li, Z.; Jia, R.; Luo, D.; Tang, M.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z. Transcriptome analysis revealed key genes and pathways related to cadmium-stress tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Niyitanga, S.; He, Q.; Chen, S.; Xu, J.; Qi, J.; Tao, A.; Fang, P.; Zhang, L. Transcriptomes of Different Tissues for Expression Characteristics Analysis of MYB gene Family in Kenaf (Hibiscus cannabinus L.). Trop. Plant Biol. 2022, 15, 261–275. [Google Scholar] [CrossRef]
- An, X.; Jin, G.; Zhang, J.; Ma, G.; Jin, L.; Luo, X.; Chen, C.; Shi, X.; Zhou, J.; Wei, W.; et al. Research Progress on Tissue Culture and Genetic Transformation of Kenaf (Hibiscus cannabinus). Open Life Sci. 2017, 12, 465–472. [Google Scholar] [CrossRef]
- Chen, M.; She, Z.; Aslam, M.; Liu, T.; Wang, Z.; Qi, J.; Niu, X. Genomic insights of the WRKY genes in kenaf (Hibiscus cannabinus L.) reveal that HcWRKY44 improves the plant’s tolerance to the salinity stress. Front. Plant Sci. 2022, 13, 984233. [Google Scholar] [CrossRef]
- An, X.; Chen, J.; Liu, T.; Li, W.; Luo, X.; Zou, L. Transcriptomic and Metabolic Profiling of Kenaf Stems under Salinity Stress. Plants 2022, 11, 1448. [Google Scholar] [CrossRef]
- Niu, X.; Chen, M.; She, Z.; Aslam, M.; Qi, J.; Qin, Y. Ectopic Expression of Kenaf (Hibiscus cannabinus L.) HcWRKY50 Improves Plants’ Tolerance to Drought Stress and Regulates ABA Signaling in Arabidopsis. Agronomy 2022, 12, 1176. [Google Scholar] [CrossRef]
- Deng, Y.; Li, D.; Huang, Y.; Huang, S. Physiological response to cadmium stress in kenaf (Hibiscus cannabinus L.) seedlings. Ind. Crops Prod. 2017, 107, 453–457. [Google Scholar] [CrossRef]
- Li, W.; Chen, C.; Deng, Y.; Luo, X.; Liu, T.; An, X.; Zou, L.; Luan, M.; Li, D. Influence of Nitrogen Supply on Growth, Antioxidant Capacity and Cadmium Absorption of Kenaf (Hibiscus cannabinus L.) Seedlings. Plants 2023, 12, 4067. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Long, T.; Chen, Z.; Liu, J.; Cui, W.; Leng, H.; Xing, Y.; Rodriguez, L.G.; Gao, Y.; Yao, Y. Genome-wide identification of NRAMP family genes in Populus trichocarpa and their roles in transport of heavy metals. Tree Genet. Genomes 2023, 19, 51. [Google Scholar] [CrossRef]
- Segond, D.; Dellagi, A.; Lanquar, V.; Rigault, M.; Patrit, O.; Thomine, S.; Expert, D. NRAMP genes function in Arabidopsis thaliana resistance to Erwinia chrysanthemi infection. Plant J. 2009, 58, 195–207. [Google Scholar] [CrossRef]
- Wei, W.; Chai, T.; Zhang, Y.; Han, L.; Xu, J.; Guan, Z. The Thlaspi caerulescens NRAMP Homologue TcNRAMP3 is Capable of Divalent Cation Transport. Mol. Biotechnol. 2008, 41, 15–21. [Google Scholar] [CrossRef]
- Cailliatte, R.; Schikora, A.; Briat, J.-F.; Mari, S.; Curie, C. High-Affinity Manganese Uptake by the Metal Transporter NRAMP1 Is Essential for Arabidopsis Growth in Low Manganese Conditions. Plant Cell 2010, 22, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, X.; Zhu, Z.; Wang, H.; Feng, S. Advances in the study of plant Nramp family involved in metal ion absorption and distribution. Plant Physiol. J. 2020, 56, 345–355. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Li, G.; Kumar, S.; Sun, Z.; Li, Y.; Guo, W.; Yang, J.; Hou, H. Genome-Wide Identification of the Nramp Gene Family in Spirodela polyrhiza and Expression Analysis under Cadmium Stress. Int. J. Mol. Sci. 2021, 22, 6414. [Google Scholar] [CrossRef]
- Qin, L.; Han, P.; Chen, L.; Walk, T.C.; Li, Y.; Hu, X.; Xie, L.; Liao, H.; Liao, X. Genome-Wide Identification and Expression Analysis of NRAMP Family Genes in Soybean (Glycine max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Tian, W.; He, G.; Qin, L.; Li, D.; Meng, L.; Huang, Y.; He, T. Genome-wide analysis of the NRAMP gene family in potato (Solanum tuberosum): Identification, expression analysis and response to five heavy metals stress. Ecotoxicol. Environ. Saf. 2021, 208, 111661. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2013, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yuan, M.; Wu, J.; Men, Y.; Jiang, M.; Gu, L.; Wang, J. Genome-wide Identification and Bioinformatics Analysis of Poplar NRAMP Gene Family. J. Anhui Agric. Sci. 2023, 51, 90–94+141. [Google Scholar] [CrossRef]
- Yan, L.; Jin, H.; Raza, A.; Huang, Y.; Gu, D.p.; Zou, X. Natural resistance-associated macrophage proteins (NRAMPs) are involved in cadmium enrichment in peanut (Arachis hypogaea L.) under cadmium stress. Plant Growth Regul. 2023, 102, 619–632. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Liu, L.-E.; Zhang, T.; Zhou, W. Identification and Analysis of the NRAMP Family in Seabuckthorn Under Lead Stress. Biotechnol. Bull. 2024, 40, 191–202. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, G.; Prasad, B.; Sharma, P.; Kumar, R. Genome wide analysis and identification of NRAMP gene family in wheat (Triticum aestivum L.). Pharma Innov. J. 2022, SP-11, 499–504. [Google Scholar]
- Zhao, Y.; Xie, Q.; Yang, Q.; Cui, J.; Tan, W.; Zhang, D.; Xiang, J.; Deng, L.; Guo, Y.; Li, M.; et al. Genome-wide identification and evolutionary analysis of the NRAMP gene family in the AC genomes of Brassica species. BMC Plant Biol. 2024, 24, 311. [Google Scholar] [CrossRef]
- Thomine, S.B.; Wang, R.W.; John, M.; Crawford, N.M.; Schroeder, J.I. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef]
- Mani, A.; Sankaranarayanan, K. In Silico Analysis of Natural Resistance-Associated Macrophage Protein (NRAMP) Family of Transporters in Rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef]
- Cailliatte, R.; Lapeyre, B.; Briat, J.-F.; Mari, S.; Curie, C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem. J. 2009, 422, 217–228. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhang, Q.; Gangjun, W.; Ye, X. Differential responses of two tomato cultivars to cadmium stress. J. Plant Nutr. Fertil. 2015, 21, 1261–1268. [Google Scholar] [CrossRef]
- Wei, T.; Sun, Y.; Yashir, N.; Li, X.; Guo, J.; Liu, X.; Jia, H.; Ren, X.; Hua, L. Inoculation with Rhizobacteria Enhanced Tolerance of Tomato (Solanum lycopersicum L.) Plants in Response to Cadmium Stress. J. Plant Growth Regul. 2021, 41, 445–460. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Song, H.; Zhao, J.; Shabala, S.; Tian, S.; Yang, X. A novel plasma membrane-based NRAMP transporter contributes to Cd and Zn hyperaccumulation in Sedum alfredii Hance. Environ. Exp. Bot. 2020, 176, 104121. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Yao, Q.; Long, D.; Fan, X.; Kang, H.; Zeng, J.; Sha, L.; Zhang, H.; Zhou, Y.; et al. Overexpression of TtNRAMP6 enhances the accumulation of Cd in Arabidopsis. Gene 2019, 696, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Ye, T.; Shang, C.; Li, S.; Khan, A.; Nkoh, J.N.; Mustafa, A.E.-Z.M.A.; Elshikh, M.S. NRAMP gene family in Kandelia obovata: Genome-wide identification, expression analysis, and response to five different copper stress conditions. Front. Plant Sci. 2024, 14, 1318383. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.-H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Li, H.; Yi, K.; Ding, N.; Li, N.; Lin, Y.; Fu, Q. Endogenous salicylic acid is required for promoting cadmium tolerance of Arabidopsis by modulating glutathione metabolisms. J. Hazard. Mater. 2016, 316, 77–86. [Google Scholar] [CrossRef]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J. Integr. Plant Biol. 2019, 62, 218–227. [Google Scholar] [CrossRef]
- Wang, F.; Tan, H.; Huang, L.; Cai, C.; Ding, Y.; Bao, H.; Chen, Z.; Zhu, C. Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice. Ecotoxicol. Environ. Saf. 2021, 207, 111198. [Google Scholar] [CrossRef]
- Oomen, R.J.F.J.; Wu, J.; Lelièvre, F.; Blanchet, S.; Richaud, P.; Barbier-Brygoo, H.; Aarts, M.G.M.; Thomine, S. Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 2008, 181, 637–650. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, C.; Yue, J.; Mubeen, S.; Cao, S.; Li, X.; Wang, M.; Zhang, H.; Wu, X.; Wang, C.; et al. Genome-wide identification of CUC gene family and functional analysis of HcCUC1 in kenaf. Plant Cell Tissue Organ Cult. 2023, 155, 91–102. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kasapoğlu, A.G.; Muslu, S.; Aygören, A.S.; Öner, B.M.; Güneş, E.; İlhan, E.; Yiğider, E.; Aydin, M. Genome-wide characterization of the GPAT gene family in bean (Phaseolus vulgaris L.) and expression analysis under abiotic stress and melatonin. Genet. Resour. Crop Evol. 2024, 25, 6101. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Battistuzzi, F.U. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Chen, Y.; Liu, Y.; Wu, Y.; Ren, S.; Li, L. Identification, evolution and expression analysis of WRKY gene family in Eucommia ulmoides. Genomics 2021, 113, 3294–3309. [Google Scholar] [CrossRef]
- Yao, Y.; He, Z.; Li, X.; Xu, J.; Han, X.; Liang, H.; Zhuo, R.; Qiu, W. Genome-wide identification of bHLH gene family and its response to cadmium stress in Populus × canescens. PeerJ 2024, 12, e17410. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, L.; Tang, B.; Fu, W.; Wang, Q.; Mo, C.; Zhang, Y.; Ao, N.; Li, Y.; Li, F.; et al. Genome-wide identification and expression analysis of the SBP-box gene family in Brassica juncea L. J. Northeast. Agric. Univ. 2023, 54, 23. [Google Scholar] [CrossRef]
- Jia, R. Comparative Morphological and Physiological Responses and Differential Gene Discovery in Kenaf (Hibiscus cannabinus L.) under Cadmium Stress. Master’s Thesis, Guangxi University, Nanning, China, 2019. [Google Scholar]
- Singh, R.; Jha, A.B.; Misra, A.N.; Sharma, P. Differential responses of growth, photosynthesis, oxidative stress, metals accumulation and NRAMP genes in contrasting Ricinus communis genotypes under arsenic stress. Environ. Sci. Pollut. Res. 2019, 26, 31166–31177. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Li, S.; Du, G.; An, X. Genome-Wide Analysis of the Nramp Gene Family in Kenaf (Hibiscus cannabinus): Identification, Expression Analysis, and Response to Cadmium Stress. Plants 2024, 13, 2514. https://doi.org/10.3390/plants13172514
Liu Q, Li S, Du G, An X. Genome-Wide Analysis of the Nramp Gene Family in Kenaf (Hibiscus cannabinus): Identification, Expression Analysis, and Response to Cadmium Stress. Plants. 2024; 13(17):2514. https://doi.org/10.3390/plants13172514
Chicago/Turabian StyleLiu, Qin, Shaocui Li, Guanghui Du, and Xia An. 2024. "Genome-Wide Analysis of the Nramp Gene Family in Kenaf (Hibiscus cannabinus): Identification, Expression Analysis, and Response to Cadmium Stress" Plants 13, no. 17: 2514. https://doi.org/10.3390/plants13172514
APA StyleLiu, Q., Li, S., Du, G., & An, X. (2024). Genome-Wide Analysis of the Nramp Gene Family in Kenaf (Hibiscus cannabinus): Identification, Expression Analysis, and Response to Cadmium Stress. Plants, 13(17), 2514. https://doi.org/10.3390/plants13172514









