Assisted Phytoremediation between Biochar and Crotalaria pumila to Phytostabilize Heavy Metals in Mine Tailings
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization and Heavy Metal Analysis of the Coconut Fiber Biochar
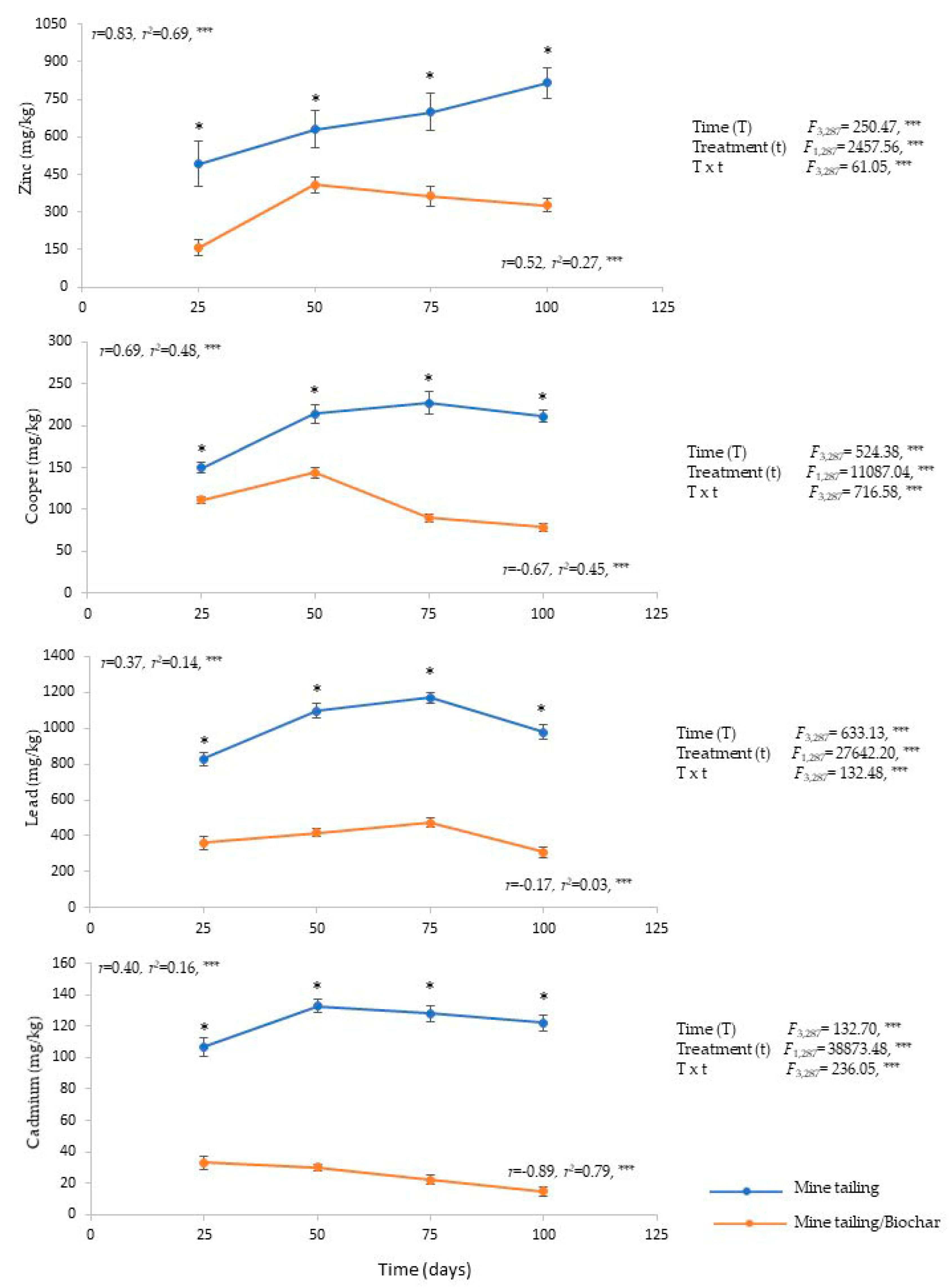
2.2. Bioaccumulation of Heavy Metals in Root and Leaf Tissue in C. pumila Individuals That Grow in Mine Tailing Substrate and Mine Tailing Substrate/Biochar
2.3. Metal Bioconcentration and Translocation Factors in Roots and Leaves in C. pumila Individuals Growing on Mine Tailing and Mine Tailing/Biochar Substrates
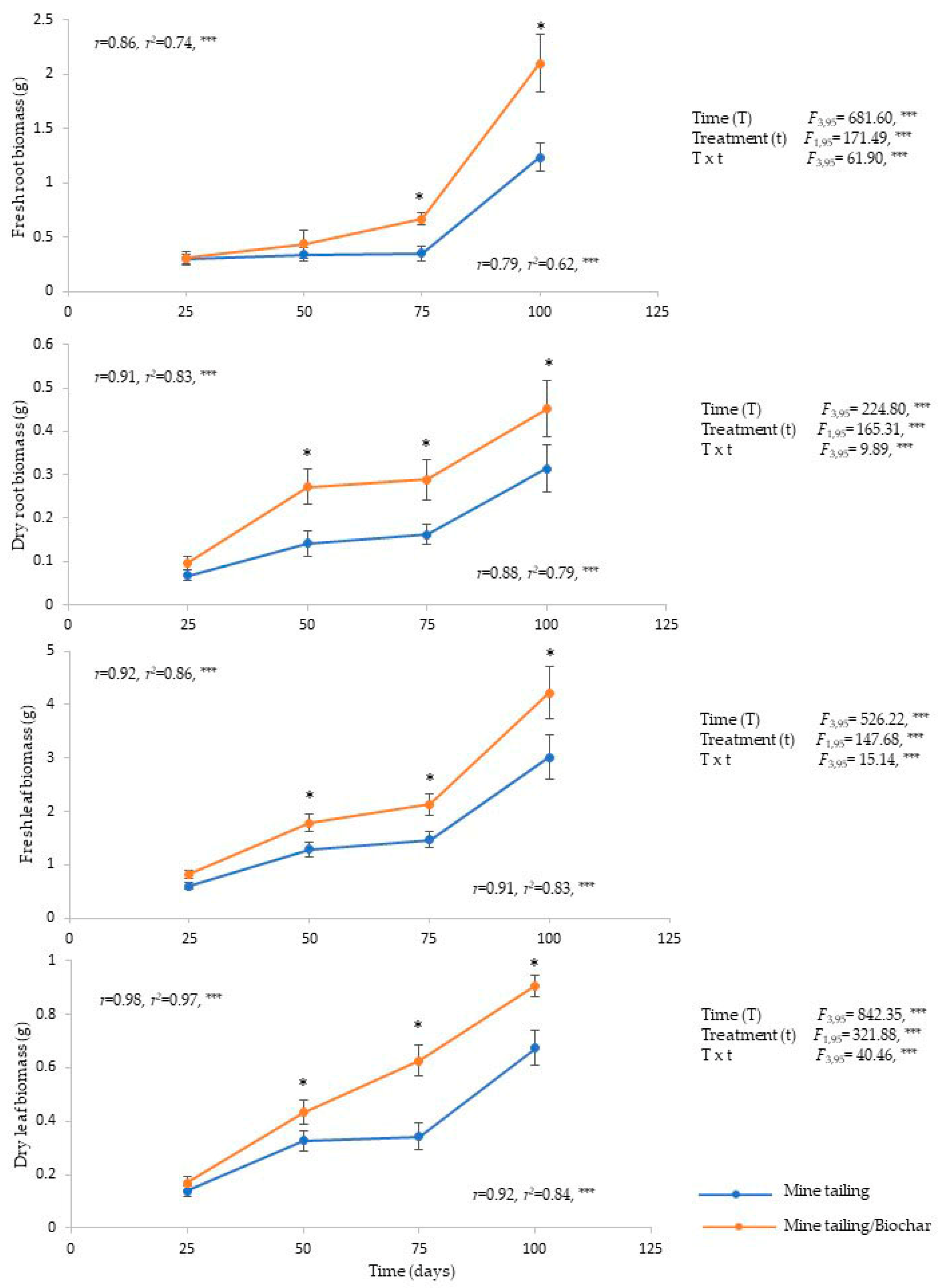
2.4. Effect of the Substrate (Mine Tailing and Mine Tailing/Biochar) on C. pumila Biomass
2.5. Effect of the Substrate (Mine Tailing and Mine Tailing/Biochar) on Chlorophyll a and b Content in C. pumila Individuals
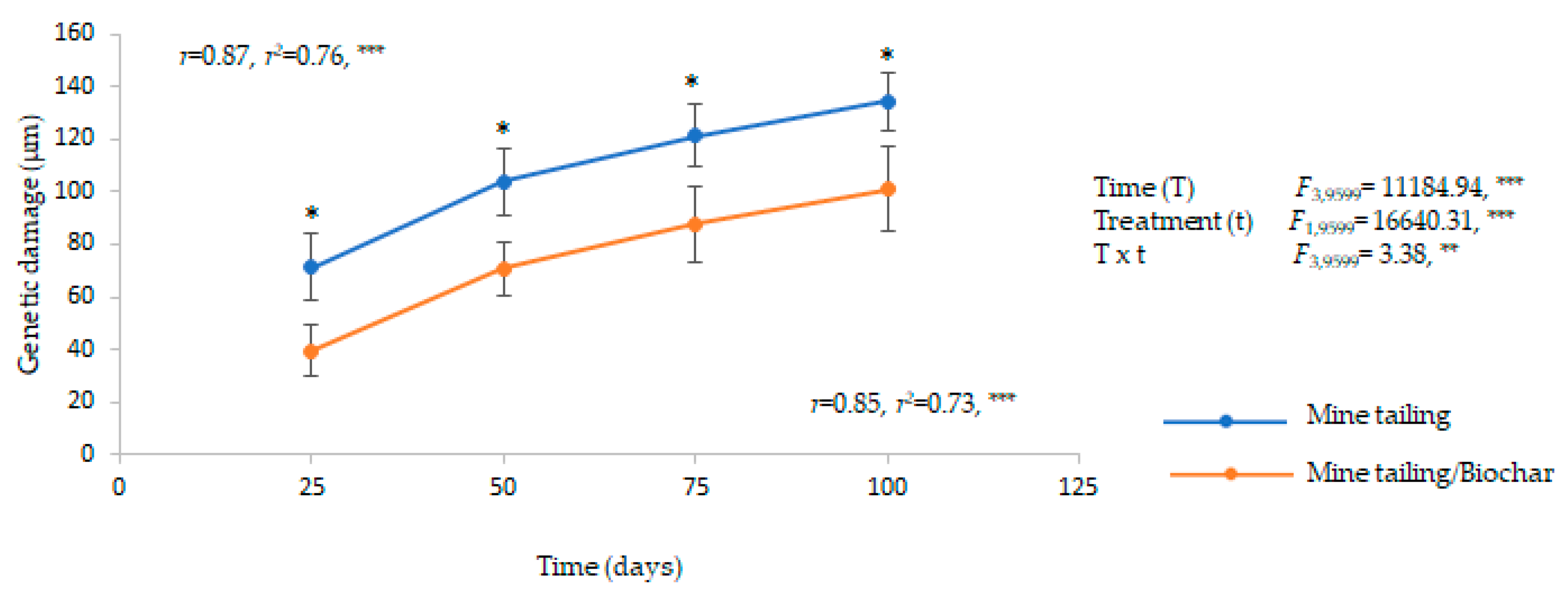
2.6. Effect of the Substrate (Mine Tailing and Mine Tailing/Biochar) on Genetic Damage in C. pumila Individuals
3. Discussion
3.1. Influence of Biochar on Mine Tailing Substrate in the Bioaccumulation of Heavy Metals in C. pumila Individuals
3.2. Influence of Biochar on Bioconcentration and Translocation of Heavy Metals in C. pumila Individuals
3.3. Influence of Biochar on Mine Tailing Substrate on the Biomass of C. pumila
3.4. Influence of Biochar on the Mine Tailing Substrate on Chlorophyll a and b Contents and Genetic Damage in C. pumila
4. Materials and Methods
4.1. Study Sites
4.2. Study Species
4.3. Seed Collection, Germination, and Obtaining Seedlings
4.4. Biochar Production and Physicochemical Characterization
4.5. Determination of Plant Biomass
4.6. Analysis of Heavy Metal Concentration
4.7. Determination of Bioconcentration Factor (BCF) and Translocation Factor (TF)
4.8. Genetic Damage: Alkaline Gel Electrophoresis
4.9. Determination of Chlorophyll Content
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akoto, R.; Anning, A.K. Heavy metal enrichment and potential ecological risks from different solid mine wastes at a mine site in Ghana. Environ. Adv. 2021, 3, 100028. [Google Scholar] [CrossRef]
- Mussali-Galante, P.; Tovar-Sánchez, E.; Valverde, M.; Rojas del Castillo, E. Biomarkers of exposure for assessing environmental metal pollution: From molecules to ecosystems. Rev. Int. Contam. Ambient. 2013, 29, 117–140. [Google Scholar] [CrossRef]
- Cuevas, J.G.; Faz, A.; Martínez, S.; Gabarrón, M.; Beltrá, J.C.; Martínez, J.; Acosta, J.A. Spatial distribution and pollution evaluation in dry riverbeds affected by mine tailings. Environ. Geochem. Health 2023, 45, 9157–9173. [Google Scholar] [CrossRef] [PubMed]
- Raji, I.B.; Palamuleni, L.G.; Toxic Heavy Metals in Soil and Plants from a Gold Mining Area, South Africa. Heavy Metals-Recent Advances. 2023. Available online: https://www.intechopen.com/books/12077#:~:text=10.5772/intechopen.104133 (accessed on 16 August 2024).
- Chileshe, M.N.; Syampungani, S.; Festin, E.S.; Tigabu, M.; Daneshvar, A.; Odén, P.C. Physico-chemical characteristics and heavy metal concentrations of copper mine wastes in Zambia: Implications for pollution risk and restoration. J. For. Res. 2023, 31, 1283–1293. [Google Scholar] [CrossRef]
- Elhag, M.; Al-Ghamdi, A.A.M.; Galal, H.K.; Dahlan, A. Evaluation of Aloe vera L. as phytoremediator of heavy metals contaminated soils in arid environments. Appl. Ecol. Environ. Res. 2018, 16, 6033–6045. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant. Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Punia, A. Role of temperature, wind, and precipitation in heavy metal contamination at copper mines: A review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanislawska, E. The Phytoremediation Potential of Local Wild Grass Versus Cultivated Grass Species for Zinc-Contaminated Soil. Agronomy 2023, 13, 160. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agronomy 2011, 112, 145–204. [Google Scholar] [CrossRef]
- Singh, H.; Pant, G. Phytoremediation: Low input-based ecological approach for sustainable environment. Appl. Water Sci. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Erakhrumen, A.A.; Agbontalor, A. Phytoremediation: An Environmentally Sound Technology for Pollution Prevention, Control and Remediation in Developing Countries. Educ. Res. Rev. 2007, 2, 151–156. Available online: https://www.academicjournals.org/ERR (accessed on 16 August 2024).
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Wang, H. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.H.; Amirahmadi, H.E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- FAO. Coconut Food and Agricultural Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 March 2023).
- Ajien, A.; Idris, J.; Sofwan, N.; Husen, R.; Seli, H. Coconut shell and husk biochar: A review of production and activation technology, economic, financial aspect and application. Waste Manag. Res. 2023, 41, 37–51. Available online: https://journals.sagepub.com/home/wmr (accessed on 16 August 2024). [CrossRef]
- Rojas, E.; Lopez, M.C.; Valverde, M. Single cell gel electrophoresis assay: Methodology and applications. J. Chromatogr. 1999, 722, 225–254. [Google Scholar] [CrossRef]
- Pernía, B.; De Sousa, A.; Reyes, R.; Castrillo, M. Biomarcadores de contaminación por cadmio en las plantas. Interciencia 2008, 33, 112–119. [Google Scholar]
- Sánchez-López, A.S.; González-Chávez, M.C.A.; Carrillo-González, R. Absorber, inmovilizar o atrapar: Funciones de las plantas en la remediación de sitios contaminados por elementos potencialmente tóxicos. Agro Product. 2017, 10, 80–86. [Google Scholar]
- Noguez-Inesta, A.; López-Sánchez, A.S.; Carrillo-González, R.; González-Chávez, M.C.A. Uso de leguminosas (Fabaceae) en fitorremediación. Agro Product. 2017, 10, 57–62. [Google Scholar]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Li, L. Effects of biochar on availability and plant uptake of heavy metals–A meta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Escalante, A.; Pérez, G.; Hidalgo, C.; López, J.; Campo, J.; Valtierra, E.; Etchevers, J.D. Biocarbón (biochar) I: Naturaleza, historia, fabricación y uso en el suelo. Terra Latinoam. 2016, 34, 367–382. [Google Scholar]
- Luna, D.; González, A.; Gordon, M.; Martín, N. Obtención de carbón activado a partir de la cáscara de coco. ContactoS 2007, 64, 39–48. [Google Scholar]
- Puentes, L.H.; Joya, E. Reconocimiento de Características, Obtención y Utilización de la Estopa de Coco. Artesanías de Col. 2005, 1–68. [Google Scholar]
- López, M.I.; Soledad, B.; Echezuría, H.; Delgado, J. Evaluación de las características físicas del biocarbón obtenido por el Centro de Investigación y Desarrollo de Ingeniería de la UCAB. Tekhné 2020, 23, 42–51. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef]
- Melo, L.C.; Coscione, A.R.; Abreu, C.A.; Puga, A.P.; Camargo, O.A. Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw–derived biochar. BioResources 2013, 8, 4992–5004. [Google Scholar] [CrossRef]
- Shen, X.; Huang, D.Y.; Ren, X.F.; Zhu, H.H.; Wang, S.; Xu, C.; Zhu, Q.H. Phytoavailability of Cd and Pb in crop straw biochar-amended soil is related to the heavy metal content of both biochar and soil. J. Environ. Manag. 2016, 168, 245–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Liao, Y.; Reid, B.J.; Chi, H.; Hou, Y.; Cai, C. Modest amendment of sewage sludge biochar to reduce the accumulation of cadmium into rice (Oryza sativa L.): A field study. Environ. Pollut. 2016, 216, 819–825. [Google Scholar] [CrossRef]
- Nawab, J.; Ghani, J.; Khan, S.; Xiaoping, W. Minimizing the risk to human health due to the ingestion of arsenic and toxic metals in vegetables by the application of biochar, farmyard manure and peat moss. J. Environ. Manag. 2018, 214, 172–183. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Wang, R. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Liu, H.T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Cea, M.; Eugenia, G.M.; Cornejo, P.; Ok, Y.; Borie, F. Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil. J. Soils Sediments 2017, 17, 741–750. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Zehetner, F. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Zhang, C.; Shan, B.; Zhu, Y.; Tang, W. Remediation effectiveness of Phyllostachys pubescens biochar in reducing the bioavailability and bioaccumulation of metals in sediments. Environ. Pollut. 2018, 242, 1768–1776. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application research of biochar for the remediation of soil heavy metals contamination: A review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512, 454–463. [Google Scholar] [CrossRef]
- Ho, S.H.; Yang, Z.K.; Nagarajan, D.; Chang, J.S.; Ren, N.Q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef]
- Ehab, I.A.; El-Sherbini, M.A.; Selim, E.M.M. Effects of biochar on soil properties, heavy metal availability and uptake, and growth of summer squash grown in metal-contaminated soil. Sci. Hortic. 2022, 301, 111097. [Google Scholar] [CrossRef]
- Eissa, M.A. Effect of cow manure biochar on heavy metals uptake and translocation by zucchini (Cucurbita pepo L.). Arabian J. Geosci. 2019, 12, 48. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Chen, Y.; Wang, S.; Wang, M.; Xie, T.; Wang, G. Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci. Rep. 2016, 6, 31616. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Fu, S.; Mendez, A.; Gasco, G.; Paz, J. Can biochar and phytoextractors be jointly used for cadmium remediation? PLoS ONE 2014, 9, e95218. [Google Scholar] [CrossRef] [PubMed]
- Smider, B.; Singh, B. Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Lebrun, M.; Macri, C.; Miard, F.; Hattab, N.; Motelica, M.; Morabito, D.; Bourgerie, S. Effect of biochar amendments on As and Pb mobility and phytoavailability in contaminated mine technosols phytoremediated by Salix. J. Geochem. Explor. 2017, 182, 149–156. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology and Development, 6th ed.; Universidad de California: Santa Cruz, CA, USA, 2015; p. 761. ISBN 978-1-60535-353-1. [Google Scholar]
- Rashid, A.; Camm, E.L.; Ekramoddoullah, A.K. Molecular mechanism of action of Pb2+ and Zn2+ on water oxidizing complex of photosystem II. FEBS Lett. 1994, 350, 296–298. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Dalurzo, H.C.; Gomez, M.; Romero-Puertas, M.C.; Del Rio, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Ali, G.; Srivastava, P.S.; Iqbal, M. Influence of cadmium and zinc on growth and photosynthesis of Bacopa monniera cultivated in vitro. Biol. Plant 2000, 43, 599–601. [Google Scholar] [CrossRef]
- Küpper, H.; Parameswaran, A.; Leitenmaier, B.; Trtílek, M.; Šetlík, I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007, 175, 655–674. [Google Scholar] [CrossRef]
- Faller, P.; Kienzler, K.; Krieger, A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gichner, T.; Patková, Z.; Száková, J.; Demnerová, K. Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotox. Environ. Saf. 2006, 65, 420–426. [Google Scholar] [CrossRef]
- Muro-González, D.A.; Mussali-Galante, P.; Valencia-Cuevas, L.; Flores-Trujillo, K.; Tovar-Sánchez, E. Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopis laevigata reveal its potential for phytoremediation. Environ. Sci. Pollut. Res. 2020, 27, 40187–40204. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Espinoza, J.; Salinas-Sánchez, D.O.; Mussali-Galante, P.; Castrejón-Godínez, M.L.; Rodríguez, A.; González-Cortazar, M.; Zamilpa, A.; Tovar-Sánchez, E. Dodonaea viscosa (Sapindaceae) as a phytoremediator for soils contaminated by heavy metals in abandoned mines. Environ. Sci. Pollut. Res. 2022, 30, 2509–2529. [Google Scholar] [CrossRef]
- Siddiqui, S.; Meghvansi, M.K.; Wani, M.A.; Jabee, F. Evaluating cadmium toxicity in the root meristem of Pisum sativum L. Acta Physiol. Plant. 2009, 31, 531–536. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Douay, F.; Dumat, C.; Pinelli, E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 121–147. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, G.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Cadmium-induced senescence in nodules of soybean (Glycine max L.) plants. Plant Soil 2004, 262, 373–381. [Google Scholar] [CrossRef]
- SEMARNAT. Evaluación de Tecnologías de Remediación Para Suelos Contaminados Con Metales, Etapa II; Secretaría del Medio Ambiente y Recursos Naturales. Instituto Nacional de Ecología, SEMARNAT-INE: México, México, 2005; p. 36. [Google Scholar]
- Mussali-Galante, P.; Santoyo-Martínez, M.; Castrejón-Godínez, M.L.; Breton-Deval, L.; Rodríguez-Solís, A.; Valencia-Cuevas, L.; Tovar-Sánchez, E. The bioaccumulation potential of heavy metals by Gliricidia sepium (Fabaceae) in mine tailings. Environ. Sci. Pollut. Res. 2023, 30, 38982–38999. [Google Scholar] [CrossRef] [PubMed]
- Rzedowski, G.C.; Rzedowski, J. Flora fanerogámica del Valle de México, 2nd ed.; Instituto de Ecología, A.C. y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Pátzcuaro, Mexico, 2001; p. 1406. [Google Scholar]
- Lindig, R.; Lara, S. Effect of scarification and growing media on seed germination of Crotalaria pumila (Ort.). Seed Sci. Technol. 2004, 32, 231–234. [Google Scholar] [CrossRef]
- Sourakov, A. You are what you eat: Native versus exotic Crotalaria species (Fabaceae) as host plants of the Ornate Bella Moth, Utetheisa ornatrix (Lepidoptera: Erebidae: Arctiinae). J. Nat. Hist. 2015, 49, 2397–2415. [Google Scholar] [CrossRef]
- Gold, K.; León, P.; Way, M. Manual de Recolección de Semillas de Plantas Silvestres; Boletín INIA; Instituto de Investigaciones Agropecuarias, Centro Regional de Investigación Intihuasi: La Serena, Chile, 2004; pp. 10–62. [Google Scholar]
- Mexicana, Norma Oficial. NOM-021-RECNAT-2000. Establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreos y análisis. D. Of. Fed. 2000, 14, 17. [Google Scholar]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Statsoft, Inc. Statistica for Windows; Statsoft, Inc.: Tulsa, OK, USA, 2007. [Google Scholar]


| Concentration (mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|
| Metals | Time (Days) | BCF (Root) | BCF (Leaf) | TF | TF | ||
| Mine Tailing | Mine Tailing/Biochar | Mine Tailing | Mine Tailing/Biochar | Mine Tailing | Mine Tailing/Biochar | ||
| Zinc | |||||||
| 25 | 1.15 | 0.37 | 0.18 | 0.14 | 0.16 | 0.37 | |
| 50 | 1.47 | 0.95 | 0.28 | 0.23 | 0.19 | 0.24 | |
| 75 | 1.63 | 0.85 | 0.54 | 0.21 | 0.33 | 0.25 | |
| 100 | 1.90 | 0.76 | 0.57 | 0.21 | 0.30 | 0.28 | |
| Average ± SD | 1.54 ± 0.32 | 0.73 ± 0.26 | 0.39 ± 0.19 | 0.20 ± 0.04 | 0.25 ± 0.08 | 0.29 ± 0.06 | |
| t-student | 24.049 *** | 12.213 *** | 3.917 *** | ||||
| Cooper | |||||||
| 25 | 18.70 | 14.00 | 12.71 | 10.50 | 0.68 | 0.75 | |
| 50 | 26.67 | 18.00 | 14.71 | 11.88 | 0.55 | 0.66 | |
| 75 | 28.29 | 11.25 | 15.46 | 6.63 | 0.55 | 0.59 | |
| 100 | 26.30 | 9.25 | 13.84 | 5.13 | 0.53 | 0.55 | |
| Average ± SD | 24.99 ± 4.28 | 13.13 ± 3.79 | 14.18 ± 1.18 | 8.53 ± 3.18 | 0.58 ± 0.07 | 0.63 ± 0.09 | |
| t-student | 28.174 *** | 22.237 *** | 5.537 *** | ||||
| Lead | |||||||
| 25 | 124.35 | 51.57 | 28.26 | 8.86 | 0.23 | 0.17 | |
| 50 | 157.20 | 59.71 | 58.09 | 11.71 | 0.37 | 0.20 | |
| 75 | 167.53 | 67.43 | 56.51 | 12.57 | 0.34 | 0.19 | |
| 100 | 140.28 | 44.00 | 55.22 | 8.14 | 0.39 | 0.19 | |
| Average ± SD | 147.34 ± 19 | 55.68 ± 10.13 | 49.52 ± 14.22 | 10.32 ± 2.15 | 0.33 ± 0.07 | 0.18 ± 0.01 | |
| t-student | 55.367 *** | 34.707 *** | 22.768 *** | ||||
| Cadmium | |||||||
| 25 | 12.79 | 3.93 | 6.69 | 1.67 | 0.52 | 0.42 | |
| 50 | 15.90 | 3.57 | 6.10 | 1.31 | 0.38 | 0.37 | |
| 75 | 15.30 | 2.62 | 5.74 | 1.07 | 0.38 | 0.41 | |
| 100 | 14.58 | 1.79 | 3.23 | 0.00 | 0.22 | 0.00 | |
| Average ± SD | 14.64 ± 1.35 | 2.98 ± 0.97 | 5.44 ± 1.53 | 1.01 ± 0.12 | 0.38 ± 0.12 | 0.30 ± 0.20 | |
| t-student | 89.419 *** | 33.574 *** | 3.462 *** | ||||
| Predicted | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Measurement | Metal | Sample []soil | Sample []plant | Adjusted BCF | Excluder | Indicator | Accumulator | Hyperaccumulator | Result |
| Root | Pb | 6.972 | 910.27 | 370.39 | Hyperaccumulator | ||||
| Cd | 8.365 | 12.62 | 42.18 | 0.810 | 3.000 | 16.155 | 61.547 | Accumulator | |
| Cu | 8.023 | 211.75 | 74.76 | Hyperaccumulator | |||||
| Zn | 428.114 | 815.54 | 39.42 | 1.675 | 6.201 | 33.395 | 127.230 | Accumulator | |
| Leaves | Pb | 6.972 | 132.3 | 145.81 | Hyperaccumulator | ||||
| Cd | 8.365 | 12.53 | 9.34 | 0.554 | 2.052 | 11.052 | 42.106 | Accumulator | |
| Cu | 8.023 | 111.05 | 39.21 | Accumulator | |||||
| Zn | 428.114 | 243.23 | 11.76 | 1.146 | 4.242 | 22.847 | 87.043 | Accumulator | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Ramírez, M.; Tovar-Sánchez, E.; Rodríguez-Solís, A.; Flores-Trujillo, K.; Castrejón-Godínez, M.L.; Mussali-Galante, P. Assisted Phytoremediation between Biochar and Crotalaria pumila to Phytostabilize Heavy Metals in Mine Tailings. Plants 2024, 13, 2516. https://doi.org/10.3390/plants13172516
Rosas-Ramírez M, Tovar-Sánchez E, Rodríguez-Solís A, Flores-Trujillo K, Castrejón-Godínez ML, Mussali-Galante P. Assisted Phytoremediation between Biochar and Crotalaria pumila to Phytostabilize Heavy Metals in Mine Tailings. Plants. 2024; 13(17):2516. https://doi.org/10.3390/plants13172516
Chicago/Turabian StyleRosas-Ramírez, Marcos, Efraín Tovar-Sánchez, Alexis Rodríguez-Solís, Karen Flores-Trujillo, María Luisa Castrejón-Godínez, and Patricia Mussali-Galante. 2024. "Assisted Phytoremediation between Biochar and Crotalaria pumila to Phytostabilize Heavy Metals in Mine Tailings" Plants 13, no. 17: 2516. https://doi.org/10.3390/plants13172516







