Quantification of Airborne Particulate Matter and Trace Element Deposition on Hedera helix and Senecio cineraria Leaves
Abstract
:1. Introduction
2. Results
2.1. Daily Meteorological Conditions and Air Quality Monitoring Stations
2.2. Gravimetric Quantification of Particulate Matter Deposited on Leaves
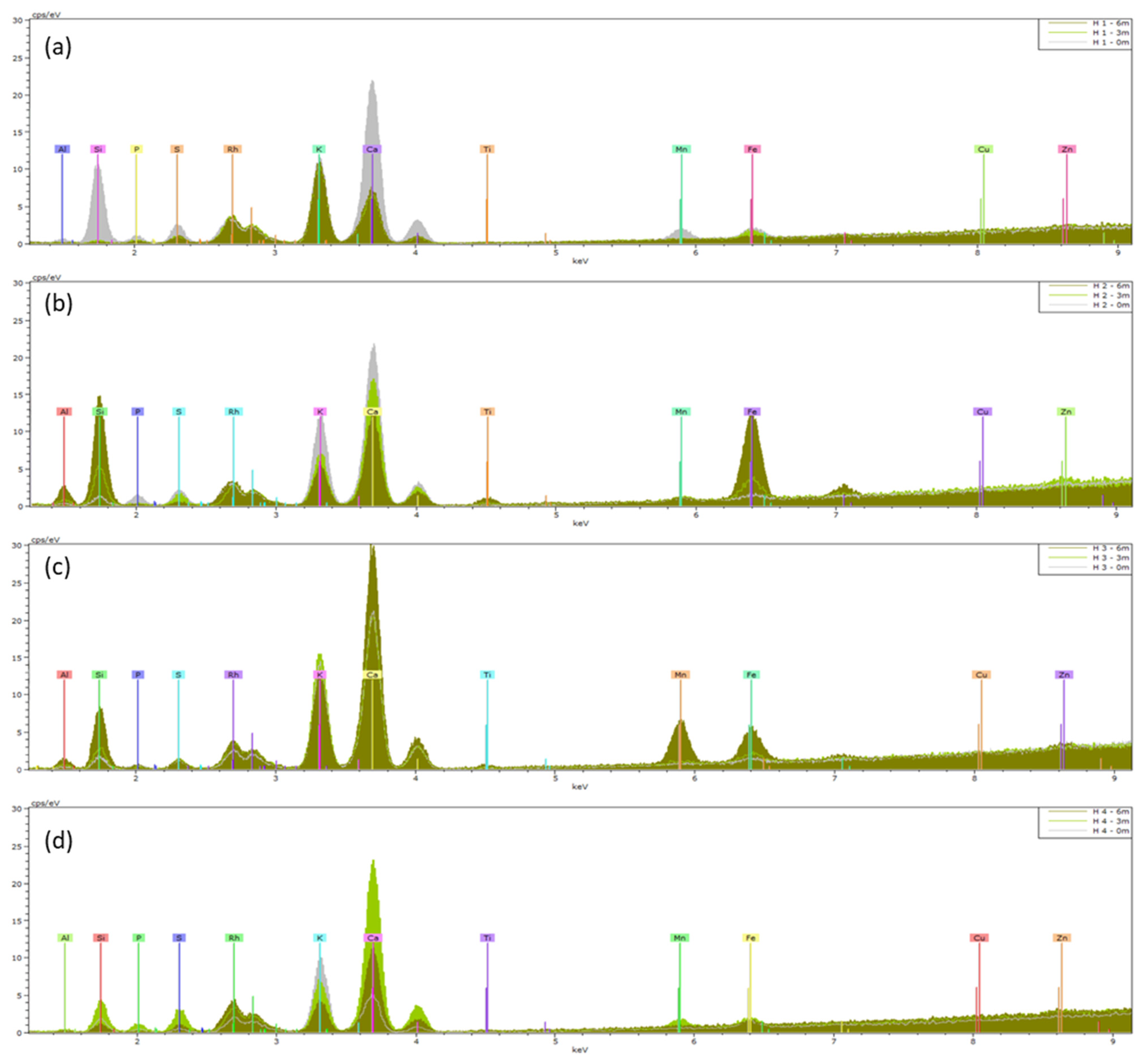
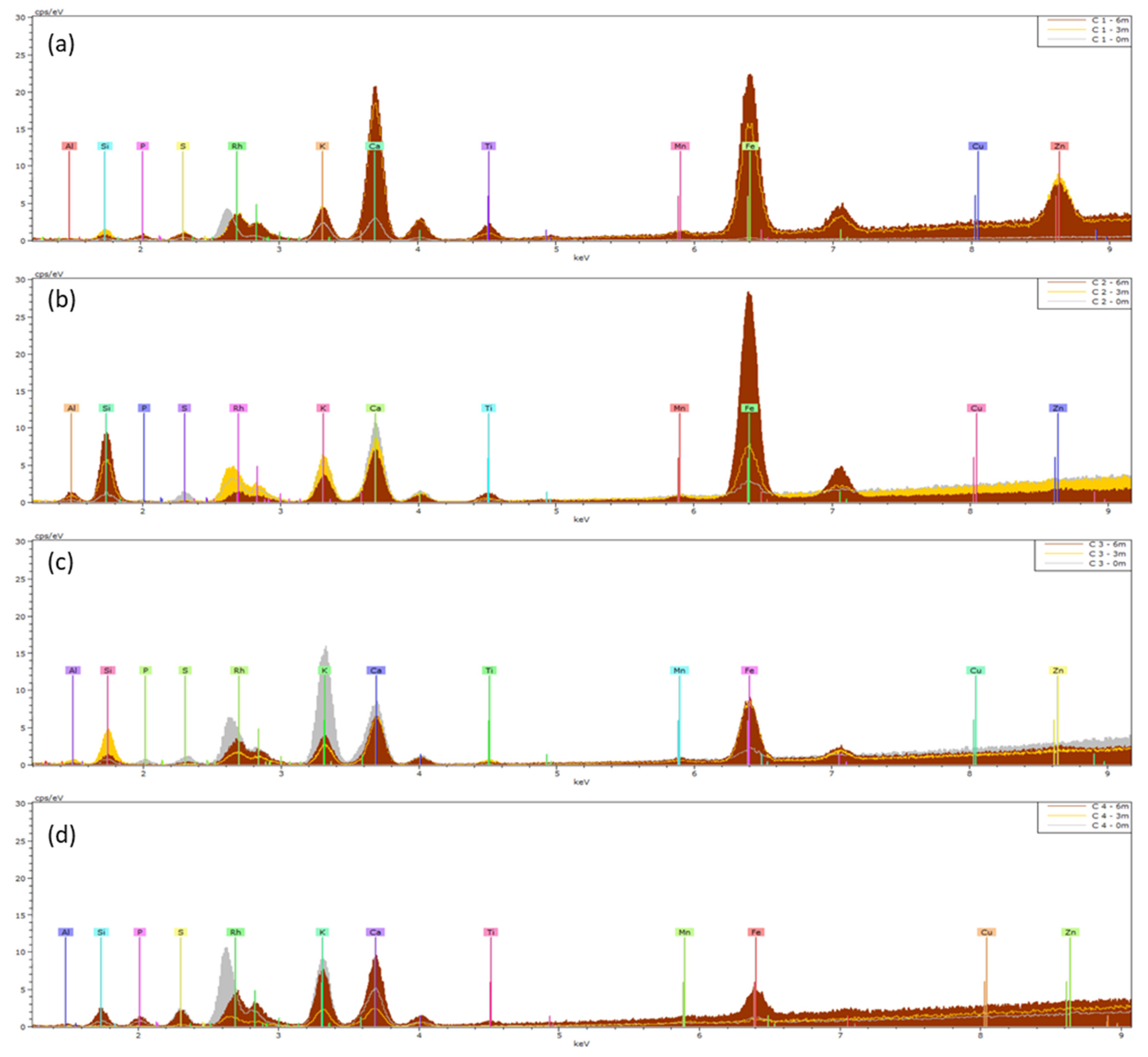
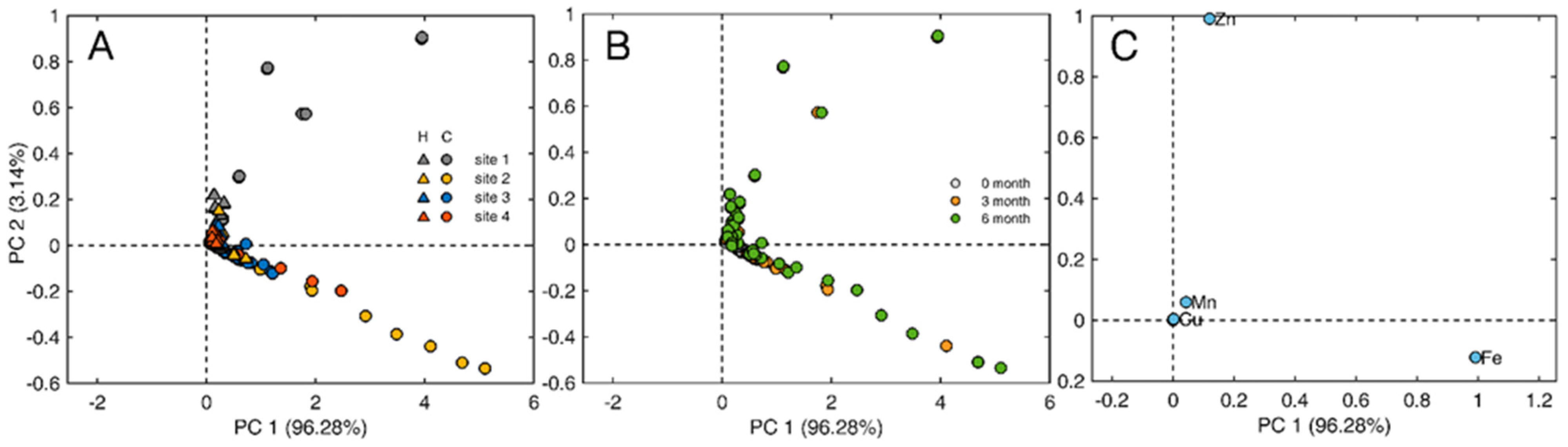
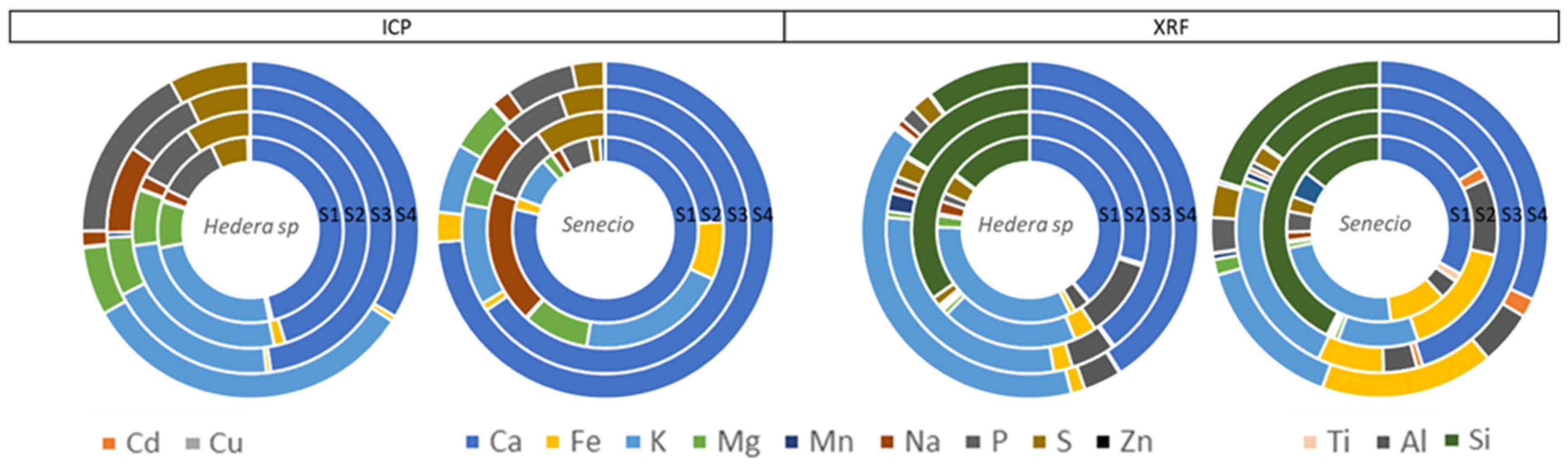
2.3. Leaf Surface Elemental Composition: XRF and ICP
3. Discussion
4. Materials and Methods
4.1. Selection of Plant Species
4.2. Sites Selected, Air Quality Monitoring Stations and Daily Meteorological Conditions
4.3. Gravimetric Quantification of Particulate Matter Deposited on Leaves
4.4. Leaf Surface Elemental Composition: XRF and ICP
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EEA. Air Quality in Europe—2017 Report; European Economic Area (EEA): Brussels, Belgium, 2017. [Google Scholar] [CrossRef]
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide: Executive Summary. Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 13 January 2024).
- Oliva, S.R.; Espinosa, A.J.F. Monitoring of Heavy Metals in Topsoils, Atmospheric Particles and Plant Leaves to Identify Possible Contamination Sources. Microchem. J. 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Ioannidou, E.; Papagiannis, S.; Manousakas, M.I.; Betsou, C.; Eleftheriadis, K.; Paatero, J.; Papadopoulou, L.; Ioannidou, A. Trace Elements Concentrations in Urban Air in Helsinki, Finland during a 44-Year Period. Atmosphere 2023, 14, 1430. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.; Zheng, X. The relationship between PM2.5 and the onset and exacerbation of childhood asthma: A short communication. Front Pediatr. 2023, 1, 1191852. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the Pathogenesis of Lung Cancer and Chronic Airway Inflammatory Diseases. Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, M.; Wang, Z.; Chen, J.; Zhao, J.; Xu, Y.; Wei, X.; Wang, J.; Xie, J. Role of PM2.5 in the Development and Progression of COPD and Its Mechanisms. Respir. Res. 2019, 20, 120. [Google Scholar] [CrossRef]
- Hantrakool, S.; Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of Particulate Matter on Inflammation and Thrombosis: Past Evidence for Future Prevention. Int. J. Environ. Res. Public Health 2022, 19, 8771. [Google Scholar] [CrossRef]
- Yi, W.Y.; Lo, K.M.; Mak, T.; Leung, K.S.; Leung, Y.; Meng, M.L. A Survey of Wireless Sensor Network Based Air Pollution Monitoring Systems. Sensors 2015, 15, 31392–31427. [Google Scholar] [CrossRef]
- Cozea, A.; Tanase, G. Green Biomonitoring Systems for Air Pollution. Eng. Proc. 2022, 19, 8. [Google Scholar] [CrossRef]
- Molnár, V.É.; Tőzsér, D.; Szabó, S.; Tóthmérész, B.; Simon, E. Use of Leaves as Bioindicator to Assess Air Pollution Based on Composite Proxy Measure (Apti), Dust Amount and Elemental Concentration of Metals. Plants 2020, 9, 1743. [Google Scholar] [CrossRef]
- Agarwal, P.; Sarkar, M.; Chakraborty, B.; Banerjee, T. Phytoremediation of Air Pollutants: Prospects and Challenges. Phytomanag. Pollut. Sites Mark. Oppor. Sustain. Phytoremediat. 2019, 9, 221–241. [Google Scholar] [CrossRef]
- Gupta, G.P.; Kumar, B.; Kulshrestha, U.C. Impact and Pollution Indices of Urban Dust on Selected Plant Species for Green Belt Development: Mitigation of the Air Pollution in NCR Delhi, India. Arab. J. Geosci. 2016, 9, 136. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Nowak, A.; Wacławek, S.; Ziembik, Z.; Rajfur, M. Is Active Moss Biomonitoring Comparable to Air Filter Standard Sampling? Int. J. Environ. Res. Public Health 2022, 19, 4706. [Google Scholar] [CrossRef] [PubMed]
- Méndez, M.; Vergara, G.; Casagrande, G.; Bongianino, S. Climate Classification of the Agricultural Region of La Pampa Province, Argentina. Semiárida Rev. Fac. Agron. UNLPam 2021, 31, 9–20. [Google Scholar] [CrossRef]
- Nguyen, T.; Yu, X.; Zhang, Z.; Liu, M.; Liu, X. Relationship between Types of Urban Forest and PM2.5 Capture at Three Growth Stages of Leaves. J. Environ. Sci. 2015, 27, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Winkler, P. The Growth of Atmospheric Aerosol Particles with Relative Humidity. Phys. Scr. 1988, 37, 223. [Google Scholar] [CrossRef]
- Mei, D.; Wen, M.; Xu, X.; Zhu, Y.; Xing, F. The Influence of Wind Speed on Airflow and Fine Particle Transport within Different Building Layouts of an Industrial City. J. Air Waste Manag. Assoc. 2018, 68, 1038–1050. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, L.; Yan, C.; Du, J.; Li, Y.; Zhao, H. Analysis of the Influence of Precipitation and Wind on PM2.5 and PM10 in the Atmosphere. Adv. Meteorol. 2020, 2020, 5039613. [Google Scholar] [CrossRef]
- Litschike, T.; Kuttler, W. On the Reduction of Urban Particle Concentration by Vegetation—A Review. Meteorol. Z. 2008, 17, 229–240. [Google Scholar] [CrossRef]
- Srivastava, N.; Bagchi, G. Influence of Micronutrient Availability on Biomass Production in Cineraria Maritima. Indian J. Pharm. Sci. 2006, 68, 238–239. [Google Scholar] [CrossRef]
- Castanheiro, A.; Hofman, J.; Nuyts, G.; Joosen, S.; Spassov, S.; Blust, R.; Lenaerts, S.; De Wael, K.; Samson, R. Leaf Accumulation of Atmospheric Dust: Biomagnetic, Morphological and Elemental Evaluation Using SEM, ED-XRF and HR-ICP-MS. Atmos. Environ. 2020, 221, 117082. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Suspended Particulate Matter Deposition and Its Impact on Urban Trees. Atmos. Pollut. Res. 2018, 9, 1072–1082. [Google Scholar] [CrossRef]
- Li, C.; Du, D.; Gan, Y.; Ji, S.; Wang, L.; Chang, M.; Liu, J. Foliar Dust as a Reliable Environmental Monitor of Heavy Metal Pollution in Comparison to Plant Leaves and Soil in Urban Areas. Chemosphere 2022, 287, 132341. [Google Scholar] [CrossRef]
- Santos, R.S.; Sanches, F.A.C.R.A.; Leitão, R.G.; Leitão, C.C.G.; Oliveira, D.F.; Anjos, M.J.; Assis, J.T. Multielemental Analysis in Nerium oleander L. Leaves as a Way of Assessing the Levels of Urban Air Pollution by Heavy Metals. Appl. Radiat. Isot. 2019, 152, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Pandolfi, M.; Moreno, T.; Furger, M.; Pey, J.; Alastuey, A.; Bukowiecki, N.; Prevot, A.S.H.; Baltensperger, U.; Querol, X. Sources and Variability of Inhalable Road Dust Particles in Three European Cities. Atmos. Environ. 2011, 45, 6777–6787. [Google Scholar] [CrossRef]
- Adachi, K.; Tainosho, Y. Characterization of Heavy Metal Particles Embedded in Tire Dust. Environ. Int. 2004, 30, 1009–1017. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Wu, S.; Deng, F.; Wei, H.; Huang, J.; Wang, H.; Shima, M.; Wang, X.; Qin, Y.; Zheng, C.; Hao, Y.; et al. Chemical Constituents of Ambient Particulate Air Pollution and Biomarkers of Inflammation, Coagulation and Homocysteine in Healthy Adults: A Prospective Panel Study. Part. Fibre Toxicol. 2012, 9, 49. [Google Scholar] [CrossRef]
- Nayebare, S.R.; Aburizaiza, O.S.; Siddique, A.; Carpenter, D.O.; Hussain, M.; Zeb, J.; Aburiziza, A.J.; Khwaja, H.A. Ambient Air Quality in the Holy City of Makkah: A Source Apportionment with 1 Elemental Enrichment Factors (EFs) and Factor Analysis (PMF). Environ. Pollut. 2018, 243, 1791–1801. [Google Scholar] [CrossRef]
- Bilo, F.; Borgese, L.; Dalipi, R.; Zacco, A.; Federici, S.; Masperi, M.; Leonesio, P.; Bontempi, E.; Depero, L. Elemental Analysis of Tree Leaves by Total Reflection X-Ray Fluorescence: New Approaches for Air Quality Monitoring. Chemosphere 2017, 178, 504–512. [Google Scholar] [CrossRef]
- Hulskotte, J.H.J.; Roskam, G.D.; Denier van der Gon, H.A.C. Elemental Composition of Current Automotive Braking Materials and Derived Air Emission Factors. Atmos. Environ. 2014, 99, 436–445. [Google Scholar] [CrossRef]
- Markert, B. Establishing of “Reference Plant” for Inorganic Characterization of Different Plant Species by Chemical Fingerprinting. Water Air Soil Pollut. 1992, 64, 533–538. [Google Scholar] [CrossRef]
- Karmakar, D.; Padhy, P.K. Air Pollution Tolerance, Anticipated Performance, and Metal Accumulation Indices of Plant Species for Greenbelt Development in Urban Industrial Area. Chemosphere 2019, 237, 124522. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, P.A.D.H.N.; Ranundeniya, R.M.N.S.; Najim, M.M.M.; Seneviratne, S. A Determination of Air Pollution in Colombo and Kurunegala, Sri Lanka, Using Energy Dispersive X-Ray Fluorescence Spectrometry on Heterodermia Speciosa. Turk. J. Bot. 2011, 35, 439–446. [Google Scholar] [CrossRef]
- Gehring, U.; Beelen, R.; Eeftens, M.; Hoek, G.; De Hoogh, K.; De Jongste, J.C.; Keuken, M.; Koppelman, G.H.; Meliefste, K.; Oldenwening, M.; et al. Particulate Matter Composition and Respiratory Health the PIAMA Birth Cohort Study. Epidemiology 2015, 26, 300–309. [Google Scholar] [CrossRef]
- Mazur, J. Plants as Natural Anti-Dust Filters—Preliminary Research. Czas. Tech. 2018, 115, 165–172. [Google Scholar] [CrossRef]
- Redondo-Bermúdez, M.d.C.; Gulenc, I.T.; Cameron, R.W.; Inkson, B.J. ‘Green Barriers’ for Air Pollutant Capture: Leaf Micromorphology as a Mechanism to Explain Plants Capacity to Capture Particulate Matter. Environ. Pollut. 2021, 288, 117809. [Google Scholar] [CrossRef]
- Pelser, P.B.; Gravendeel, B.; Van Der Meijden, R. Tackling Speciose Genera: Species Composition and Phylogenetic Position of Senecio Sect. Jacobaea (Asteraceae) Based on Plastid and NrDNA Sequences. Am. J. Bot. 2002, 89, 929–939. [Google Scholar] [CrossRef]
- Imperato, V.; Kowalkowski, L.; Portillo-Estrada, M.; Gawronski, S.W.; Vangronsveld, J.; Thijs, S. Characterisation of the Carpinus betulus L. Phyllomicrobiome in Urban and Forest Areas. Front. Microbiol. 2019, 10, 1110. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]




| Plant | Time | Site 1 | Site 2 | Site 3 | Site 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | ||
| Hedera helix | 0 m | 485 ± 96 a | 681 ± 125 a | 930 ± 175 a | 115 ± 44 a | 60 ± 26 a | 30 ± 23 a | 151 ± 51 a | 109 ± 90 a |
| 3 m | 341 ± 83 a | 552 ± 588 a | 699 ± 274 ab | 670 ± 577 ab | 946 ± 312 b | 965 ± 343 b | 175 ± 81 a | 215 ± 90 a | |
| 6 m | 412 ± 43 a | 505 ± 465 a | 501 ± 330 ab | 682 ± 198 b | 697 ± 92 b | 1016 ± 682 b | 752 ± 435 b | 601 ± 359 b | |
| Senecio cineraria | 0 m | 429 ± 176 a | 691 ± 235 a | 260 ± 153 a | 231 ± 159 a | 55 ± 26 a | 43 ± 50 a | 234 ± 138 a | 152 ± 48 a |
| 3 m | 2893 ± 3634 ab | 140 ± 54 b | 1686 ± 2174 ab | 344 ± 159 ab | 2450 ± 264 b | 2450 ± 343 c | 158 ± 83 a | 231 ± 114 a | |
| 6 m | 2800 ± 884 b | 132 ± 44 b | 649 ± 242 b | 383 ± 55 ab | 1056 ± 538 b | 1047 ± 582 b | 623 ± 329 b | 548 ± 94 b | |
| Plant | Site | Time | Ca | Cd | Cu | Fe | K | Mg | Mn | Na | P | S | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hedera helix | Site 1 | 0 m | 32,998.98 ± 27,408.30 a | Nd | 3.47 ± 0.62 a | 282.60 ± 84.91 a | 10,177.23 ± 2375.02 a | 2878.20 ± 632.54 a | 141.70 ± 106.07 a | 1960.00± 1227.32 a | 2860.35 ± 398.44 a | 2818.02± 613.94 a | 38.31 ± 20.02 a |
| 3 m | 24,550.20 ± 8769.40 a | 2.89 ± 0.28 a | 5.27 ± 2.37 ab | 278.83 ± 118.10 a | 13,758.05 ± 3142.70 a | 4427.88 ± 775.66 b | 170.41 ± 117.23 a | 1032.23 ± 342.76 a | 5956.04 ± 435.09 b | 3523.38 ± 767.15 a | 259.68 ± 73.00 b | ||
| 6 m | 25,406.80 ± 8920.33 a | 2.94 ± 0.27 a | 5.29 ± 2.37 ab | 283.83 ± 114.90 a | 13,846.05 ± 3147.12 a | 4445.88 ± 779.02 b | 171.21 ± 117.40 a | 1040.63 ± 343.05 a | 6002.24 ± 443.03 b | 3551.38 ± 793.25 a | 266.88 ± 71.05 b | ||
| Site 2 | 0 m | 23,197.17 ± 7330.51 a | Nd | 4.81 ± 1.68 a | 383.47 ± 225.47 a | 9875.71 ± 1534.85 | 3350.94 ± 483.98 a | 249.40 ± 115.99 a | 1961.76 ± 895.02 a | 5049.78 ± 2329.52 b | 3380.00 ± 1388.36 a | 56.00 ± 22.86 a | |
| 3 m | 48,032.26 ± 51,400.51 a | 4.23 ± 0.87 b | 7.32 ± 4.38 ab | 321.38 ± 70.78 a | 13,599.53 ± 4004.43 a | 3787.71 ± 1443.64 a | 90.95 ± 70.04 a | 902.90 ± 820.12 a | 5355.60 ± 1904.17 b | 2865.20 ± 973.93 a | 112.76 ± 90.17 a | ||
| 6 m | 19,401.31 ± 5444.44 a | Nd | 4.67 ± 1.29 a | 649.47 ± 317.21 ab | 11,185.56 ± 1650.25 a | 3288.92 ± 965.82 a | 75.17 ± 29.09 a | 797.30 ± 742.60 a | 3611.54 ± 719.13 a | 3833.01 ± 992.85 a | 31.64 ± 6.22 a | ||
| Site 3 | 0 m | 25,929.29 ± 23,126.85 a | Nd | 3.21 ± 1.34 a | 221.79 ± 54.85 a | 11,111.98 ± 1172.28 a | 2355.56 ± 493.09 a | 46.03 ± 13.86 a | 3046.21 ± 2273.69 a | 3146.16 ± 636.60 a | 2010.47 ± 661.11 a | 43.11 ± 25.10 a | |
| 3 m | 33,938.87 ± 38,909.77 a | Nd | 2.48 ± 0.80 a | 179.69 ± 52.53 a | 9353.99 ± 729.09 a | 2163.34 ± 570.93 a | 56.97 ± 12.56 a | 1236.40 ± 687.53 a | 1741.14 ± 400.99 a | 2453.05 ± 536.44 a | 49.58 ± 17.04 a | ||
| 6 m | 30,095.36 ± 22,843.27 a | Nd | 3.96 ± 2.98 a | 309.19 ± 95.20 a | 12,189.52 ± 3214.23 a | 3452.43 ± 1190.64 a | 349.01 ± 227.08 b | 1267.34 ± 1277.31 a | 5211.00 ± 1801.86 b | 4396.05 ± 2204.44 a | 85.82 ± 41.79 a | ||
| Site 4 | 0 m | 43,931.55 ± 37,549.14 a | Nd | 2.20 ± 0.81 a | 247.16 ± 77.11 a | 13,281.14 ± 1698.19 a | 2130.03 ± 688.09 a | 101.30 ± 130.88 a | 2260.15 ± 1059.64 a | 4830.25 ± 2304.66 a | 1998.10 ± 1479.04 a | 41.17 ± 21.86 a | |
| 3 m | 18,168.02 ± 6224.95 a | Nd | 2.25 ± 0.57 a | 215.74 ± 57.74 a | 13,351.28 ± 3530.92 a | 2526.43 ± 626.98 a | 107.40 ± 147.84 a | 1221.94 ± 602.59 a | 4110.69 ± 931.49 a | 2191.61 ± 1014.37 a | 63.94 ± 18.51 a | ||
| 6 m | 13,619.08 ± 5789.33 a | 0.32 ± 0.47 c | 6.03 ± 3.36 ab | 203.90 ± 57.85 a | 13,245.27 ± 5173.38 a | 2653.63 ± 507.95 a | 54.49 ± 60.57 a | 600.77 ± 560.78 a | 6978.94 ± 1613.66 a | 3121.97 ± 554.38 a | 72.78 ± 23.90 a | ||
| Senecio cineraria | Site 1 | 0 m | 43,003.87 ± 41,889.75 b | Nd | 3.47 ± 1.04 c | 235.83 ± 203.76 b | 13,576.72 ± 1680.00 b | 3377.58 ± 890.75 b | 91.99 ± 37.43 c | 7524.43 ± 4040.73 b | 3918.28 ± 1802.97 c | 3081.96 ± 739.87 b | 19.60 ± 2.89 c |
| 3 m | 87,656.37 ± 19,066.81 ab | 3.53 ± 0.54 d | 8.78 ± 2.91 d | 2190.97 ± 2106.59 d | 8507.05 ± 4559.51 b | 2050.04 ± 223.34 bc | 78.46 ± 65.85 c | 1896.24 ± 2402.75 c | 5738.16 ± 1423.92 c | 2119.96 ± 552.69 b | 1064.59 ± 712.99 d | ||
| 6 m | 88,316.37 ± 19,645.60 ab | 3.69 ± 0.63 d | 9.46 ± 3.17 d | 2216.97 ± 2112.24 d | 8565.05 ± 4585.26 b | 2098.04 ± 191.59 bc | 86.90 ± 72.92 c | 1941.84 ± 2435.27 c | 5822.16 ± 1434.75 c | 2185.96 ± 575.67 b | 1074.59 ± 712.99 d | ||
| Site 2 | 0 m | 88,507.02 ± 16,117.84 ab | Nd | 4.24 ± 1.90 c | 730.86 ± 164.83 c | 10,782.14 ± 1315.85 b | 3018.09 ± 548.62 b | 73.32 ± 21.72 c | 8541.73 ± 2537.09 b | 3559.17 ± 310.35 c | 2963.39 ± 355.52 b | 17.86 ± 10.12 c | |
| 3 m | 59,156.13 ± 38,898.75 b | 4.14 ± 0.64 d | 6.24 ± 2.60 cd | 2818.65 ± 2098.26 d | 16,404.67 ± 2925.56 c | 3944.36 ± 1815.69 b | 101.35 ± 65.43 c | 9709.67 ± 5393.01 b | 5175.05 ± 1605.05 c | 4113.68 ± 2126.64 b | 52.44 ± 29.48 c | ||
| 6 m | 18,171.57 ± 7147.07 b | 0.31 ± 0.20 e | 11.64 ± 3.28 d | 6084.31 ± 1533.21 d | 15,767.24 ± 3801.27 c | 6859.51 ± 1959.25 d | 162.11 ± 49.24 c | 14,061.89 ± 7121.0 b | 7615.10 ± 5031.09 c | 7365.76 ± 2770.72 c | 83.95 ± 29.79 d | ||
| Site 3 | 0 m | 47,180.13 ± 33,223.00 b | Nd | 2.34 ± 1.05 c | 778.30 ± 188.21 c | 12,669.43 ± 2541.18 b | 3200.89 ± 901.71 b | 84.08 ± 33.99 c | 8664.28 ± 7280.34 b | 5181.14 ± 3379.99 c | 3079.68 ± 1357.29 b | 18.20 ± 4.22 c | |
| 3 m | 39,425.96 ± 35,092.92 b | Nd | 4.36 ± 3.20 c | 1146.82 ± 394.87 d | 11,196.75 ± 3419.12 b | 3716.50 ± 1002.07 b | 81.51 ± 19.01 c | 7279.63 ± 1295.19 b | 3892.59 ± 827.82 c | 4391.16 ± 725.90 b | 29.80 ± 8.65 c | ||
| 6 m | 83,017.81 ± 78,166.32 ab | Nd | 6.98 ± 5.01 cd | 1109.90 ± 614.07 d | 14,541.05 ± 12,435.81 b | 4379.37 ± 2126.98 b | 109.61 ± 61.35 c | 7949.80 ± 3392.24 b | 8947.63 ± 6062.20 c | 6508.40 ± 3249.88 b | 61.93 ± 41.55 d | ||
| Site 4 | 0 m | 37,841.02 ± 37,178.86 b | Nd | 4.23 ± 2.40 c | 324.66 ± 71.99 b | 14,974.45 ± 1196.15 b | 3917.13 ± 862.73 b | 126.87 ± 65.10 c | 12,689.41 ± 4629.01 b | 7830.11 ± 2236.98 c | 5344.27 ± 1095.24 b | 26.25 ± 7.07 c | |
| 3 m | 50,865.89 ± 41,348.93 b | Nd | 4.76 ± 3.16 c | 282.47 ± 83.96 b | 14,674.16 ± 1360.47 b | 3796.20 ± 748.57 b | 54.37 ± 17.25 c | 9090.10 ± 2127.22 b | 5650.92 ± 1327.42 c | 5662.42 ± 1925.06 b | 53.36 ± 13.71 c | ||
| 6 m | 54,148.55 ± 39,324.89 b | 0.69 ± 0.13 e | 4.34 ± 2.33 c | 2084.22 ± 1236.25 d | 4875.76 ± 1035.63 d | 3567.87 ± 1182.56 b | 120.40 ± 67.42 c | 1360.68 ± 338.54 c | 4823.98 ± 1367.35 c | 2243.05 ± 529.26 b | 87.31 ± 45.13 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saran, A.; Mendez, M.J.; Much, D.G.; Imperato, V.; Thijs, S.; Vangronsveld, J.; Merini, L.J. Quantification of Airborne Particulate Matter and Trace Element Deposition on Hedera helix and Senecio cineraria Leaves. Plants 2024, 13, 2519. https://doi.org/10.3390/plants13172519
Saran A, Mendez MJ, Much DG, Imperato V, Thijs S, Vangronsveld J, Merini LJ. Quantification of Airborne Particulate Matter and Trace Element Deposition on Hedera helix and Senecio cineraria Leaves. Plants. 2024; 13(17):2519. https://doi.org/10.3390/plants13172519
Chicago/Turabian StyleSaran, Anabel, Mariano Javier Mendez, Diego Gabriel Much, Valeria Imperato, Sofie Thijs, Jaco Vangronsveld, and Luciano Jose Merini. 2024. "Quantification of Airborne Particulate Matter and Trace Element Deposition on Hedera helix and Senecio cineraria Leaves" Plants 13, no. 17: 2519. https://doi.org/10.3390/plants13172519






