Auxin-Mediated Lateral Root Development in Root Galls of Cucumber under Meloidogyne incognita Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Nematodes
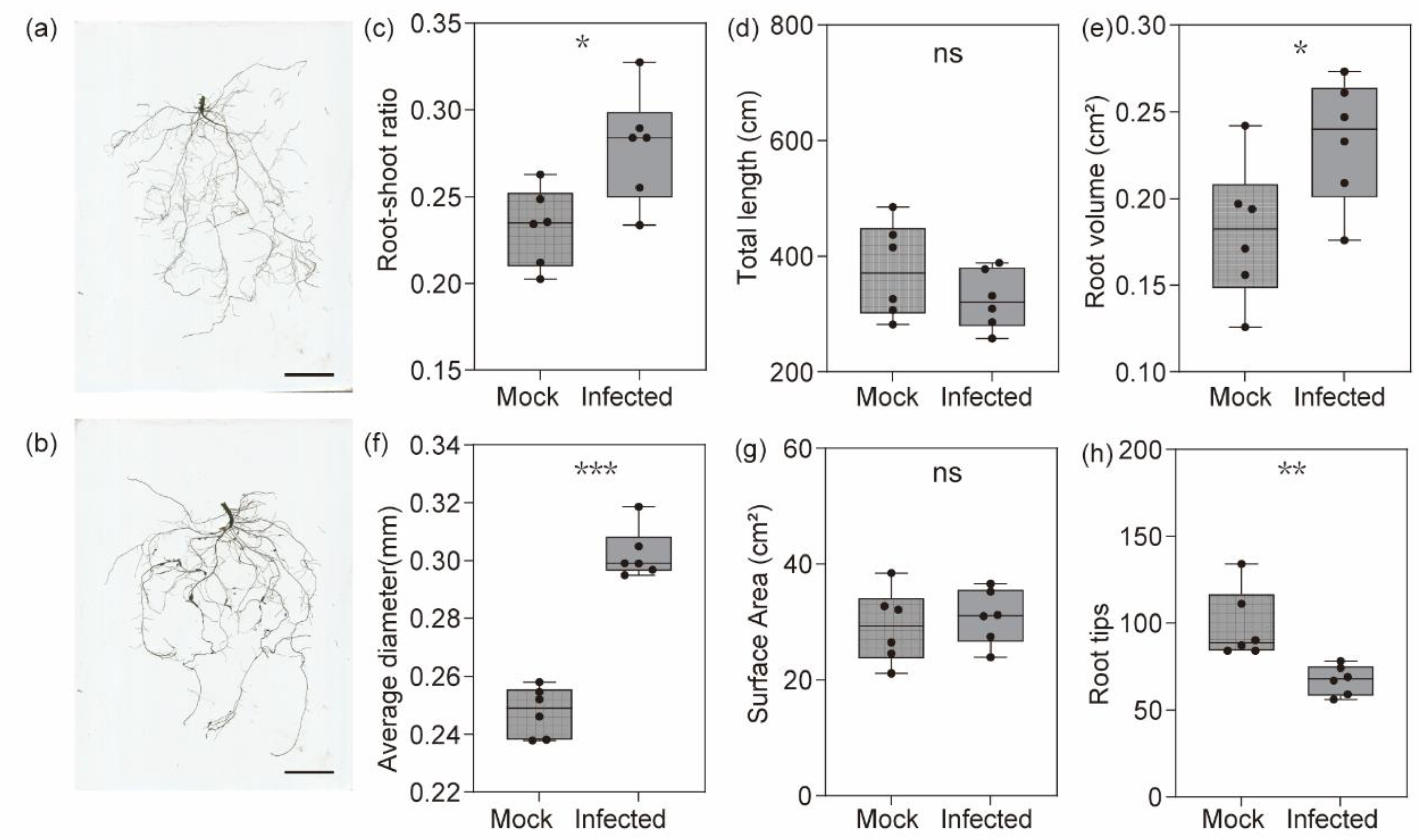
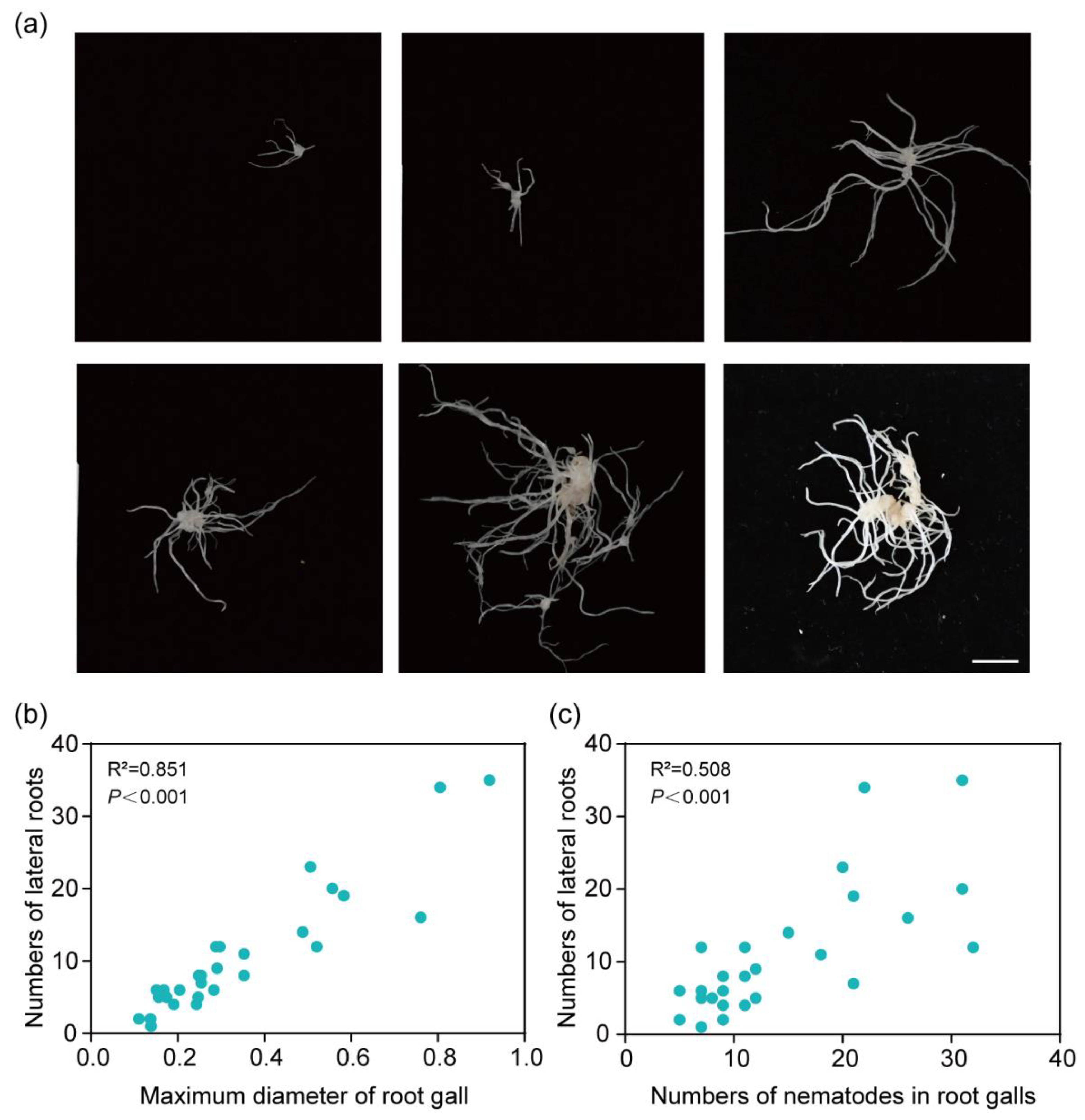
2.2. Analysis of Root Architecture and Lateral Root Numbers on the Root Galls
2.3. Promoter Activity Analysis Using Cucumber Transgenic Hairy Roots
2.4. Relative Expression of CsPIN1 and CsAUX1
2.5. GUS Staining of the Transgenic Roots
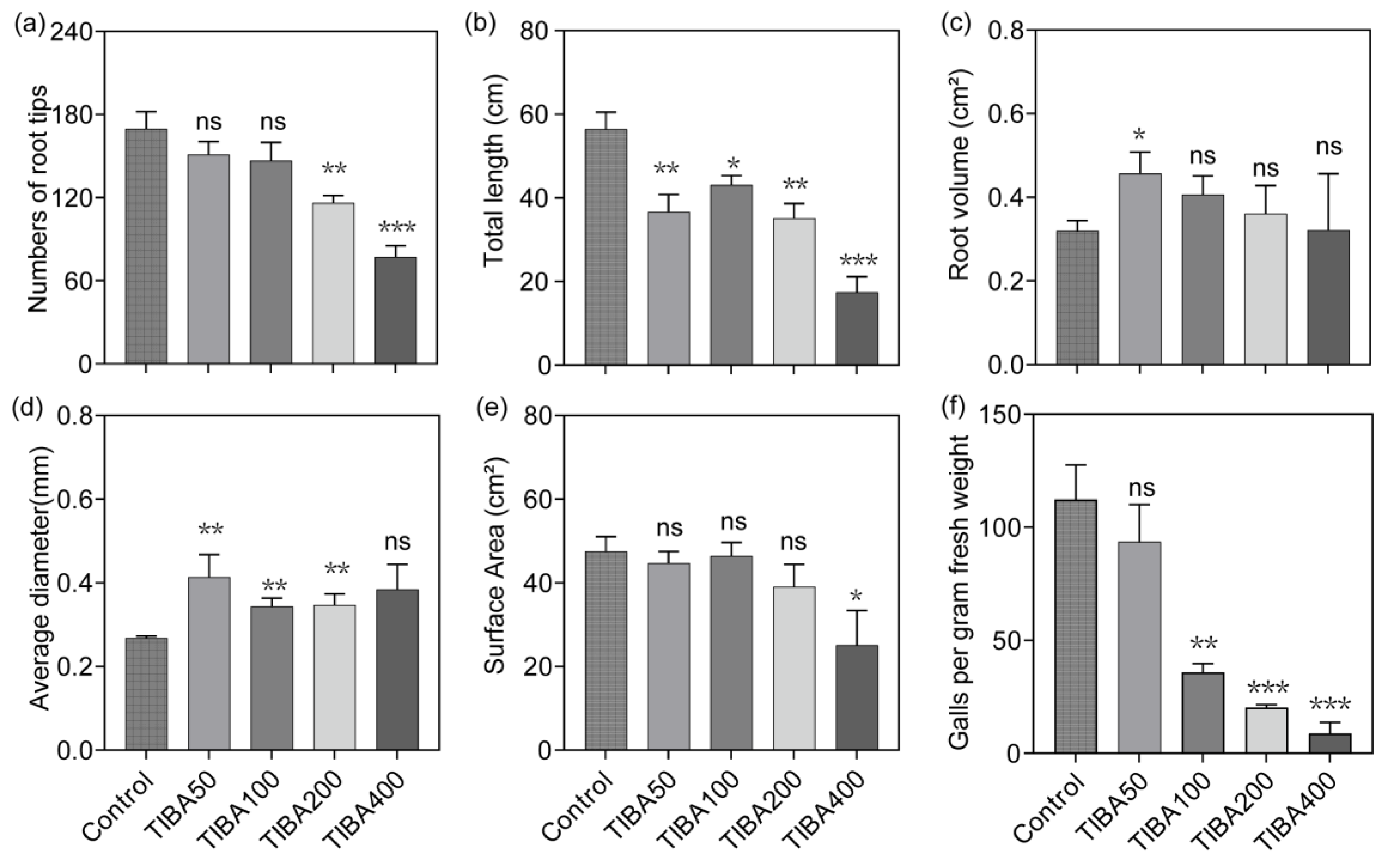
2.6. TIBA Treatment of Cucumber under M. incognita Stress
2.7. Statistical Analysis
3. Results
3.1. Nematode Infection Affects the Lateral Root Development
3.2. DR5 GUS Indicated the Auxin Distribution in Roots and Root Galls
3.3. Nematode Infection Activated Cucumber Auxin Transport Genes
3.4. TIBA Treatment Affects Cucumber Root Growth and Nematode Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holbein, J.; Franke, R.B.; Marhavý, P.; Fujita, S.; Górecka, M.; Sobczak, M.; Geldner, N.; Schreiber, L.; Grundler, F.M.W.; Siddique, S. Root Endodermal Barrier System Contributes to Defence against Plant-Parasitic Cyst and Root-Knot Nematodes. Plant J. 2019, 100, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Williamson, V.M. Plant Nematode Interaction: A Sophisticated Dialogue. In Advances in Botanical Research; Kader, J.-C., Delseny, M., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 53, pp. 147–192. [Google Scholar]
- Jones, M.G.K.; Goto, D.B. Root-Knot Nematodes and Giant Cells. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 83–100. ISBN 978-94-007-0434-3. [Google Scholar]
- Mathesius, U. Conservation and Divergence of Signalling Pathways between Roots and Soil Microbes—The Rhizobium-Legume Symbiosis Compared to the Development of Lateral Roots, Mycorrhizal Interactions and Nematode-Induced Galls. Plant Soil. 2003, 255, 105–119. [Google Scholar] [CrossRef]
- Barthels, N.; van der Lee, F.M.; Klap, J.; Goddijn, O.J.; Karimi, M.; Puzio, P.; Grundler, F.M.; Ohl, S.A.; Lindsey, K.; Robertson, L.; et al. Regulatory Sequences of Arabidopsis Drive Reporter Gene Expression in Nematode Feeding Structures. Plant Cell 1997, 9, 2119–2134. [Google Scholar] [PubMed]
- Absmanner, B.; Stadler, R.; Hammes, U.Z. Phloem Development in Nematode-Induced Feeding Sites: The Implications of Auxin and Cytokinin. Front. Plant Sci. 2013, 4, 241. [Google Scholar] [CrossRef]
- Weijers, D.; Wagner, D. Transcriptional Responses to the Auxin Hormone. Annu. Rev. Plant Biol. 2016, 67, 539–574. [Google Scholar] [CrossRef]
- De Smet, I.; Lau, S.; Voss, U.; Vanneste, S.; Benjamins, R.; Rademacher, E.H.; Schlereth, A.; De Rybel, B.; Vassileva, V.; Grunewald, W.; et al. Bimodular Auxin Response Controls Organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 2705–2710. [Google Scholar] [CrossRef]
- Karczmarek, A.; Overmars, H.; Helder, J.; Goverse, A. Feeding Cell Development by Cyst and Root-Knot Nematodes Involves a Similar Early, Local and Transient Activation of a Specific Auxin-Inducible Promoter Element. Mol. Plant Pathol. 2004, 5, 343–346. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium Triggers Lateral Root Branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-Dependent Manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral Root Formation Is Blocked by a Gain-of-Function Mutation in the SOLITARY-ROOT/IAA14 Gene of Arabidopsis. Plant J. 2002, 29, 153–168. [Google Scholar] [CrossRef]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Kyndt, T.; Goverse, A.; Haegeman, A.; Warmerdam, S.; Wanjau, C.; Jahani, M.; Engler, G.; De Almeida Engler, J.; Gheysen, G. Redirection of Auxin Flow in Arabidopsis Thaliana Roots after Infection by Root-Knot Nematodes. EXBOTJ 2016, 67, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Friml, J.; Marchant, A.; Ljung, K.; Sandberg, G.; Palme, K.; Bennett, M. Localization of the Auxin Permease AUX1 Suggests Two Functionally Distinct Hormone Transport Pathways Operate in the Arabidopsis Root Apex. Genes. Dev. 2001, 15, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, L.; Estelle, M. The Axr4 Auxin-Resistant Mutants of Arabidopsis Thaliana Define a Gene Important for Root Gravitropism and Lateral Root Initiation. Plant J. 1995, 7, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklöf, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 Promotes Lateral Root Formation by Facilitating Indole-3-Acetic Acid Distribution between Sink and Source Tissues in the Arabidopsis Seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef]
- Benjamins, R.; Malenica, N.; Luschnig, C. Regulating the Regulator: The Control of Auxin Transport. BioEssays 2005, 27, 1246–1255. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN Auxin Efflux Facilitator Network Controls Growth and Patterning in Arabidopsis Roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Watanabe, C.; Fujii, N.; Yanai, K.; Hotta, T.; Kim, D.-H.; Kamada, M.; Sasagawa-Saito, Y.; Nishimura, T.; Koshiba, T.; Miyazawa, Y.; et al. Gravistimulation Changes the Accumulation Pattern of the CsPIN1 Auxin Efflux Facilitator in the Endodermis of the Transition Zone in Cucumber Seedlings. Plant Physiol. 2012, 158, 239–251. [Google Scholar] [CrossRef]
- Olmo, R.; Cabrera, J.; Moreno-Risueno, M.A.; Fukaki, H.; Fenoll, C.; Escobar, C. Molecular Transducers from Roots Are Triggered in Arabidopsis Leaves by Root-Knot Nematodes for Successful Feeding Site Formation: A Conserved Post-Embryogenic De Novo Organogenesis Program? Front. Plant Sci. 2017, 8, 875. [Google Scholar] [CrossRef]
- Levin, K.A.; Tucker, M.R.; Bird, D.M.; Mather, D.E. Infection by Cyst Nematodes Induces Rapid Remodelling of Developing Xylem Vessels in Wheat Roots. Sci. Rep. 2020, 10, 9025. [Google Scholar] [CrossRef]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-Induced Recruitment of Specific Root-Associated Bacterial Consortium Capable of Enhancing Plant Adaptability to Salt Stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, X.; Song, M.; Ma, S.; Tian, Y.; Gao, L. Peat-Based Hairy Root Transformation Using Rhizobium Rhizogenes as a Rapid and Efficient Tool for Easily Exploring Potential Genes Related to Root-Knot Nematode Parasitism and Host Response. Plant Methods 2023, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Jásik, J.; Bokor, B.; Stuchlík, S.; Mičieta, K.; Turňa, J.; Schmelzer, E. Effects of Auxins on PIN-FORMED2 (PIN2) Dynamics Are Not Mediated by Inhibiting PIN2 Endocytosis. Plant Physiol. 2016, 172, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Ruess, L.; Neumann, G.; Marhan, S.; Kandeler, E. Low-Level Herbivory by Root-Knot Nematodes (Meloidogyne Incognita) Modifies Root Hair Morphology and Rhizodeposition in Host Plants (Hordeum Vulgare). Plant Soil. 2007, 301, 151–164. [Google Scholar] [CrossRef]
- Zwart, R.S.; Thudi, M.; Channale, S.; Manchikatla, P.K.; Varshney, R.K.; Thompson, J.P. Resistance to Plant-Parasitic Nematodes in Chickpea: Current Status and Future Perspectives. Front. Plant Sci. 2019, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Hoth, S.; Stadler, R.; Sauer, N.; Hammes, U.Z. Differential Vascularization of Nematode-Induced Feeding Sites. Proc. Natl. Acad. Sci. USA 2008, 105, 12617–12622. [Google Scholar] [CrossRef]
- Xu, P.; Li, H.; Wang, X.; Zhao, G.; Lu, X.; Dai, S.; Cui, X.; Yuan, M.; Liu, Z. Integrated Analysis of the lncRNA/circRNA-miRNA-mRNA Expression Profiles Reveals Novel Insights into Potential Mechanisms in Response to Root-Knot Nematodes in Peanut. BMC Genom. 2022, 23, 239. [Google Scholar] [CrossRef]
- Jammes, F.; Lecomte, P.; de Almeida-Engler, J.; Bitton, F.; Martin-Magniette, M.-L.; Renou, J.P.; Abad, P.; Favery, B. Genome-Wide Expression Profiling of the Host Response to Root-Knot Nematode Infection in Arabidopsis. Plant J. 2005, 44, 447–458. [Google Scholar] [CrossRef]
- Barcala, M.; García, A.; Cabrera, J.; Casson, S.; Lindsey, K.; Favery, B.; García-Casado, G.; Solano, R.; Fenoll, C.; Escobar, C. Early Transcriptomic Events in Microdissected Arabidopsis Nematode-induced Giant Cells. Plant J. 2010, 61, 698–712. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk Complexities between Auxin, Cytokinin, and Ethylene in Arabidopsis Root Development: From Experiments to Systems Modeling, and Back Again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandberg, G.; Casero, P.J.; et al. Auxin Transport Promotes Arabidopsis Lateral Root Initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA Proteins Repress Expression of Reporter Genes Containing Natural and Highly Active Synthetic Auxin Response Elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-X.; Xu, H.-H.; Gong, W.; Jin, Y.; Shi, Y.-Y.; Yuan, T.-T.; Li, J.; Lu, Y.-T. Proper PIN1 Distribution Is Needed for Root Negative Phototropism in Arabidopsis. PLoS ONE 2014, 9, e85720. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; Cannoot, B.; Friml, J.; Gheysen, G. Parasitic Nematodes Modulate PIN-Mediated Auxin Transport to Facilitate Infection. PLoS Pathog. 2009, 5, e1000266. [Google Scholar] [CrossRef] [PubMed]
- Kamada, M.; Yamasaki, S.; Fujii, N.; Higashitani, A.; Takahashi, H. Gravity-Induced Modification of Auxin Transport and Distribution for Peg Formation in Cucumber Seedlings: Possible Roles for CS-AUX1 and CS-PIN1. Planta 2003, 218, 15–26. [Google Scholar] [CrossRef]
- Krecek, P.; Skupa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zazímalová, E. The PIN-FORMED (PIN) Protein Family of Auxin Transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef]
- Mazarei, M.; Lennon, K.A.; Puthoff, D.P.; Rodermel, S.R.; Baum, T.J. Expression of an Arabidopsis Phosphoglycerate Mutase Homologue Is Localized to Apical Meristems, Regulated by Hormones, and Induced by Sedentary Plant-Parasitic Nematodes. Plant Mol. Biol. 2003, 53, 513–530. [Google Scholar] [CrossRef]
- Hammes, U.Z.; Schachtman, D.P.; Berg, R.H.; Nielsen, E.; Koch, W.; McIntyre, L.M.; Taylor, C.G. Nematode-Induced Changes of Transporter Gene Expression in Arabidopsis Roots. Mol. Plant Microbe Interact. 2005, 18, 1247–1257. [Google Scholar] [CrossRef]
- Ng, J.L.P.; Perrine-Walker, F.; Wasson, A.P.; Mathesius, U. The Control of Auxin Transport in Parasitic and Symbiotic Root–Microbe Interactions. Plants 2015, 4, 606–643. [Google Scholar] [CrossRef]
- Lv, G.; Zheng, X.; Duan, Y.; Wen, Y.; Zeng, B.; Ai, M.; He, B. The GRAS Gene Family in Watermelons: Identification, Characterization and Expression Analysis of Different Tissues and Root-Knot Nematode Infestations. PeerJ 2021, 9, e11526. [Google Scholar] [CrossRef]
- Damiani, I.; Baldacci-Cresp, F.; Hopkins, J.; Andrio, E.; Balzergue, S.; Lecomte, P.; Puppo, A.; Abad, P.; Favery, B.; Hérouart, D. Plant Genes Involved in Harbouring Symbiotic Rhizobia or Pathogenic Nematodes. New Phytol. 2012, 194, 511–522. [Google Scholar] [CrossRef]
- Arieti, R.S.; Staiger, C.J. Auxin-Induced Actin Cytoskeleton Rearrangements Require AUX1. New Phytol. 2020, 226, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Rengasamy, B.; Ambasht, N.K.; Sinha, A.K. Characterization and Expression Profiling of PIN Auxin Efflux Transporters Reveal Their Role in Developmental and Abiotic Stress Conditions in Rice. Front. Plant Sci. 2022, 13, 1059559. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, J.G.; Doerner, P.W.; Colón-Carmona, A.; Rost, T.L. Pericycle Cell Proliferation and Lateral Root Initiation in Arabidopsis. Plant Physiol. 2000, 124, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.C.; Chen, G.L.; Bartel, B. Ethylene Directs Auxin to Control Root Cell Expansion. Plant J. 2010, 64, 874–884. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Guo, X.; Liu, J.; Feng, G.; Hao, X.; Zhang, X.; Chen, Z. Auxin-Mediated Lateral Root Development in Root Galls of Cucumber under Meloidogyne incognita Stress. Plants 2024, 13, 2679. https://doi.org/10.3390/plants13192679
Ren B, Guo X, Liu J, Feng G, Hao X, Zhang X, Chen Z. Auxin-Mediated Lateral Root Development in Root Galls of Cucumber under Meloidogyne incognita Stress. Plants. 2024; 13(19):2679. https://doi.org/10.3390/plants13192679
Chicago/Turabian StyleRen, Baoling, Xin Guo, Jingjing Liu, Guifang Feng, Xiaodong Hao, Xu Zhang, and Zhiqun Chen. 2024. "Auxin-Mediated Lateral Root Development in Root Galls of Cucumber under Meloidogyne incognita Stress" Plants 13, no. 19: 2679. https://doi.org/10.3390/plants13192679
APA StyleRen, B., Guo, X., Liu, J., Feng, G., Hao, X., Zhang, X., & Chen, Z. (2024). Auxin-Mediated Lateral Root Development in Root Galls of Cucumber under Meloidogyne incognita Stress. Plants, 13(19), 2679. https://doi.org/10.3390/plants13192679




