Effect of Environmental and Anthropic Conditions on the Development of Solanum peruvianum: A Case of the Coastal Lomas, Lima-Peru
Abstract
:1. Introduction
2. Results
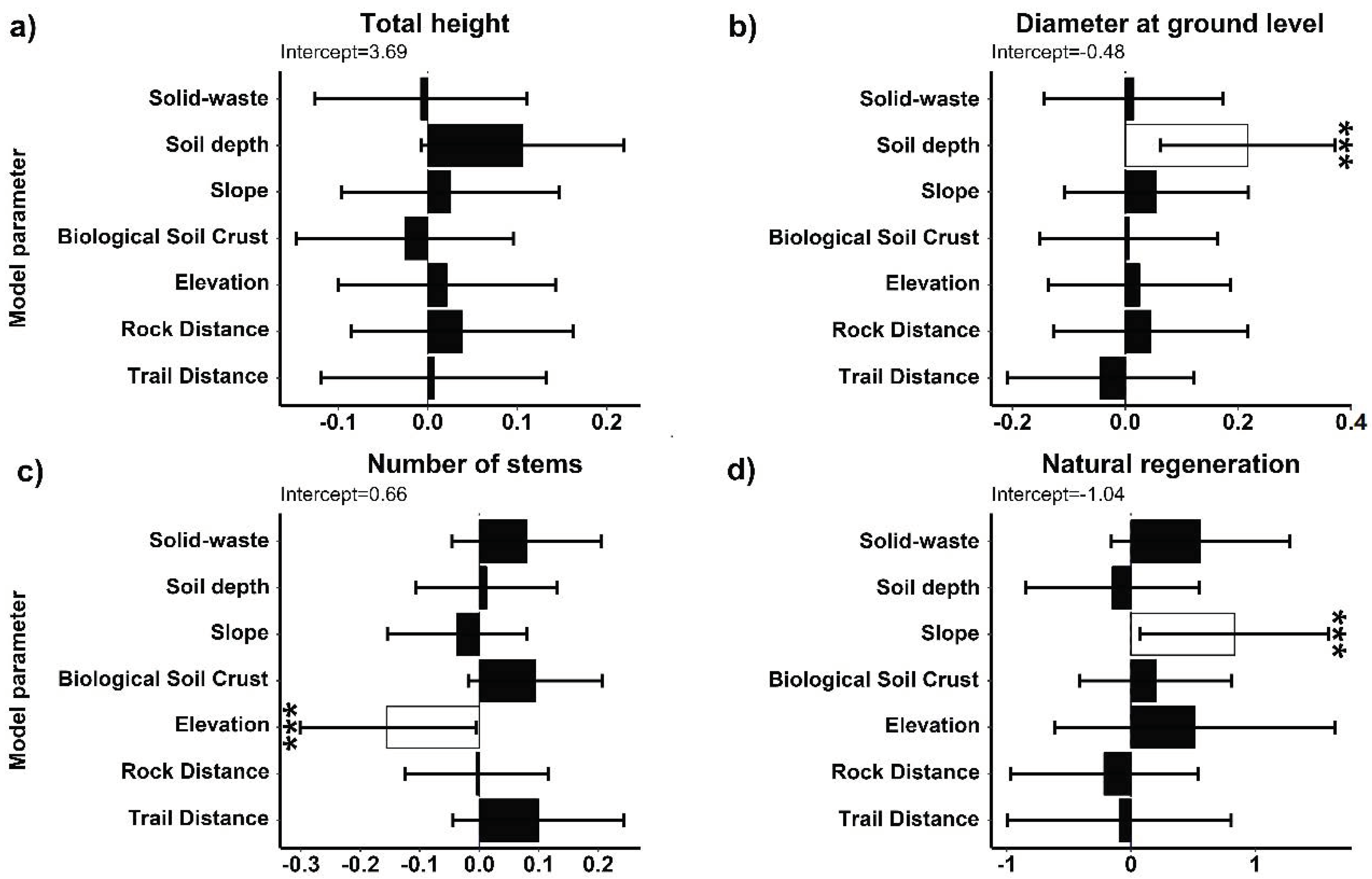
2.1. Population Structure of S. peruvianum
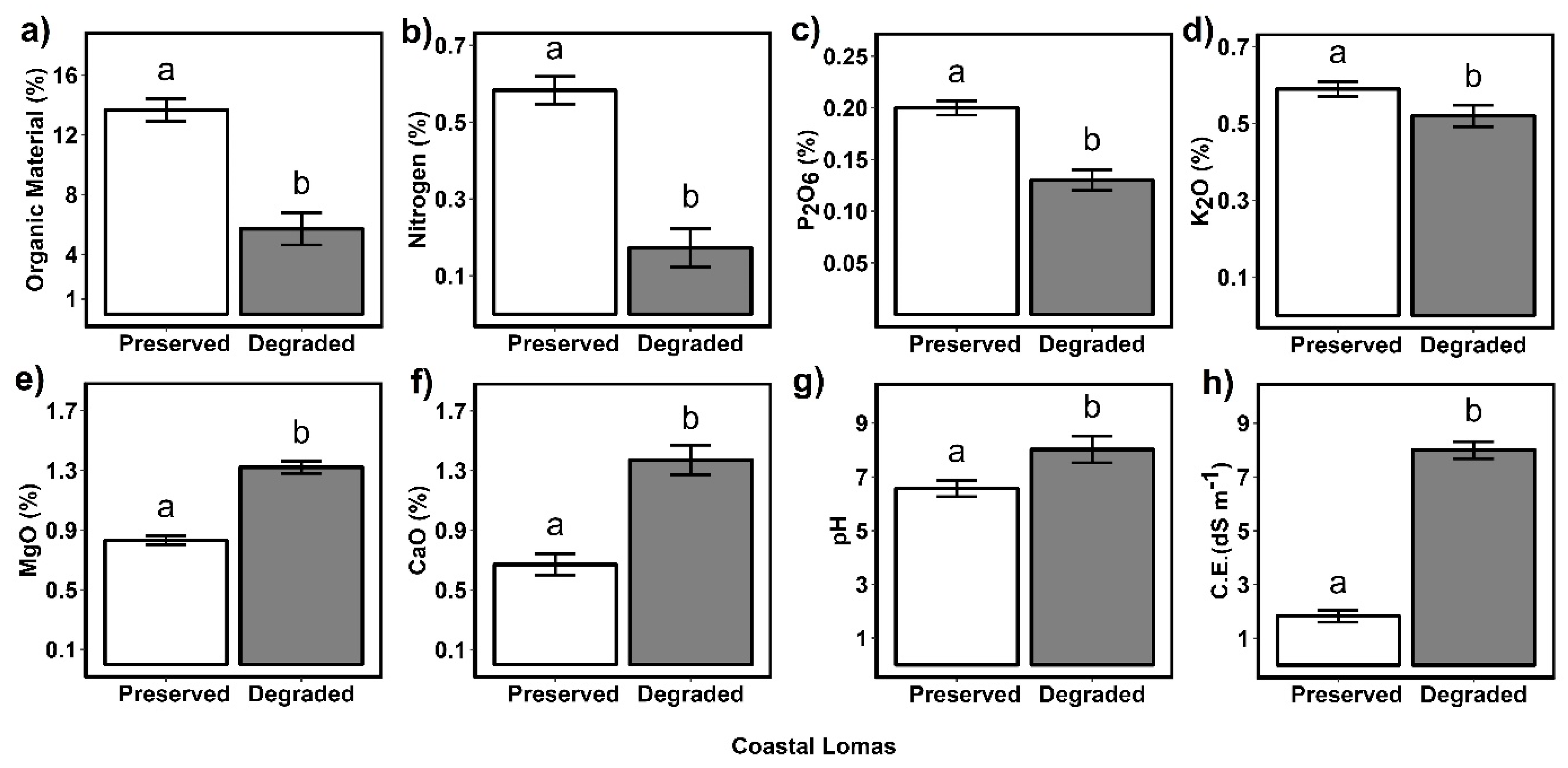
2.2. Differences in Physicochemical Properties between Preserved and Degraded Soils in Coastal Lomas
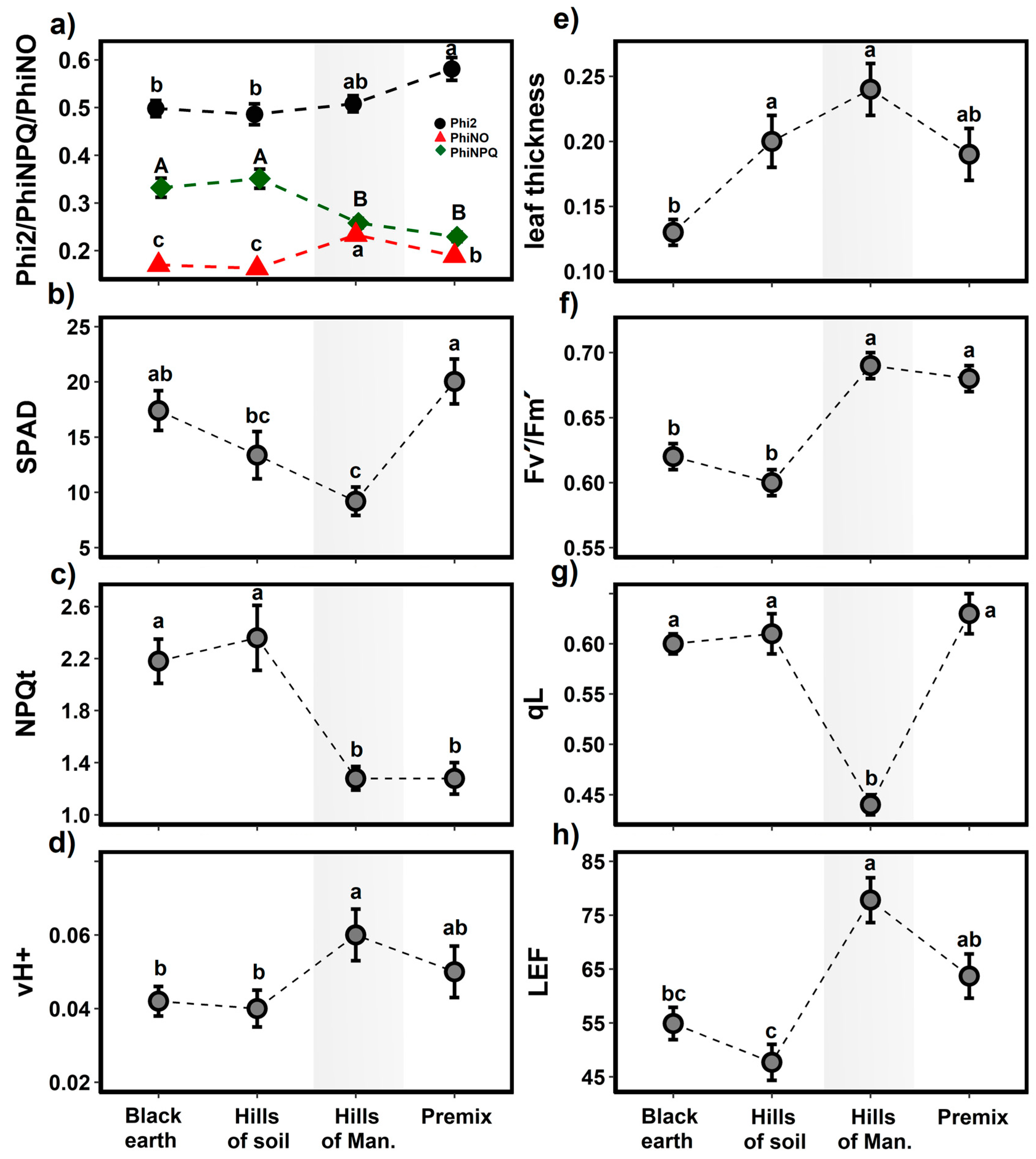
2.3. Application of Organic Fertilizers on the Chemical and Biological Properties of the Degraded Soil of the Mangomarca Lomas
3. Discussion
4. Materials and Methods
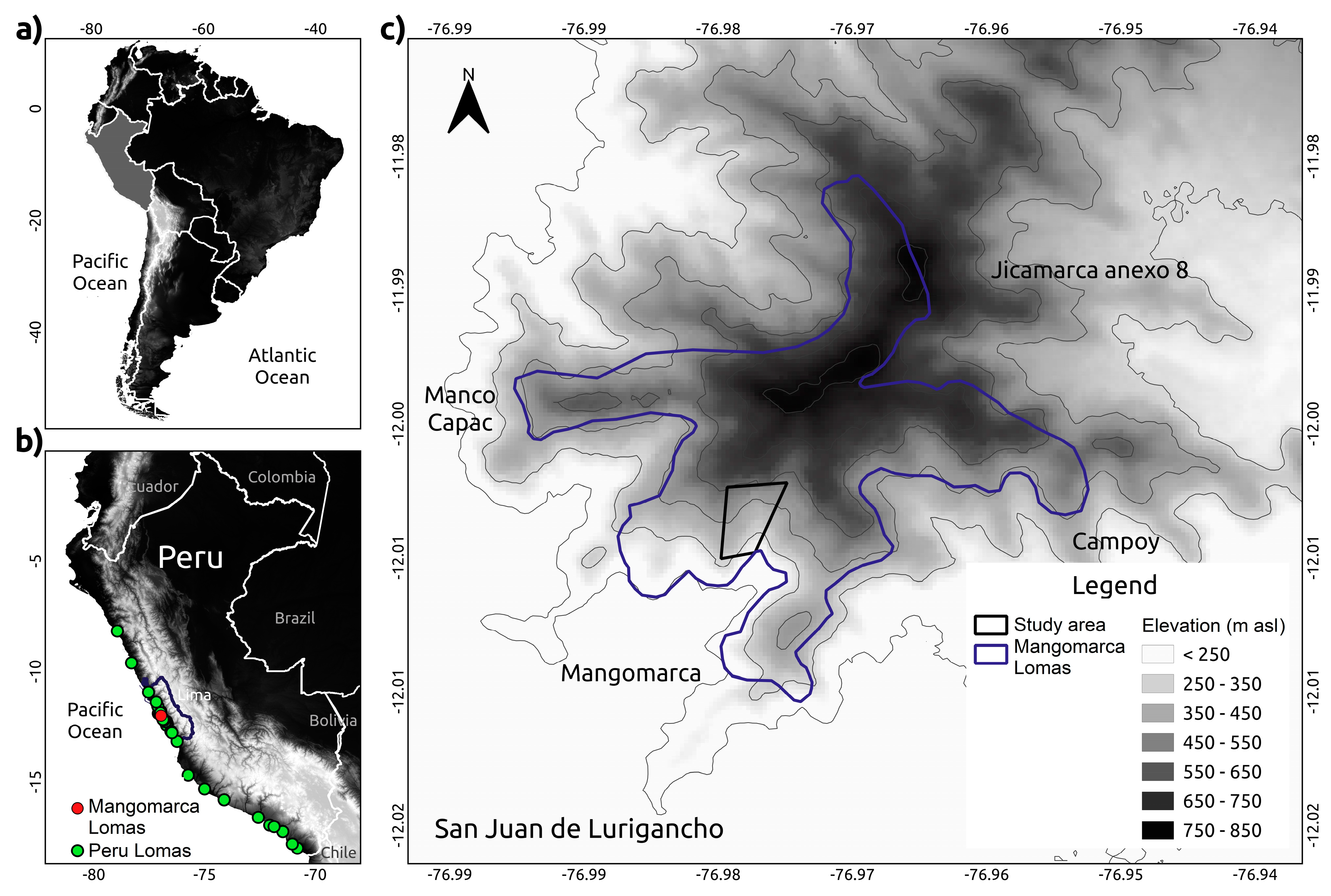
4.1. Study Area
4.2. Soil Sample Collection
4.3. Treatment of Degraded Soils in the Development of S. peruvianum Plants
4.4. Growth Evaluation and Photosynthetic Phenotyping of S. peruvianum
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being; Sarukhán, J., Whyte, A., MA Board of Review Editors, Adeel, Z., Safriel, U., Niemeijer, D., White, R., Eds.; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Leal, W.; Marisa, A.; Brandli, L.; Lange, A.; Wall, T. Encyclopedia of the UN Sustainable Development Goals; Filho, W.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Norman, L.M.; Lal, R.; Wohl, E.; Fairfax, E.; Gellis, A.C.; Pollock, M.M. Natural Infrastructure in Dryland Streams (NIDS) Can Establish Regenerative Wetland Sinks That Reverse Deserti Fi Cation and Strengthen Climate Resilience. Sci. Total Environ. 2022, 849, 157738. [Google Scholar] [CrossRef]
- Lins, A.; Henschel, J.; Nóbrega, V.; De Cássia, R.; Da Silca, K.; Moreira, E.; Tejo, M.; Silva, D.; Da Costa, F. Irrigation Level and Substrate Type on the Acclimatization and Development of Mandacaru (Cereus jamacaru DC.): An Emblematic Cactus from Brazilian Semiarid Region. Sci. Rep. 2023, 13, 20547. [Google Scholar] [CrossRef]
- United Nations New York. Trends in Sustainable Development; United Nations: New York, NY, USA, 2008. [Google Scholar]
- Rodrigues, F. Global Environmental Changes, Desertification and Sustainability SpringerBriefs in Latin American Studies; SpringerBriefs in Latin American Studies; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Behnke, R. The Socio-Economic Causes and Consequences of Desertifi Cation in Central Asia; Macaulay Institute Craigiebuckler, Ed.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- United Nations General Assembly. Resolution 73/284; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2019; pp. 1–7. [Google Scholar]
- Galán, A.; Linares, E.; Campos, J.; Vicente, J. Interpretación Fitosociológica de La Vegetación de Las Lomas Del Desierto Peruano. Rev. Biol. Trop. 2011, 59, 809–828. [Google Scholar] [CrossRef]
- Sotomayor, D.; Jiménez, P. Condiciones Meteorológicas y Dinámica Vegetal del Ecosistema Costero Lomas de Atiquipa (Caravelí-Arequipa) en el sur de Perú. Ecol. Apl. 2008, 7, 1–9. [Google Scholar] [CrossRef]
- González, F.; Craven, D.; Armesto, J. Islands in the mist: A systematic review of the coastal lomas of South America. J. Arid Environ. 2023, 211, 104942. [Google Scholar] [CrossRef]
- Padilla, D. Estudio de la Variación Espacio-Temporal de la Comunidad Vegetal de las Lomas de Mangomarca Durante el 2013 Como Contribución a Su Gestión. Bachelor’s Thesis, Universidad Nacional Agraria La Molina, La Molina, Peru, 2018. [Google Scholar]
- Rundel, P.W.; Dillon, M.O.; Palma, B.; Mooney, H.A.; Gulmon, S.L. The Phytogeography and Ecology of the Coastal Atacama and Peruvian Deserts. Aliso J. Syst. Florist. Bot. 1991, 13, 1–49. [Google Scholar] [CrossRef]
- Lee, C.; Harvey, J.T.; Qin, K.; Joshi, V.; Leskovar, D.I. Exploring the Potential of Solanum Pennellii and Solanum peruvianum as Rootstocks for Enhancing Thermotolerance of Tomato Plants. Environ. Exp. Bot. 2024, 221, 16. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Pertuzé, R.A.; Faúndez, L.; Graham, E.B.; Jones, C.M. Distribution, Ecology and Reproductive Biology of Wild Tomatoes and Related Nightshades from the Atacama Desert Region of Northern Chile. Euphytica 2009, 167, 77–93. [Google Scholar] [CrossRef]
- Tapia, G.; Méndez, J.; Inostroza, L. Different Combinations of Morpho-Physiological Traits Are Responsible for Tolerance to Drought in Wild Tomatoes Solanum Chilense and Solanum Peruvianum. Plant Biol. 2016, 18, 406–416. [Google Scholar] [CrossRef]
- Jewehan, A.; Salem, N.; Tóth, Z.; Salamon, P.; Szabó, Z. Evaluation of Responses to Tomato Brown Rugose Fruit Virus (ToBRFV) and Selection of Resistant Lines in Solanum Habrochaites and Solanum Peruvianum Germplasm. J. Gen. Plant Pathol. 2022, 88, 187–196. [Google Scholar] [CrossRef]
- Kaushal, A.; Sadashiva, A.T.; Ravishankar, K.V.; Singh, T.H. Rapid Disease Resistance Breeding in Tomato (Solanum lycopersicum L.). Accel. Plant Breed. 2020, 2, 17–55. [Google Scholar] [CrossRef]
- Carucci, F.; Bregaglio, S.; Caldarola, D.P.; Gatta, G.; Giuliani, M.M. An Agro-Physiological Dataset on Industrial Tomatoes from Nine Years of Field Experiments Conducted with Alternative Water-Saving Strategies in Mediterranean Environments. Data Brief 2024, 53, 110225. [Google Scholar] [CrossRef]
- Utasi, L.; Kovács, V.; Gulyás, Z.; Marcek, T.; Janda, T.; Darko, E. Threshold or Not: Spectral Composition and Light-Intensity Dependence of Growth and Metabolism in Tomato Seedlings, Scientia. Sci. Hortic. 2023, 313, 5–24. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants. In Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; p. 649. [Google Scholar]
- Olagunju, S.O.; Sosanya, O.S.; Oguntade, O.A.; Adewusi, K.M.; Soremi, P.A.S.; Joda, A.O.; Nassir, A.L. Effect of NPK Fertiliser on Upper and Basal Stem Diameters and Implication on Growth Habit of Tomato. J. Saudi Soc. Agric. Sci. 2024, 23, 55–66. [Google Scholar] [CrossRef]
- Medina, S. Evaluación de La Capacidad Fitoestabilizadora de Solanum peruvianum L. en Suelos Contaminados Con Cobre, Plomo y Cadmio, Moquegua, 2019. Bachelor’s Thesis, Universidad Nacional de Moquegua, Moquegua, Peru, 2022. [Google Scholar]
- Hernández, A.; Vera, L.; Naveda, C.; Guzmán, Á.M.; Vivar, M.; Roberto, T.; Mesías, F.; Ormanza, K.; Aguilar, R.; López, G. Variaciones en Algunas Propiedades Membrillo, Manabí, Ecuador Variations in Some Soil Properties Because of the Land Use Change in the Middle and Low Parts of the Membrillo Micro-Watershed, Manabi, Ecuador. Inst. Nac. Ciencias Agrícolas 2017, 38, 50–56. [Google Scholar]
- Montoya, H.; Gómez, J.; Mariano, M.; Jara, E.; Carrasco, N.; Tarazona, R.; Vela, M. Comunidades Aereoterrestres de La Microalga Klebsormidium(Klebsormidiophyceae, Streptophyta) en Costras Biológicas del Desierto Costero Peruano. Arnaldoa 2019, 26, 1105–1124. [Google Scholar] [CrossRef]
- Alonso, C.; Solórzano, R. Problemática Socioambiental de Las Lomas. Rev. Cienc. Soc. 2021, 2, 18–28. [Google Scholar]
- Ordoñez, J.A.B.; De Jong, B.H.J.; García, F.; Aviña, F.L.; Pérez, J.V.; Guerrero, G.; Martínez, R.; Masera, O. Carbon Content in Vegetation, Litter, and Soil under 10 Different Land-Use and Land-Cover Classes in the Central Highlands of Michoacan, Mexico. For. Ecol. Manag. 2008, 255, 2074–2084. [Google Scholar] [CrossRef]
- Madsen, E.L. Microorganisms and Their Roles in Fundamental Biogeochemical Cycles. Curr. Opin. Biotechnol. 2011, 22, 456–464. [Google Scholar] [CrossRef]
- Gion, A.; Bleuler, M.; Krähenbühl, N.; Schönborn, A. Quality and Suitability of Fecal Biochar in Structurally Stable Urban Tree Substrates. Sci. Total Environ. 2022, 838, 12. [Google Scholar] [CrossRef]
- Jaramillo, P.; Ramírez, M.; Pérez, D. Impacts of Bokashi on Survival and Growth Rates of Pinus Pseudostrobus in Community Reforestation Projects. J. Environ. Manag. 2015, 150, 48–56. [Google Scholar] [CrossRef]
- Killi, D.; Kavdir, Y. Effects of Olive Solid Waste and Olive Solid Waste Compost Application on Soil Properties and Growth of Solanum lycopersicum. Int. Biodeterior. Biodegrad. 2013, 82, 157–165. [Google Scholar] [CrossRef]
- Meena, R.S. Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Guerrero, J.; Suni, J. Análisis Termodinámico de La Ciudad de Lima. Int. J. Environ. Resil. Res. Sci. 2021, 3, 12. [Google Scholar]
- Alata, P.; Quispe, H.; Mercado, T.; Cordova, G.; Pacora, L. ¿Cómo Vamos en Lima y Callao? Reporte Urbano de Indicadores de Calidad de Vida 2021; Sistema Urbano: Lima, Peru, 2022; Volume 4. [Google Scholar]
- Havik, G.; Buizer, M.; Daalder, R. City Portrait Amsterdam; EU: Brussels, Belgium, 2015. [Google Scholar]
- Chi, W.; Jia, J.; Pan, T.; Jin, L.; Bai, X. Multi-Scale Analysis of Green Space for Human Settlement Sustainability in Urban Areas of the Inner Mongolia Plateau, China. Sustainability 2020, 12, 6783. [Google Scholar] [CrossRef]
- Senetra, A.; Krzywnicka, I.; Mielke, M. An Analysis of the Spatial Distribution, Influence and Quality of Urban Green Space—A Case Study of the Polish City of Tczew. Bull. Geogr. Socio-economic Ser. 2018, 42, 129–149. [Google Scholar] [CrossRef]
- Matus, F.J.; Maire, C.R. Relación Entre la Materia Orgánica del Suelo, Textura del Suelo y Tasas de Mineralización de Carbono y Nitrógeno. Agric. Técnica 2000, 60, 112–126. [Google Scholar] [CrossRef]
- Sharanappa. Soil Fertility and Nutrient Management; CRC Press: London, UK, 2023. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Silva Oliveira, M.L.; Silva Oliveira, L.F.; Homrich Schneider, I.A.; Kautzmann, R.M. Application of Andesite Rock as a Clean Source of Fertilizer for Eucalyptus Crop: Evidence of Sustainability. J. Clean. Prod. 2020, 256, 120432. [Google Scholar] [CrossRef]
- Ángel, R.R.; Lauro, L.M.; Juan Antonio, C.R. Rocks Are Safe Sites for Establishment of Bursera Seedlings in a Seasonally Dry Tropical Forest of Mexico. J. Arid Environ. 2021, 186, 104395. [Google Scholar] [CrossRef]
- Conver, J.L.; Yarwood, E.; Hetherington, L.D.; Swann, D.E. Nurse Rock Microclimates Significantly Buffer Exposure to Freezing Temperature and Moderate Summer Temperature. J. Arid Environ. 2020, 177, 104140. [Google Scholar] [CrossRef]
- Peters, E.M.; Martorell, C.; Ezcurra, E. Nurse Rocks Are More Important than Nurse Plants in Determining the Distribution and Establishment of Globose Cacti (Mammillaria) in the Tehuacán Valley, Mexico. J. Arid Environ. 2008, 72, 593–601. [Google Scholar] [CrossRef]
- Puente, M.E.; Li, C.Y.; Bashan, Y. Microbial Populations and Activities in the Rhizoplane of Rock-Weathering Desert Plants. II. Growth Promotion of Cactus Seedlings. Plant Biol. 2004, 6, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, N.S.; McGeoch, M.A.; Boelhouwers, J.C. Contrasting Nurse Plants and Nurse Rocks: The Spatial Distribution of Seedlings of Two Sub-Antarctic Species. Acta Oecologica 2010, 36, 299–305. [Google Scholar] [CrossRef]
- Campbell, P.; van Staden, J.; Stevens, C.; Whitwell, M.I. The Effects of Locality, Season and Year of Seed Collection on the Germination of Bugweed (Solanum mauritianum Scop.) Seeds. S. Afr. J. Bot. 1992, 58, 310–316. [Google Scholar] [CrossRef]
- Fabre, A.; Gauquelin, T.; Vilasante, F.; Ortega, A.; Puig, H. Phosphorus Content in Five Representative Landscape Units of the Lomas de Arequipa (Atacama Desert-Peru). Catena 2006, 65, 80–86. [Google Scholar] [CrossRef]
- Balaguer, L.; Arroyo-García, R.; Jiménez, P.; Jiménez, M.D.; Villegas, L.; Cordero, I.; de Casas, R.R.; Fernández-Delgado, R.; Ron, M.E.; Manrique, E.; et al. Forest Restoration in a Fog Oasis: Evidence Indicates Need for Cultural Awareness in Constructing the Reference. PLoS ONE 2011, 6, e23004. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Van Meerbeek, K. Endangered Lomas Plant Communities and Their Potential on Green Roofs in Peru. Landsc. Urban Plan. 2024, 247, 105061. [Google Scholar] [CrossRef]
- Kakeh, J.; Gorji, M.; Sohrabi, M.; Tavili, A.; Pourbabaee, A.A. Effects of Biological Soil Crusts on Some Physicochemical Characteristics of Rangeland Soils of Alagol, Turkmen Sahra, NE Iran. Soil Tillage Res. 2018, 181, 152–159. [Google Scholar] [CrossRef]
- Wang, F.; Michalski, G.; Luo, H.; Caffee, M. Role of Biological Soil Crusts in Affecting Soil Evolution and Salt Geochemistry in Hyper-Arid Atacama Desert, Chile. Geoderma 2017, 307, 54–64. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Li, X.Y.; Wei, J.Q.; Chen, H.Y.; Li, Z.C.; Liu, L.; Hu, X. Contrasting Surface Soil Hydrology Regulated by Biological and Physical Soil Crusts for Patchy Grass in the High-Altitude Alpine Steppe Ecosystem. Geoderma 2018, 326, 201–209. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Sinha, D.; Tandon, P.K. An Overview of Nitrogen, Phosphorus and Potassium: Key Players of Nutrition Process in Plants. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Bailón, H.; Castillo, I.; Pablo, M.; Topiño, Y.; Rosa, R.L. Respuestas Fisiológicas de Raphanus sativus L. Frente a Physiological Responses of Raphanus sativus L. to Three Concentrations of Mg+2. Biologist 2007, 5, 11–18. [Google Scholar]
- Hao, D.; Li, X.; Kong, W.; Chen, R.; Liu, J.; Guo, H.; Zhou, J. Phosphorylation Regulation of Nitrogen, Phosphorus, and Potassium Uptake Systems in Plants. Crop J. 2023, 11, 1034–1047. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial Interactions within the Plant Holobiont. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Baliran, V.; Chavez, F.; Fermoselle, G.; Perrone, A.; Pellegrini, A.; Balagué, L. Caracterización y Evaluación de la Calidad del Bokashi Para su Empleo en un Vivero Forestal. In Proceedings of the VII Congreso Latinoamericano de Agroecología, Quito, Ecuador, Quito, 2–5 October 2019. [Google Scholar]
- Ibrahim, E.A.; El-sherbini, M.A.A.; Selim, E.M. Effects of Biochar on Soil Properties, Heavy Metal Availability and Uptake, and Growth of Summer Squash Grown in Metal-Contaminated Soil. Sci. Hortic. 2022, 301, 10. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar a Guide to Analytical Methods; de Kretser, A., Writing, R., Eds.; Csiro Publishing: Clayton, Australia, 2017. [Google Scholar]
- Cabello-Torres, R.J.; Espinoza Farfán, E.R.; Castañeda-Olivera, C.A.; Romero-Cabello, E.A.; Alegría-Arnedo, M.C. Challenges and Opportunities of Collaborative Management and University Social Responsibility of Adaptation to Climate Change in the Community of Mangomarca (Oasis of Fog) in Lima, Peru. In Proceedings of the LACCEI International Multi-Conference for Engineering, Education and Technology, Buenos Aires, Argentina, 17–21 July 2023; pp. 1–7. [Google Scholar] [CrossRef]


| Treatment | OM | Nitrogen | P2O5 | K2O | CaO | MgO | EC | pH |
|---|---|---|---|---|---|---|---|---|
| CS | 13.67 c ± 0.49 | 0.58 d ± 0.03 | 0.2 f ± 0.005 | 0.60 d ± 0.4 | 0.67 d ± 0.06 | 0.8 b ± 0.03 | 2.08 b ±0.27 | 6.56 e ± 0.3 |
| Pre | 50.82 d ± 0.69 | 2.25 e ± 0.04 | 0.05 g ± 0.007 | 0.18 e ± 0.03 | 0.76 d ± 0.09 | 1.06 c ± 0.05 | 0.90 c ± 0.19 | 5.44 f ± 0.4 |
| DS | 5.71 b ± 0.69 | 0.17 c ± 0.04 | 0.12 e ± 0.007 | 0.52 d ± 0.04 | 1.37 c ± 0.09 | 1.32 a ± 0.05 | 7.99 a ± 0.27 | 8.02 d ± 0.4 |
| DSB | 12.91 c ± 0.69 | 0.75 b ± 0.04 | 0.43 d ± 0.007 | 1.97 c ± 0.04 | 1.87 b ± 0.09 | 1.28 a ± 0.05 | 14.47 a ± 0.27 | 9.07 c ± 0.4 |
| DSC | 12.81 c ± 0.69 | 0.63 d ± 0.04 | 0.23 c ± 0.007 | 0.88 b ± 0.04 | 1.88 b ± 0.09 | 1.20 a ± 0.05 | 7.76 a ± 0.27 | 8.17 b ± 0.4 |
| DSC-B | 14.01 c ± 0.69 | 0.69 bd ± 0.04 | 0.26 b ± 0.007 | 1.20 a ± 0.04 | 2.15 a ± 0.09 | 1.28 a ± 0.05 | 8.29 a ± 0.27 | 8.52 a ± 0.4 |
| DSPBS | 72.28 a ± 0.69 | 0.91 a ± 0.04 | 0.16 a ± 0.007 | 0.54 d ± 0.04 | 1.57 c ± 0.09 | 1.03 c ± 0.05 | 0.91 c ± 0.27 | 5.53 f ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camel, V.; Quispe-Huañahue, J.; Felix, E.; Ninanya-Parra, Z.; Mendoza, Y.; Peralta-Yalta, S.; Pillpa, F.; Cabello-Torres, R. Effect of Environmental and Anthropic Conditions on the Development of Solanum peruvianum: A Case of the Coastal Lomas, Lima-Peru. Plants 2024, 13, 2683. https://doi.org/10.3390/plants13192683
Camel V, Quispe-Huañahue J, Felix E, Ninanya-Parra Z, Mendoza Y, Peralta-Yalta S, Pillpa F, Cabello-Torres R. Effect of Environmental and Anthropic Conditions on the Development of Solanum peruvianum: A Case of the Coastal Lomas, Lima-Peru. Plants. 2024; 13(19):2683. https://doi.org/10.3390/plants13192683
Chicago/Turabian StyleCamel, Vladimir, July Quispe-Huañahue, Edwin Felix, Zulema Ninanya-Parra, Yngrid Mendoza, Sebastian Peralta-Yalta, Freddy Pillpa, and Rita Cabello-Torres. 2024. "Effect of Environmental and Anthropic Conditions on the Development of Solanum peruvianum: A Case of the Coastal Lomas, Lima-Peru" Plants 13, no. 19: 2683. https://doi.org/10.3390/plants13192683
APA StyleCamel, V., Quispe-Huañahue, J., Felix, E., Ninanya-Parra, Z., Mendoza, Y., Peralta-Yalta, S., Pillpa, F., & Cabello-Torres, R. (2024). Effect of Environmental and Anthropic Conditions on the Development of Solanum peruvianum: A Case of the Coastal Lomas, Lima-Peru. Plants, 13(19), 2683. https://doi.org/10.3390/plants13192683







