Effect of Different Basal Media and Organic Supplements on In Vitro Seedling Development of the Endangered Orchid Species Dendrobium moniliforme (L.) Swartz
Abstract
1. Introduction
2. Results and Discussion
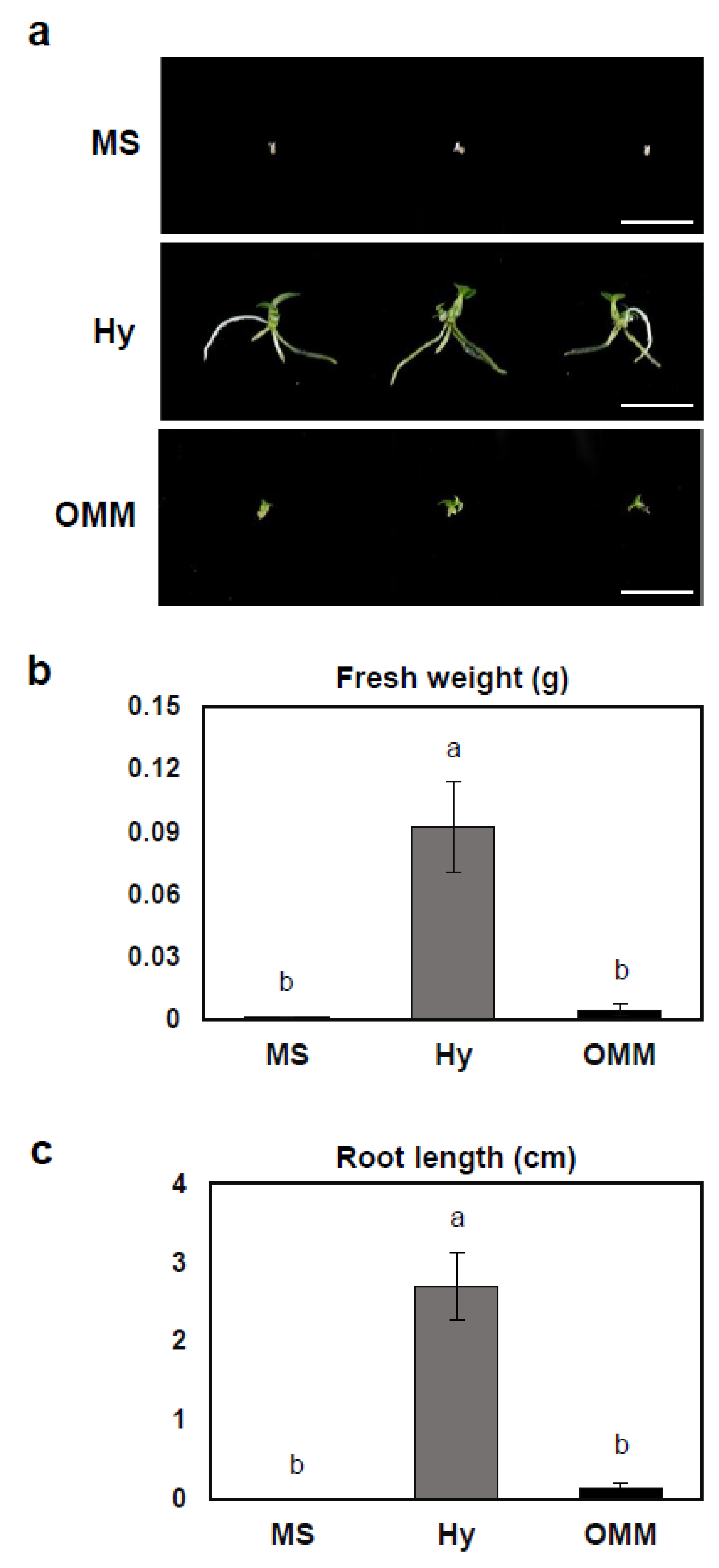
2.1. Effects of Various Basal Media on Seedling Growth
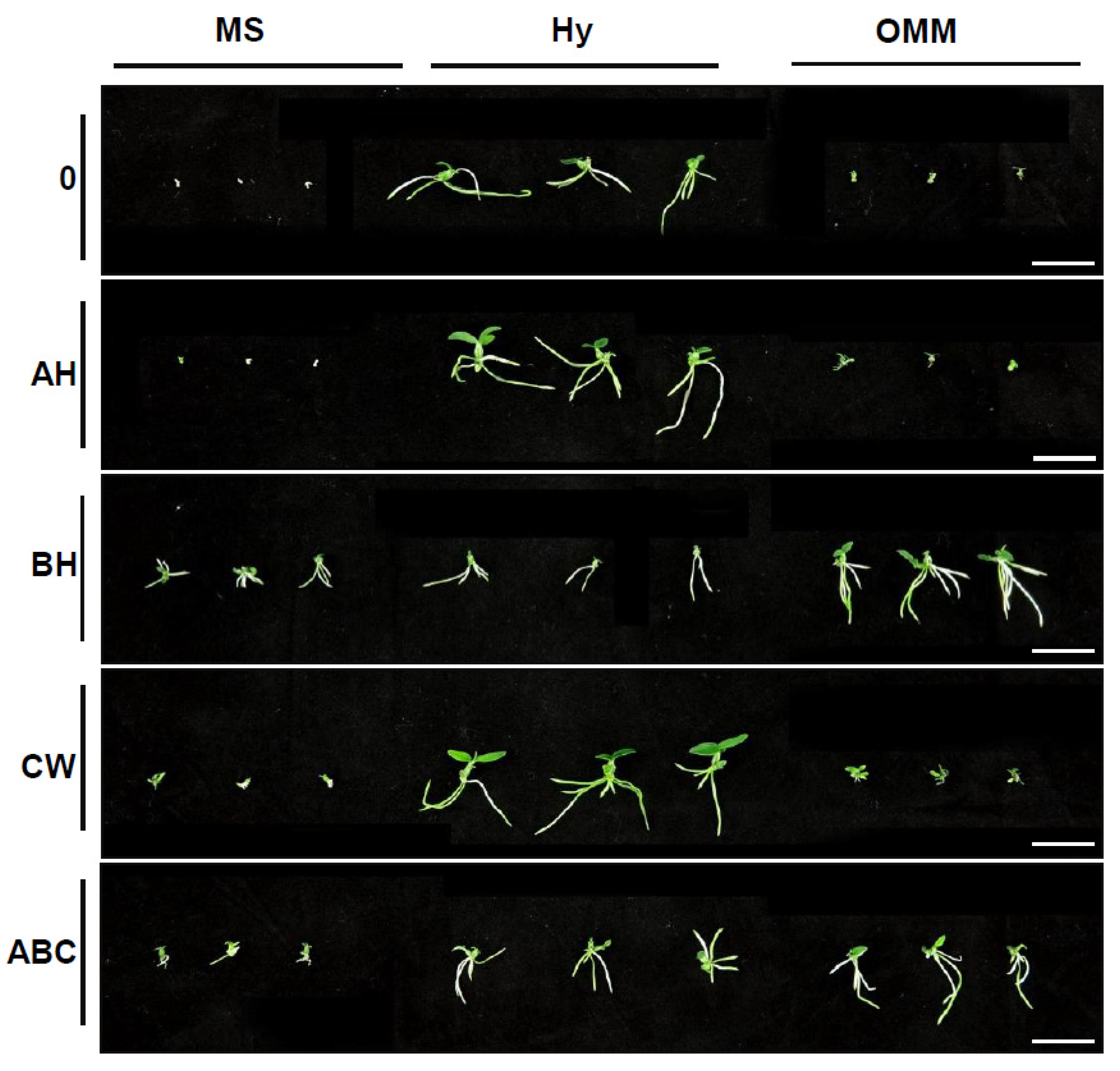
2.2. Effects of Organic Supplements on Seedling Growth
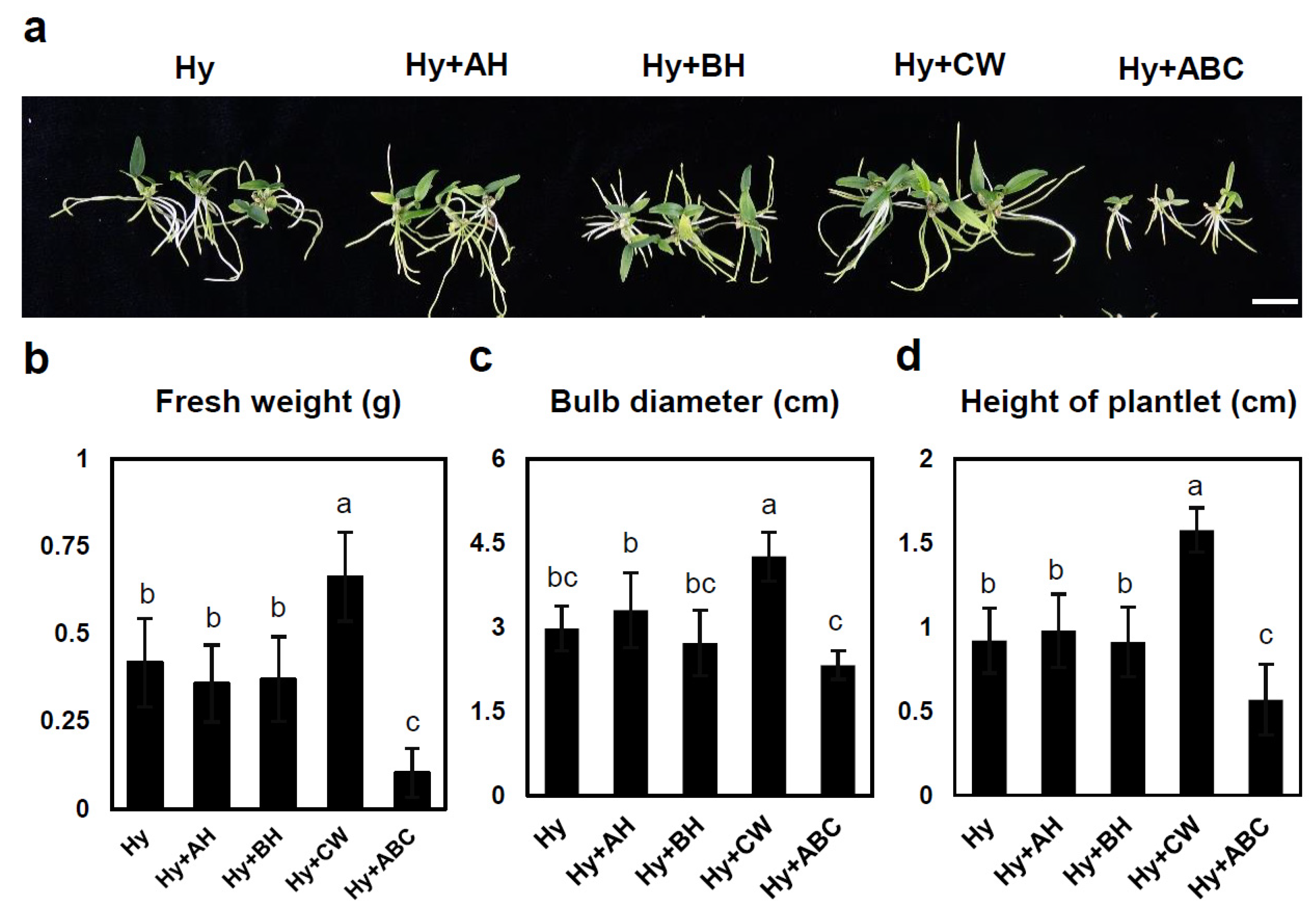
2.3. Effects of Various Media on Plant Growth at Later Development Stages
3. Materials and Methods
3.1. Plant Material and Surface Sterilization of Capsules
3.2. Culture Media for In Vitro Seedling Growth
3.3. Measurement of Seedling Development and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van den Berg, C.; Schuiteman, A. An Updated Classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Willis, K.J. State of the World’s Plants Report-2017; Royal Botanic Gardens: Sydney, Australia, 2017; ISBN 1-84246-647-X. [Google Scholar]
- Govaerts, R.; Nic Lughadha, E.; Black, N.; Turner, R.; Paton, A. The World Checklist of Vascular Plants, a Continuously Updated Resource for Exploring Global Plant Diversity. Sci. Data 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Wraith, J.; Pickering, C. Quantifying Anthropogenic Threats to Orchids Using the IUCN Red List. Ambio 2018, 47, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef]
- Arcidiacono, M.; Catalano, C.; Motisi, A.; Sajeva, M.; Carimi, F.; Carra, A. Influence of Culture Conditions on in Vitro Asymbiotic Germination of Anacamptis longicornu and Ophrys panormitana (Orchidaceae). Plants 2021, 10, 2543. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sharma, M.; Pathak, P. Cost Effective Protocol for in Vitro Mass Propagation of Cymbidium Aloifolium (L.) Sw. a Medicinally Important Orchid. Eng. Life Sci. 2009, 9, 444–453. [Google Scholar] [CrossRef]
- Arditti, J. Orchid Biology, Reviews and Perspectives, I; Cornell University Press: Ithaca, NY, USA, 1977. [Google Scholar]
- Pathak, P.; Piri, H.; Vij, S.; Mahant, K.; Chauhan, S. In Vitro Propagation and Mass Scale Multiplication of a Critically Endangered Epiphytic Orchid, Gastrochilus calceolaris (Buch.-Ham Ex JE Sm.) D. Don. Indian J. Exp. Biol. 2011, 49, 711–716. [Google Scholar]
- Islam, M.O.; Rahman, A.R.M.M.; Matsui, S. Effects of Complex Organic Extracts on Callus Growth and PLB Regeneration through Embryogenesis in the Doritaenopsis Orchid. Jpn. Agric. Res. Q. JARQ 2003, 37, 229–235. [Google Scholar] [CrossRef]
- Murdad, R.; Hwa, K.S.; Seng, C.K.; Latip, M.A.; Aziz, Z.A.; Ripin, R. High Frequency Multiplication of Phalaenopsis Gigantea Using Trimmed Bases Protocorms Technique. Sci. Hortic. 2006, 111, 73–79. [Google Scholar] [CrossRef]
- Arditti, J. Factors Affecting the Germination of Orchid Seeds. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef]
- Arditti, J.; Ernst, R.; Yam, T.W.; Glabe, C. The Contribution of Orchid Mycorrhizal Fungi to Seed Germination: A Speculative Review. Lindleyana 1990, 5, 249–255. [Google Scholar]
- Huh, Y.S.; Lee, J.K.; Nam, S.Y.; Paek, K.Y.; Suh, G.U. Improvement of Asymbiotic Seed Germination and Seedling Development of Cypripedium Macranthos Sw. with Organic Additives. J. Plant Biotechnol. 2016, 43, 138–145. [Google Scholar] [CrossRef]
- An, J.; Kim, P.B.; Park, H.B.; Kim, S.; Park, H.J.; Lee, C.W.; Lee, B.-D.; Kim, N.Y.; Hwang, J.E. Effects of Different Growth Media on In Vitro Seedling Development of an Endangered Orchid Species Sedirea japonica. Plants 2021, 10, 1193. [Google Scholar] [CrossRef] [PubMed]
- Henrich, J.E.; Stimart, D.P.; Ascher, P.D. Terrestrial Orchid Seed Germination in Vitro on a Defined Medium. J. Am. Soc. Hortic. Sci. 1981, 106, 193–196. [Google Scholar] [CrossRef]
- Arditti, J.; Ernst, R. Physiology of Germinating Orchid Seeds. In Orchidbiology, Reviews and Perspectives III; Cornell University Press: Ithaca, NY, USA, 1984. [Google Scholar]
- Fonnesbech, M. Organic Nutrients in the Media for Propagation of Cymbidium in Vitro. Physiol. Plant. 1972, 27, 360–364. [Google Scholar] [CrossRef]
- Shah, S.; Shrestha, R.; Maharjan, S.; Selosse, M.-A.; Pant, B. Isolation and Characterization of Plant Growth-Promoting Endophytic Fungi from the Roots of Dendrobium Moniliforme. Plants 2018, 8, 5. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Fan, X.-L.; Zhou, L.-R.; Shao, S.-C.; Liu, Q.; Selosse, M.-A.; Gao, J.-Y. Symbiotic Fungi Undergo a Taxonomic and Functional Bottleneck during Orchid Seeds Germination: A Case Study on Dendrobium moniliforme. Symbiosis 2019, 79, 205–212. [Google Scholar] [CrossRef]
- Lee, K. The Wild Orchids of Korea; Jungsung Publisher: Seoul, Republic of Korea, 1980; p. 193. [Google Scholar]
- Kim, Y.-G.; Yang, G.-H.; Cho, S.-I. Anti-Oxidative Effects of Dendrobii Herba on Toxic Agent Induced Kidney Cell Injury. Korea J. Herbol. 2005, 20, 53–60. [Google Scholar]
- Yoon, M.-Y.; Kim, J.-Y.; Hwang, J.-H.; Cha, M.-R.; Lee, M.-R.; Jo, K.-J.; Park, H.-R. Protective Effect of Methanolic Extracts from Dendrobium nobile Lindl. on H2O2-Induced Neurotoxicity in PC12 Cells. Appl. Biol. Chem. 2007, 50, 63–67. [Google Scholar]
- Lo, S.-F.; Nalawade, S.M.; Kuo, C.-L.; Chen, C.-L.; Tsay, H.-S. Asymbiotic Germination of Immature Seeds, Plantlet Development and Ex Vitro Establishment of Plants of Dendrobium tosaense Makino—A Medicinally Improrant Orchid. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 528–535. [Google Scholar] [CrossRef]
- Bae, K.H.; Kim, N.Y.; Song, J.M.; Song, G. In Vitro Propagation and Protocorm-like Body Formation of Endangered Species, Dendrobium moniliforme. J. For. Environ. Sci. 2014, 30, 126–132. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Sun, L.; Nie, T.; Chen, Y.; Yin, Z. In Vitro Rapid Propagation Technology System of Dendrobium Moniliforme (L.) Sw., a Threatened Orchid Species in China. Plant Biotechnol. Rep. 2023, 17, 369–378. [Google Scholar] [CrossRef]
- Kundson, L. A New Nutrient Solution for the Germination of Orchid Seeds; American Orchid Society Bulletin: Cambridge, MA, USA, 1946; Volume 15, pp. 214–217. [Google Scholar]
- Vacin, E.F.; Went, F. Some pH Changes in Nutrient Solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Kano, K. Studies on the Media for Orchid Seed Germination; Memoirs of the Faculty of Agriculture, Kagawa University: Takamatsu, Japan, 1965; Volume 20, p. 68. [Google Scholar]
- Brundrett, M.; Sivasithamparam, K.; Ramsay, M.; Krauss, S.; Taylor, R.; Bunn, E.; Hicks, A.; Karim, N.; Debeljak, N.; Mursidawati, S. Orchid conservation techniques manual. In First International Orchid Conservation Congress-Training Course; Plant Science, King Park & Botanic Garden: Perth, Australia, 2001. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Park, H.B.; An, J.; Bae, K.-H.; Hong, S.H.; Park, H.J.; Kim, S.; Lee, C.W.; Lee, B.-D.; Baek, J.H.; Kim, N.Y. Asymbiotic Seed Germination and In Vitro Seedling Development of the Endangered Orchid Species Cypripedium Guttatum. Plants 2023, 12, 3788. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, Y.; Yang, J.; Wan, X.; Yin, Z. Post-Embryonic Development and Seedling Morphogenesis of Dendrobium moniliforme (L.) Sw. under Asymbiotic Culture Conditions. S. Afr. J. Bot. 2022, 149, 240–246. [Google Scholar] [CrossRef]
- Dwiyani, R. The Response to The Growth of Dendrobium Sp. Seedling at The Time of Acclimatization to The Various Foliar Fertilizer Application Frequencies. J. Agrotrop 2012, 2, 171–175. [Google Scholar]
- Thepsithar, C.; Thongpukdee, A.; Kukieatdetsakul, K. Enhancement of Organic Supplements and Local Fertilisers in Culture Medium on Growth and Development of Phalaenopsis ‘Silky Moon’Protocorm. Afr. J. Biotechnol. 2009, 8, 4433–4440. [Google Scholar]
- Alam, M.; Rashid, M.; Hossain, M.; Salam, M.; Rouf, M. In Vitro Seed Propagation of Dendrobium (Dendrobium transparens) Orchid as Influenced by Different Media. Int. J. Biotechnol. Pak. 2002, 1, 111–115. [Google Scholar] [CrossRef]
- Nitsch, J.; Nitsch, C.; Rossini, L.; Ha, D.B.D. The Role of Adenine in Bud Differentiation. Phytomorphology 1967, 17, 446–453. [Google Scholar]
- Stenberg, M.; Kane, M. In Vitro Seed Germination and Greenhouse Cultivation of Encyclia Boothiana Var. Erythronioides, an Endangered Florida Orchid. Lindleyana 1998, 13, 101–112. [Google Scholar]
- Kauth, P.J.; Vendrame, W.A.; Kane, M.E. In Vitro Seed Culture and Seedling Development of Calopogon Tuberosus. Plant Cell Tissue Organ Cult. 2006, 85, 91–102. [Google Scholar] [CrossRef]
- Stewart, S.L.; Kane, M.E. Asymbiotic Seed Germination and in Vitro Seedling Development of Habenaria Macroceratitis (Orchidaceae), a Rare Florida Terrestrial Orchid. Plant Cell Tissue Organ Cult. 2006, 86, 147–158. [Google Scholar] [CrossRef]
- Chung, J.; Chun, C.; Kim, S. Asymbiotic Germination of Aerides Japonicum, 1; Determination of Optimal Medium and Cultural Condition for Germination of Seeds and Growth of Seedlings Korea. J. Korean Soc. Hortic. Sci. Korea R 1984, 25, 4. [Google Scholar]
- Zhang, Y.; Lee, Y.-I.; Deng, L.; Zhao, S. Asymbiotic Germination of Immature Seeds and the Seedling Development of Cypripedium Macranthos Sw., an Endangered Lady’s Slipper Orchid. Sci. Hortic. 2013, 164, 130–136. [Google Scholar] [CrossRef]
- Winarto, B.; da Silva, J.A.T. Use of Coconut Water and Fertilizer for in Vitro Proliferation and Plantlet Production of Dendrobium ‘Gradita 31’. In Vitro Cell. Dev. Biol.-Plant 2015, 51, 303–314. [Google Scholar] [CrossRef]
- Baque, M.A.; Shin, Y.-K.; Elshmari, T.; Lee, E.-J.; Paek, K.-Y. Effect of Light Quality, Sucrose and Coconut Water Concentration on the Microporpagation of Calanthe Hybrids (’Bukduseong’×’Hyesung’and’Chunkwang’×’Hyesung’). Aust. J. Crop Sci. 2011, 5, 1247–1254. [Google Scholar]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef]
- Kende, H.; Zeevaart, J. The Five “Classical” Plant Hormones. Plant Cell 1997, 9, 1197. [Google Scholar] [CrossRef]
- Utami, E.S.W.; Hariyanto, S. Organic Compounds: Contents and Their Role in Improving Seed Germination and Protocorm Development in Orchids. Int. J. Agron. 2020, 2020, e2795108. [Google Scholar] [CrossRef]
- Vyas, S.; Guha, S.; Bhattacharya, M.; Rao, I.U. Rapid Regeneration of Plants of Dendrobium lituiflorum Lindl. (Orchidaceae) by Using Banana Extract. Sci. Hortic. 2009, 121, 32–37. [Google Scholar] [CrossRef]
- Arditti, J. Micropropagation of Orchids; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 1-4443-0040-7. [Google Scholar]
- Tawaro, S. Tissue Culture and Gene Transformation in Cymbidium Finlaysonianum Lindl. Master’s Thesis, Walailak Universiyt, Nakhon Si Thammarat, Thailand, 2005; p. 120. [Google Scholar]
- Arditti, J. Germination and Growth of Orchids on Banana Fruit Tissue and Some of Its Extracts. Am. Orchid. Soc. Bull. 1968, 37, 112–116. [Google Scholar]
- Zeng, S.; Wu, K.; da Silva, J.A.T.; Zhang, J.; Chen, Z.; Xia, N.; Duan, J. Asymbiotic Seed Germination, Seedling Development and Reintroduction of Paphiopedilum wardii Sumerh., an Endangered Terrestrial Orchid. Sci. Hortic. 2012, 138, 198–209. [Google Scholar] [CrossRef]
- Wu, K.; Zeng, S.; Lin, D.; da Silva, J.A.T.; Bu, Z.; Zhang, J.; Duan, J. In Vitro Propagation and Reintroduction of the Endangered Renanthera Imschootiana Rolfe. PLoS ONE 2014, 9, e110033. [Google Scholar] [CrossRef]
- Utami, E.S.W.; Hariyanto, S. In Vitro Seed Germination and Seedling Development of a Rare Indonesian Native Orchid Phalaenopsis Amboinensis JJ Sm. Scientifica 2019, 2019, 1–6. [Google Scholar] [CrossRef]


| Medium | Organic Additive | Fresh Weight (mg) | Root Length(cm) | ||||
|---|---|---|---|---|---|---|---|
| 4 Months | 5 Months | 6 Months | 4 Months | 5 Months | 6 Months | ||
| MS | - | 2 g | 1 d | 1 h | 0 f | 0 e | 0 h |
| AH | 1 g | 1 d | 1 h | 0 f | 0 e | 0 h | |
| BH | 2 fg | 12 cd | 29 fh | 0.29 cdef | 0.68 de | 1.08 efg | |
| CW | 4 defg | 4 d | 7 gh | 0.1 ef | 0.1 e | 0.26 fgh | |
| ABC | 3 efg | 10 d | 19 gh | 0.32 cdef | 0.56 de | 0.72 fgh | |
| Hyponex | - | 20 a | 92 a | 83 cd | 0.9 ab | 2.7 a | 3.12 ab |
| AH | 15 ab | 53 b | 152 b | 1 a | 1.98 ab | 2.72 bc | |
| BH | 7 cdefg | 15 cd | 16 gh | 0.6 bcd | 1.22 cd | 1.3 def | |
| CW | 18 a | 92 a | 201 a | 0.66 abc | 2.6 a | 4.0 a | |
| ABC | 9 bcde | 14 cd | 53 ef | 0.47 cde | 1.22 cd | 1.92 cde | |
| OMM | - | 7 cdefg | 5 d | 4 gh | 0.1 ef | 0.14 e | 0.17 gh |
| AH | 8 cdef | 11 d | 11 gh | 0.13 ef | 0.26 e | 0.3 fgh | |
| BH | 10 bcd | 17 cd | 107 c | 0.53 cd | 1.19 cd | 3.1 ab | |
| CW | 11 bc | 16 cd | 24 gh | 0.27 def | 0.22 e | 0.36 fgh | |
| ABC | 9 bcdef | 34 bc | 67 de | 0.42 cde | 1.42 bc | 2.26 bcd | |
| Two-way ANOVA | Medium | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Organic additive | <0.001 | <0.001 | <0.001 | 0.164 | 0.082 | <0.001 | |
| Medium organic additive | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.E.; Park, H.B.; Jeon, D.Y.; Park, H.J.; Kim, S.; Lee, C.W.; Kim, Y.-J.; Yoon, Y.-J. Effect of Different Basal Media and Organic Supplements on In Vitro Seedling Development of the Endangered Orchid Species Dendrobium moniliforme (L.) Swartz. Plants 2024, 13, 2721. https://doi.org/10.3390/plants13192721
Hwang JE, Park HB, Jeon DY, Park HJ, Kim S, Lee CW, Kim Y-J, Yoon Y-J. Effect of Different Basal Media and Organic Supplements on In Vitro Seedling Development of the Endangered Orchid Species Dendrobium moniliforme (L.) Swartz. Plants. 2024; 13(19):2721. https://doi.org/10.3390/plants13192721
Chicago/Turabian StyleHwang, Jung Eun, Hyeong Bin Park, Dae Young Jeon, Hwan Joon Park, Seongjun Kim, Chang Woo Lee, Young-Joong Kim, and Young-Jun Yoon. 2024. "Effect of Different Basal Media and Organic Supplements on In Vitro Seedling Development of the Endangered Orchid Species Dendrobium moniliforme (L.) Swartz" Plants 13, no. 19: 2721. https://doi.org/10.3390/plants13192721
APA StyleHwang, J. E., Park, H. B., Jeon, D. Y., Park, H. J., Kim, S., Lee, C. W., Kim, Y.-J., & Yoon, Y.-J. (2024). Effect of Different Basal Media and Organic Supplements on In Vitro Seedling Development of the Endangered Orchid Species Dendrobium moniliforme (L.) Swartz. Plants, 13(19), 2721. https://doi.org/10.3390/plants13192721






