Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions
Abstract
:1. Introduction
2. Control of PPF by Antibiotics
2.1. Fungal Pathogens and Diseases
2.2. Factors in Spreading PPF
2.3. Antifungal Agrochemicals in PPF Control
3. Mechanisms of Antifungal Resistance
| Fungicide Classes | Example | Resistant Fungal Pathogens | Mechanisms of Resistance | References |
|---|---|---|---|---|
| Polyenes | Amphotericin B, Candicidin, Hamycin, Natamycin, Nystatin, Rimocidin | Aspergillus spp., C. albicans, C. glabrata, C. auris, C. neoformans, Fusarium spp. | Target alteration by AmB resistance, ERG gene mutation Mutations in FCY1, FCY2, and FUR1 genes | [5,76] |
| Benzimidazoles | Carbendazim, Thiabendazole | B. cinerea | Point mutations in the β-tubulin gene resulting in altered benzimidazole-binding site | [77,78] |
| Benomyl | Venturia inaequalis, V. pirina, Monilinia fructicola, Sclerotinia homoeocarpa, Penicillium | Point mutations in the β-tubulin gene (mutation at position 198) | [106] | |
| SDHIs | Bixafen, Boscalid, Carboxin, Fluaxapyroxad, Fluopyram, Isopyrazam, Penthiopyrad, Sedaxane | B. cinerea, Corynespora cassicola, D. bryoniae, Botrytis elliptica, Podosphaera xanthii, P. pachyrhizi, Stemphylium botryosum, Aspergillus flavus, Alternaria alternata | Mutations in sdh genes | [73,107,108,109,110,111,112] |
| QoIs | Pyraclostrobin, Azoxystrobin, Mandestrobin, Kresoxim-Methyl, Dimoxystrobin, Famoxadone, Fluoxastrobin, Fenamidone, Pyribencarb | B. cinerea, P. pachyrhizi | Mutation in cytb | [108,111] |
| Morpholines | Aldomorph, Enpropimorph, Dodemorph, Tridemorph, Fenpropimorph, Fenpropidin Spiroxamine, | E. necator, E. graminis, Sphaerotheca fuliginea, | Unknown | [113,114] |
| Anilinopyrimidines | Cyprodinil, Mepanipyrim, Pyrimethanil | B. cinerea, V. inaequalis, | All identified resistance-conferring genes encode proteins that are involved in mitochondrial processes, suggesting that anilinopyrimidines primarily target the mitochondria. | [115,116,117] |
| DMIs | Cyproconazole, Propiconazole, Tebuconazole | P. pachyrhizi | Mutation on cyp51 gene | [111] |
| Azoles | Abafungin, Bifonazole, Butoconazole, Lotrimazole, Econazole, Fenticonazole, Ketoconazole, Miconazole, Oxiconazole, Sulconazole, Albaconazole, Inaconazole, Epoxiconazole, Luconazole, Prothioconazole | Z. tritici, V. inaequalis, P. digitatum, C. beticola, M. fructicola, E. necator, Brumeriela jaapii, B. cinérea, B. graminis, M. graminicola, O. acuformis, O. yallundae, P. fusca | Mutations in cyp51, upregulation of cyp51 and the genes encoding membrane transporters, efflux pump activity, ABC transporter PMR1 over-expression, mutations in erg11 or erg3 | [5,118,119,120] |
| Echinocandins | Anidulafungin, Aspofungin, Micafungin | C. albicans, C. krusei, C. auris, C. tropicalis, A. fumigatus | Mutations in fks1 gene | [4,5] |
| Others | Azoxystrobin, Difenoconazole | Alternaria solani | Mutations in cytb are associated with azoxystrobin resistance | [121] |
| Thiophanate-Methyl | Lasiodiplodia theobromae | Mutations in β-tubulin gene | [122] |
4. Impact of Antifungal Chemicals on Humans, Plants, and Environment
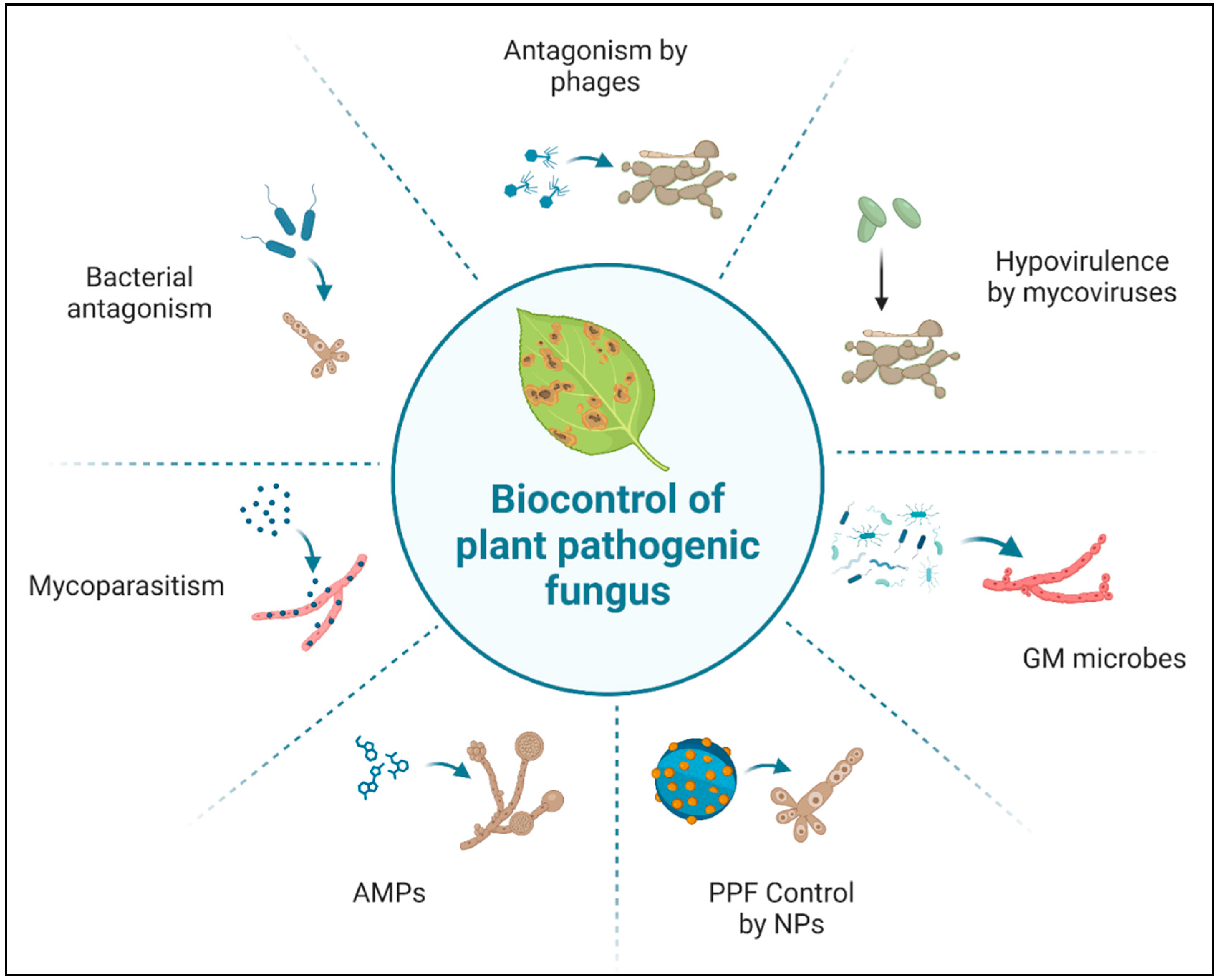
5. BCAs and Nanotechnologies in Controlling PPF
5.1. Use of Antagonistic Microorganisms against PPF
5.1.1. Use of Antagonistic Bacteria
5.1.2. Use of Antagonistic Fungi
5.1.3. Use of Genetically Modified (GM) Organisms
5.1.4. Use of Viral Vectors/Phages
5.2. Use of AMPs
5.3. Use of Nano-Based Technologies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Islam, T.; Haque, M.A.; Barai, H.R.; Istiaq, A.; Kim, J.-J. Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants 2024, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.T.; Gifford, H.; Rhodes, J. Emerging Antifungal Resistance in Fungal Pathogens. Curr. Clin. Microbiol. Rep. 2024, 11, 43–50. [Google Scholar] [CrossRef]
- Boyce, K.J. The Microevolution of Antifungal Drug Resistance in Pathogenic Fungi. Microorganisms 2023, 11, 2757. [Google Scholar] [CrossRef]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular Mechanisms Governing Antifungal Drug Resistance. NPJ Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Thangaraj, K.; Deng, C.; Deng, W.W.; Zhang, Z.Z. Phyllosticta capitalensis Causes Leaf Spot on Tea Plant (Camellia sinensis) in China. Plant Dis. 2019, 103, 2964. [Google Scholar] [CrossRef]
- Chen, F.; Ma, R.; Chen, X.L. Advances of Metabolomics in Fungal Pathogen–Plant Interactions. Metabolites 2019, 9, 169. [Google Scholar] [CrossRef]
- Al-Sadi, A.M. Bipolaris Sorokiniana-Induced Black Point, Common Root Rot, and Spot Blotch Diseases of Wheat: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 584899. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Dong, Y.; Ranjitkar, S.; Schaefer, D.A.; Karunarathna, S.C.; Hyde, K.D.; Jayawardena, R.S.; Manawasinghe, I.S.; Bebber, D.P.; Promputtha, I.; et al. Climate-Fungal Pathogen Modeling Predicts Loss of Up to One-Third of Tea Growing Areas. Front. Cell. Infect. Microbiol. 2021, 11, 610567. [Google Scholar] [CrossRef]
- Karunarathna, S.C.; Maharachchikumbura, S.S.N.; Ariyawansa, H.A.; Shenoy, B.D.; Jeewon, R. Editorial: Emerging Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2021, 11, 765549. [Google Scholar] [CrossRef]
- McLean, M.A.; Angilletta, M.J.; Williams, K.S. If You Can’t Stand the Heat, Stay out of the City: Thermal Reaction Norms of Chitinolytic Fungi in an Urban Heat Island. J. Therm. Biol. 2005, 30, 384–391. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 284486. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus Atrophaeus Gbsc56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne Incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus Strain S2 Shows High Nematicidal Activity against Meloidogyne Incognita by Producing Sphingosine. Sci. Rep. 2016, 6, 28756. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile Compounds of Endophytic bacillus Spp. Have Biocontrol Activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef]
- Zubair, M.; Farzand, A.; Mumtaz, F.; Khan, A.R.; Sheikh, T.M.M.; Haider, M.S.; Yu, C.; Wang, Y.; Ayaz, M.; Gu, Q.; et al. Novel Genetic Dysregulations and Oxidative Damage in Fusarium graminearum Induced by Plant Defense Eliciting Psychrophilic Bacillus atrophaeus TS1. Int. J. Mol. Sci. 2021, 22, 12094. [Google Scholar] [CrossRef] [PubMed]
- Schoina, C.; Stringlis, I.A.; Pantelides, I.S.; Tjamos, S.E.; Paplomatas, E.J. Evaluation of Application Methods and Biocontrol Efficacy of Paenibacillus Alvei Strain K-165, against the Cotton Black Root Rot Pathogen Thielaviopsis Basicola. Biol. Control 2011, 58, 68–73. [Google Scholar] [CrossRef]
- Zaker, M. Natural Plant Products as Eco-Friendly Fungicides for Plant Diseases Control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, N.; Barbetti, M.J.; You, M.P.; Burrell, D.; Neate, S. White Leaf Spot Caused by Neopseudocercosporella capsellae: A Re-Emerging Disease of Brassicaceae. Front. Cell. Infect. Microbiol. 2020, 10, 588090. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, N.; Barbetti, M.J.; You, M.P.; Dehigaspitiya, P.; Neate, S. Dimorphism in Neopseudocercosporella capsellae, an Emerging Pathogen Causing White Leaf Spot Disease of Brassicas. Front. Cell. Infect. Microbiol. 2021, 11, 678231. [Google Scholar] [CrossRef]
- Gao, H.; Pan, M.; Tian, C.; Fan, X. Cytospora and Diaporthe Species Associated with Hazelnut Canker and Dieback in Beijing, China. Front. Cell. Infect. Microbiol. 2021, 11, 664366. [Google Scholar] [CrossRef]
- Karunarathna, A.; Tibpromma, S.; Jayawardena, R.S.; Nanayakkara, C.; Asad, S.; Xu, J.; Hyde, K.D.; Karunarathna, S.C.; Stephenson, S.L.; Lumyong, S.; et al. Fungal Pathogens in Grasslands. Front. Cell. Infect. Microbiol. 2021, 11, 695087. [Google Scholar] [CrossRef]
- Wilson, S. Grasses and Grassland Ecology. Ann. Bot. 2009, 104, ix. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Saha, O.; Rahman Sid, A.; Saha, A.; Hussain, M.S.; Islam, T. Molecular Detection of Multidrug Resistance Pathogenic Bacteria from Protective Materials Used By Healthcare Workers (HCW); Bangladesh Scenario. J. Appl. Sci. 2018, 18, 48–55. [Google Scholar] [CrossRef]
- Sagor, M.S.; Hossain, M.S.; Islam, T.; Mahmud, M.A.; Miah, M.S.; Karim, M.R.; Giasuddin, M.; Samad, M.A. Phenotypic and Genotypic Antibiotic Resistance and Virulence Profiling of Enterococcus Faecalis Isolated from Poultry at Two Major Districts in Bangladesh. Pak. Vet. J. 2022, 42, 153–160. [Google Scholar] [CrossRef]
- Islam, T.; Kubra, K.; Chowdhury, M.M.H. Prevalence of Methicillin-Resistant Staphylococcus Aureus in Hospitals in Chittagong, Bangladesh: A Threat of Nosocomial Infection. J. Microsc. Ultrastruct. 2018, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Saha, O.; Sultana, S.; Hridoy, M.; Hasan, M.; Marzan, S.; Musfiqur Rahman, M. Comparison between Reduced Susceptibility to Disinfectants and Multidrug Resistance among Hospital Isolates of Pseudomonas aeruginosa and Staphylococcus aureus in Bangladesh. Bagcilar Med. Bull. 2017, 2, 88–97. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to Global Food Security from Emerging Fungal and Oomycete Crop Pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.N.; Eyles, C.J.; Kay, W.; Cowper, J.; Gurr, S.J. A Role for Random, Humidity-Dependent Epiphytic Growth Prior to Invasion of Wheat by Zymoseptoria tritici. Fungal Genet. Biol. 2017, 106, 51–60. [Google Scholar] [CrossRef]
- Suffert, F.; Ravigné, V.; Sachec, I. Seasonal Changes Drive Short-Term Selection for Fitness Traits in the Wheat Pathogen Zymoseptoria tritici. Appl. Environ. Microbiol. 2015, 81, 6367. [Google Scholar] [CrossRef] [PubMed]
- Renato Echeveste da Rosa, C. Asian Soybean Rust Resistance: An Overview. J. Plant Pathol. Microbiol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Li, X.; Esker, P.D.; Pan, Z.; Dias, A.P.; Xue, L.; Yang, X.B. The Uniqueness of the Soybean Rust Pathosystem: An Improved Understanding of the Risk in Different Regions of the World. Plant Dis. 2010, 94, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.N.; Fisher, M.C.; Gurr, S.J. Emerging Fungal Threats to Plants and Animals Challenge Agriculture and Ecosystem Resilience. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A. Rapid Emergence of Hybrids between the Two Subspecies of Ophiostoma Novo-Ulmi with a High Level of Pathogenic Fitness. Plant Pathol. 2010, 59, 186–199. [Google Scholar] [CrossRef]
- Mottaleb, K.A.; Singh, P.K.; Sonder, K.; Kruseman, G.; Tiwari, T.P.; Barma, N.C.D.; Malaker, P.K.; Braun, H.J.; Erenstein, O. Threat of Wheat Blast to South Asia’s Food Security: An Ex-Ante Analysis. PLoS ONE 2018, 13, e0197555. [Google Scholar] [CrossRef] [PubMed]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Ribas e Ribas, A.D.; Spolti, P.; Del Ponte, E.M.; Donato, K.Z.; Schrekker, H.; Fuentefria, A.M. Is the Emergence of Fungal Resistance to Medical Triazoles Related to Their Use in the Agroecosystems? A Mini Review. Braz. J. Microbiol. 2016, 47, 793–799. [Google Scholar] [CrossRef]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini-Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Pasquali, M.; Pallez-Barthel, M.; Beyer, M. Searching Molecular Determinants of Sensitivity Differences towards Four Demethylase Inhibitors in Fusarium Graminearum Field Strains. Pestic. Biochem. Physiol. 2020, 164, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Yadav, A.; Wadhwa, K.; Jain, K.; Ghorai, S.M. Azoles Used in Agriculture as Possible Cause of Azole-Resistance in Clinical Candida Isolates. Biosci. Biotechnol. Res. Asia 2021, 17, 789–799. [Google Scholar] [CrossRef]
- Cappelletty, D.; Eiselstein-McKitrick, K. The Echinocandins. Pharmacotherapy 2007, 27, 369–388. [Google Scholar] [CrossRef]
- Klittich, C.J. Milestones in Fungicide Discovery: Chemistry That Changed Agriculture. Plant Health Prog. 2018, 9, 31. [Google Scholar] [CrossRef]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia Solani in Sugar Beet and Effect of Fungicide Application and Plant Cultivar on Inoculum Potential in the Soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef]
- Buysens, C.; Dupré de Boulois, H.; Declerck, S. Do Fungicides Used to Control Rhizoctonia Solani Impact the Non-Target Arbuscular Mycorrhizal Fungus Rhizophagus Irregularis? Mycorrhiza 2015, 25, 277–288. [Google Scholar] [CrossRef] [PubMed]
- IDM for Important Diseases of Maize—ICAR-Indian Institute of Maize Research. Available online: https://iimr.icar.gov.in/?page_id=2134 (accessed on 25 May 2024).
- Ayed, F.; Daami-Remadi, M.; Jabnoun-Khiareddine, H.; Hibar, K.; El Mahjoub, M. Evaluation of Fungicides for Control of Fusarium Wilt of Potato. Plant Pathol. J. 2006, 5, 239–243. [Google Scholar] [CrossRef]
- Ben Naim, Y.; Cohen, Y. Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato. J. Fungi 2023, 9, 1046. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Sharma, S.; Hansen, Z.R.; Vaghefi, N.; Hanson, L.E.; Kikkert, J.R. Improving Fungicide-Based Management of Cercospora Leaf Spot in Table Beet in New York, USA. Can. J. Plant Pathol. 2020, 42, 353–366. [Google Scholar] [CrossRef]
- Amini, J.; Fevzi Sidovich, D. The Effects of Fungicides on Fusarium oxysporum f. sp. lycopersici Associated with Fusarium Wilt of Tomato. J. Plant Prot. Res. 2010, 50, 172–178. [Google Scholar]
- Ahmad, S.; Yousaf, M.; Anjum, R.; Raza, W.; Ali, Y.; Rehman, M.A. Evaluation of Fungicides against Fusarium oxysporum f.Sp. lycopersici the Cause of Fusarium Wilt of Tomato. J. Plant Environ. 2021, 3, 125–135. [Google Scholar] [CrossRef]
- Veloukas, T.; Bardas, G.A.; Karaoglanidis, G.S.; Tzavella-Klonari, K. Management of Tomato Leaf Mould Caused by Cladosporium Fulvum with Trifloxystrobin. Crop Prot. 2007, 26, 845–851. [Google Scholar] [CrossRef]
- Ishaq, H.; Khan, M.A.; Ahmed, I.; Shah, S.M.A.; Ali, U.; Jatoi, G.H.; Sultan, A.; Akhtar, A.; Usmani, M.M.; Iftikhar, S. Antifungal Exploitation of Fungicides and Plant Extracts against Fusarium oxysporum f.sp. melongenae causing fusarium wilt of eggplant. Pakistan J. Biotechnol. 2023, 20, 59–67. [Google Scholar] [CrossRef]
- Acosta-Gonz Alez, U.; Silva-Rojas, H.V.; Fuentes-Arag, D.; Us Hern Andez-Castrej, J.; Romero-Bautista, A.; Rebollar-Alviter, A. Comparative Performance of Fungicides and Biocontrol Products in the Management of Fusarium Wilt of Blackberry. Plant Dis. 2022, 106, 1419–1427. [Google Scholar] [CrossRef]
- Steentjes, M.B.F.; Scholten, O.E.; van Kan, J.A.L. Peeling the Onion: Towards a Better Understanding of Botrytis Diseases of Onion. Phytopathology 2021, 111, 464–473. [Google Scholar] [CrossRef]
- Sanglard, D.; Coste, A.; Ferrari, S. Antifungal Drug Resistance Mechanisms in Fungal Pathogens from the Perspective of Transcriptional Gene Regulation. FEMS Yeast Res. 2009, 9, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Seblani, R.; Keinath, A.P.; Munkvold, G. Gummy Stem Blight: One Disease, Three Pathogens. Mol. Plant Pathol. 2023, 24, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Benigni, M.; Bompeix, G. Chemical and Biological Control of Sclerotinia sclerotiorum in Witloof Chicory Culture. Pest Manag. Sci. 2010, 66, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Song, Y.F.; Li, B.X.; Mu, W.; Liu, F. Baseline Sensitivity of Isopyrazam against Sclerotinia Sclerotiorum and Its Efficacy for the Control of Sclerotinia Stem Rot in Vegetables. Crop Prot. 2019, 122, 42–48. [Google Scholar] [CrossRef]
- Rajput, M.A.; Rajput, N.A.; Syed, R.N.; Lodhi, A.M.; Que, Y. Sugarcane Smut: Current Knowledge and the Way Forward for Management. J. Fungi 2021, 7, 1095. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Hansen, J.G.; Semaskiene, R.; Korbas, M.; Danielewicz, J.; Glazek, M.; Maumene, C.; Rodemann, B.; Weigand, S.; et al. Four Azoles’ Profile in the Control of Septoria, Yellow Rust and Brown Rust in Wheat across Europe. Crop Prot. 2018, 105, 16–27. [Google Scholar] [CrossRef]
- Ramanauskienė, J.; Gaurilčikienė, I.; Supronienė, S. Effects of Fungicides on the Occurrence of Winter Wheat Eyespot Caused by Fungi Oculimacula acuformis and O. yallundae. Crop Prot. 2016, 90, 90–95. [Google Scholar] [CrossRef]
- Xu, S.; Wang, B.; Li, L.; Zhou, Q.; Tian, M.; Zhao, X.; Peng, J.; Liu, F.; Chen, Y.; Xu, Y.; et al. Effects of Camptothecin on the Rice Blast Fungus Magnaporthe Oryzae. Pestic. Biochem. Physiol. 2020, 163, 108–116. [Google Scholar] [CrossRef]
- Juliatti, F.C.; Azevedo, L.A.; Juliatti, F.C. Strategies of Chemical Protection for Controlling Soybean Rust. In Soybean: The Basis of Yield, Biomass and Productivity; Books on Demand: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef]
- Baginski, M.; Czub, J. Amphotericin B and Its New Derivatives—Mode of Action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef]
- Chapeland, F.; Fritz, R.; Lanen, C.; Gredt, M.; Leroux, P. Inheritance and Mechanisms of Resistance to Anilinopyrimidine Fungicides in Botrytis Cinerea (Botryotinia fuckeliana). Pestic. Biochem. Physiol. 1999, 64, 85–100. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in Understanding Molecular Mechanisms and Evolution of Resistance to Succinate Dehydrogenase Inhibiting (SDHI) Fungicides in Phytopathogenic Fungi. Crop. Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Davidse, L.C. Benzimidazole Fungicides: Mechanism of Action and Biological Impact. Annu. Rev. Phytopathol. 1986, 24, 43–65. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Tores, J.A.; Pérez-García, A. Mechanisms of Resistance to QoI Fungicides in Phytopathogenic Fungi. Int. Microbiol. 2008, 11, 1–9. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Advances in Understanding Molecular Mechanisms of Fungicide Resistance and Molecular Detection of Resistant Genotypes in Phytopathogenic Fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Leroux, P.; Chapeland, F.; Desbrosses, D.; Gredt, M. Patterns of Cross-Resistance to Fungicides in Botryotinia Fuckeliana (Botrytis cinerea) Isolates from French Vineyards. Crop Prot. 1999, 18, 687–697. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental Hotspots for Azole Resistance Selection of Aspergillus Fumigatus, the Netherlands. Emerg. Infect. Dis. 2019, 25, 1347–1353. [Google Scholar] [CrossRef]
- Hurst, S.F.; Berkow, E.L.; Stevenson, K.L.; Litvintseva, A.P.; Lockhart, S.R. Isolation of Azole-Resistant Aspergillus Fumigatus from the Environment in the South-Eastern USA. J. Antimicrob. Chemother. 2017, 72, 2443–2446. [Google Scholar] [CrossRef]
- Sewell, T.R.; Zhu, J.; Rhodes, J.; Hagen, F.; Meis, J.F.; Fisher, M.C.; Jombart, T. Nonrandom Distribution of Azole Resistance across the Global Population of Aspergillus fumigatus. mBio 2019, 10, e00392-19. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-Resistant Aspergillus fumigatus Harboring TR34/L98H, TR46/Y121F/T289A and TR53 Mutations Related to Flower Fields in Colombia. Sci. Rep. 2017, 7, 45631. [Google Scholar] [CrossRef]
- Etienne, K.A.; Berkow, E.L.; Gade, L.; Nunnally, N.; Lockhart, S.R.; Beer, K.; King Jordan, I.; Rishishwar, L.; Litvintseva, A.P. Genomic Diversity of Azole-Resistant Aspergillus Fumigatus in the United States. mBio 2021, 12, e0180321. [Google Scholar] [CrossRef] [PubMed]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A. A Sterol-Regulatory Element Binding Protein Is Required for Cell Polarity, Hypoxia Adaptation, Azole Drug Resistance, and Virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Bien, C.M.; Lee, H.; Espenshade, P.J.; Kwon-Chung, K.J. Sre1p, a Regulator of Oxygen Sensing and Sterol Homeostasis, Is Required for Virulence in Cryptococcus neoformans. Mol. Microbiol. 2007, 64, 614–629. [Google Scholar] [CrossRef]
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A Novel Zn2-Cys6 Transcription Factor AtrR Plays a Key Role in an Azole Resistance Mechanism of Aspergillus fumigatus by Co-Regulating Cyp51A and Cdr1B Expressions. PLoS Pathog. 2017, 13, e1006096. [Google Scholar] [CrossRef]
- Paul, S.; Stamnes, M.; Thomas, G.H.; Liu, H.; Hagiwara, D.; Gomi, K.; Filler, S.G.; Moye-Rowley, W.S. AtrR Is an Essential Determinant of Azole Resistance in Aspergillus fumigatus. mBio 2019, 10, e02563-18. [Google Scholar] [CrossRef]
- Du, W.; Zhai, P.; Wang, T.; Bromley, M.J.; Zhang, Y.; Lu, L. The C2H2 Transcription Factor SltA Contributes to Azole Resistance by Coregulating the Expression of the Drug Target Erg11A and the Drug Efflux Pump Mdr1 in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2021, 65, e01839-20. [Google Scholar] [CrossRef]
- Park, S.; Kelly, R.; Kahn, J.N.; Robles, J.; Hsu, M.J.; Register, E.; Li, W.; Vyas, V.; Fan, H.; Abruzzo, G.; et al. Specific Substitutions in the Echinocandin Target Fks1p Account for Reduced Susceptibility of Rare Laboratory and Clinical Candida Sp. Isolates. Antimicrob. Agents Chemother. 2005, 49, 3264–3273. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida Glabrata FKS1 and FKS2 Mutations on Echinocandin Sensitivity and Kinetics of 1,3-Beta-D-Glucan Synthase: Implication for the Existing Susceptibility Breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Tronchin, G.; Larcher, G.; Ernoult, E.; Bergès, T.; Chabasse, D.; Bouchara, J.P. A Nonsense Mutation in the ERG6 Gene Leads to Reduced Susceptibility to Polyenes in a Clinical Isolate of Candida Glabrata. Antimicrob. Agents Chemother. 2008, 52, 3701–3709. [Google Scholar] [CrossRef]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness Trade-Offs Restrict the Evolution of Resistance to Amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar] [CrossRef]
- Rybak, J.M.; Barker, K.S.; Muñoz, J.F.; Parker, J.E.; Ahmad, S.; Mokaddas, E.; Abdullah, A.; Elhagracy, R.S.; Kelly, S.L.; Cuomo, C.A.; et al. In Vivo Emergence of High-Level Resistance during Treatment Reveals the First Identified Mechanism of Amphotericin B Resistance in Candida Auris. Clin. Microbiol. Infect. 2022, 28, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Turner, V.; Ischer, F.; Morschhäuser, J.; Forche, A.; Selmecki, A.; Berman, J.; Bille, J.; Sanglard, D. A Mutation in Tac1p, a Transcription Factor Regulating CDR1 and CDR2, Is Coupled with Loss of Heterozygosity at Chromosome 5 to Mediate Antifungal Resistance in Candida albicans. Genetics 2006, 172, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, N.; Blaß, J.; Rogers, P.D.; Morschhäuser, J. Mutations in the Multi-Drug Resistance Regulator MRR1, Followed by Loss of Heterozygosity, Are the Main Cause of MDR1 Overexpression in Fluconazole-Resistant Candida Albicans Strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Coste, A.T.; Bachmann, D.; Sanglard, D.; Lamoth, F. Deciphering the Mrr1/Mdr1 Pathway in Azole Resistance of Candida Auris. Antimicrob. Agents Chemother. 2022, 66, e00067-22. [Google Scholar] [CrossRef]
- Rybak, J.M.; Muñoz, J.F.; Barker, K.S.; Parker, J.E.; Esquivel, B.D.; Berkow, E.L.; Lockhart, S.R.; Gade, L.; Palmer, G.E.; White, T.C.; et al. Mutations in TAC1B: A Novel Genetic Determinant of Clinical Fluconazole Resistance in Candida Auris. mBio 2020, 11, e00365-20. [Google Scholar] [CrossRef]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and Isochromosome Formation in Drug-Resistant Candida Albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef]
- Selmecki, A.; Gerami-Nejad, M.; Paulson, C.; Forche, A.; Berman, J. An Isochromosome Confers Drug Resistance in Vivo by Amplification of Two Genes, ERG11 and TAC1. Mol. Microbiol. 2008, 68, 624–641. [Google Scholar] [CrossRef]
- Yang, F.; Gritsenko, V.; Futterman, Y.S.; Gao, L.; Zhen, C.; Lu, H.; Jiang, Y.Y.; Berman, J. Tunicamycin Potentiates Antifungal Drug Tolerance via Aneuploidy in Candida Albicans. mBio 2021, 12, e02272-21. [Google Scholar] [CrossRef]
- Yang, F.; Teoh, F.; Tan, A.S.M.; Cao, Y.; Pavelka, N.; Berman, J.; Malik, H. Aneuploidy Enables Cross-Adaptation to Unrelated Drugs. Mol. Biol. Evol. 2019, 36, 1768–1782. [Google Scholar] [CrossRef]
- Poláková, S.; Blume, C.; Zárate, J.Á.; Mentel, M.; Jørck-Ramberg, D.; Stenderup, J.; Piškur, J. Formation of New Chromosomes as a Virulence Mechanism in Yeast Candida glabrata. Proc. Natl. Acad. Sci. USA 2009, 106, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Sionov, E.; Lee, H.; Chang, Y.C.; Kwon-Chung, K.J. Cryptococcus neoformans Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes. PLoS Pathog. 2010, 6, e1000848. [Google Scholar] [CrossRef] [PubMed]
- Morogovsky, A.; Handelman, M.; Abou Kandil, A.; Shadkchan, Y.; Osherov, N. Horizontal Gene Transfer of Triazole Resistance in Aspergillus fumigatus. Microbiol. Spectr. 2022, 10, e01112-22. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, H. Characterization of Mutations in the Beta-Tubulin Gene of Benomyl-Resistant Field Strains of Venturia inaequalis and Other Plant Pathogenic Fungi. Phytopathology 1992, 82, 1348. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A Review of Current Knowledge of Resistance Aspects for the Next-Generation Succinate Dehydrogenase Inhibitor Fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple Resistance of Botrytis Cinerea from Kiwifruit to SDHIs, QoIs and Fungicides of Other Chemical Groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Resistance to Boscalid Fungicide in Alternaria Alternata Isolates from Pistachio in California. Plant Dis. 2007, 91, 1345–1350. [Google Scholar] [CrossRef]
- McKay, A.H.; Hagerty, G.C.; Follas, G.B.; Moore, M.S.; Christie, M.S.; Beresford, R.M. Succinate Dehydrogenase Inhibitor (SDHI) Fungicide Resistance Prevention Strategy. N. Z. Plant Prot. 2011, 64, 119–124. [Google Scholar] [CrossRef]
- Müller, M.A.; Stammler, G.; May De Mio, L.L. Multiple Resistance to DMI, QoI and SDHI Fungicides in Field Isolates of Phakopsora pachyrhizi. Crop Prot. 2021, 145, 105618. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Haidukowski, M.; Logrieco, A.F.; Moretti, A. Genetic Polymorphisms Associated to SDHI Fungicides Resistance in Selected Aspergillus Flavus Strains and Relation with Aflatoxin Production. Int. J. Food Microbiol. 2020, 334, 108799. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Latorre, B.A.; Spadaro, I.; Rioja, M.E. Occurrence of Resistant Strains of Botrytis Cinerea to Anilinopyrimidine Fungicides in Table Grapes in Chile. Crop Prot. 2002, 21, 957–961. [Google Scholar] [CrossRef]
- Mosbach, A.; Edel, D.; Farmer, A.D.; Widdison, S.; Barchietto, T.; Dietrich, R.A.; Corran, A.; Scalliet, G. Anilinopyrimidine Resistance in Botrytis Cinerea Is Linked to Mitochondrial Function. Front. Microbiol. 2017, 8, 304532. [Google Scholar] [CrossRef]
- Fiaccadori, R.; Collina, M.; Brunelli, A. Study on the Sensitivity of Venturia inaequalis to Anilinopyrimidine Fungicides in Italy. Commun. Agric. Appl. Biol. Sci. 2007, 71, 997–1001. [Google Scholar]
- Cools, H.J.; Hawkins, N.J.; Fraaije, B.A. Constraints on the Evolution of Azole Resistance in Plant Pathogenic Fungi. Plant Pathol. 2013, 62, 36–42. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole Fungicides—Understanding Resistance Mechanisms in Agricultural Fungal Pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Hokken, M.W.J.; Zwaan, B.J.; Melchers, W.J.G.; Verweij, P.E. Facilitators of Adaptation and Antifungal Resistance Mechanisms in Clinically Relevant Fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef]
- Nuwamanya, A.M.; Runo, S.; Mwangi, M. In-Vitro Sensitivity of Alternaria Solani Isolates to Azoxystrobin and Difenoconazole Fungicides in Kenya and Detection of Cyt b Mutations Associated with Azoxystrobin Resistance. Crop Prot. 2022, 158, 106010. [Google Scholar] [CrossRef]
- Chen, F.; Tsuji, S.S.; Li, Y.; Hu, M.; Bandeira, M.A.; Câmara, M.P.S.; Michereff, S.J.; Schnabel, G. Reduced Sensitivity of Azoxystrobin and Thiophanate-Methyl Resistance in Lasiodiplodia Theobromae from Papaya. Pestic. Biochem. Physiol. 2020, 162, 60–68. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, F.; Zhao, J.; Fan, H.; Qin, C.; Li, R.; Verweij, P.E.; Zheng, Y.; Han, L. High Azole Resistance in Aspergillus Fumigatus Isolates from Strawberry Fields, China, 2018—Volume 26, Number 1—January 2020—Emerging Infectious Diseases Journal—CDC. Emerg. Infect. Dis. 2020, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Beer, K.D.; Kuivila, K.M.; Chiller, T.M.; Jackson, B.R. Trends in Agricultural Triazole Fungicide Use in the United States, 1992–2016 and Possible Implications for Antifungal-Resistant Fungi in Human Disease. Environ. Health Perspect. 2021, 129, 055001. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (COVID-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and Mitigating the Risk of COVID-19 Associated Pulmonary Aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef] [PubMed]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive Pulmonary Aspergillosis Is a Frequent Complication of Critically Ill H1N1 Patients: A Retrospective Study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Fisher, M.C.; Rannala, B.; Chaturvedi, V.; Taylor, J.W. Disease Surveillance in Recombining Pathogens: Multilocus Genotypes Identify Sources of Human Coccidioides Infections. Proc. Natl. Acad. Sci. USA 2002, 99, 9067–9071. [Google Scholar] [CrossRef]
- Farrer, R.A.; Chang, M.; Davis, M.J.; van Dorp, L.; Yang, D.H.; Shea, T.; Sewell, T.R.; Meyer, W.; Balloux, F.; Edwards, H.M.; et al. A New Lineage of Cryptococcus Gattii (VGV) Discovered in the Central Zambezian Miombo Woodlands. mBio 2019, 10, e02306-19. [Google Scholar] [CrossRef]
- Ashu, E.E.; Hagen, F.; Chowdhary, A.; Meis, J.F.; Xu, J. Global Population Genetic Analysis of Aspergillus fumigatus. mSphere 2017, 2, e00019-17. [Google Scholar] [CrossRef]
- Van Rhijn, N.; Bromley, M. The Consequences of Our Changing Environment on Life Threatening and Debilitating Fungal Diseases in Humans. J. Fungi 2021, 7, 367. [Google Scholar] [CrossRef]
- Carneiro, H.C.S.; Bastos, R.W.; Ribeiro, N.Q.; Gouveia-Eufrasio, L.; Costa, M.C.; Magalhães, T.F.F.; Oliveira, L.V.N.; Paixão, T.A.; Joffe, L.S.; Rodrigues, M.L.; et al. Hypervirulence and Cross-Resistance to a Clinical Antifungal Are Induced by an Environmental Fungicide in Cryptococcus gattii. Sci. Total Environ. 2020, 740, 140135. [Google Scholar] [CrossRef] [PubMed]
- Wroński, M.; Trawiński, J.; Skibiński, R. Antifungal Drugs in the Aquatic Environment: A Review on Sources, Occurrence, Toxicity, Health Effects, Removal Strategies and Future Challenges. J. Hazard. Mater. 2024, 465, 133167. [Google Scholar] [CrossRef] [PubMed]
- Islam, T. Heavy Metal Tolerance Pattern of Textile Dye Degrading Native Bacteria: A Bioremediation Viewpoint. Ann. Med. Health Sci. Res. 2017, 7, 67–73. [Google Scholar]
- Juksu, K.; Zhao, J.L.; Liu, Y.S.; Yao, L.; Sarin, C.; Sreesai, S.; Klomjek, P.; Jiang, Y.X.; Ying, G.G. Occurrence, Fate and Risk Assessment of Biocides in Wastewater Treatment Plants and Aquatic Environments in Thailand. Sci. Total Environ. 2019, 690, 1110–1119. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, P.; Yu, J.; Jiang, Z.; Guo, X. Experimental and Molecular Docking Study on Graphene/Fe3O4 Composites as a Sorbent for Magnetic Solid-Phase Extraction of Seven Imidazole Antifungals in Environmental Water Samples Prior to LC-MS/MS for Enantiomeric Analysis. Microchem. J. 2018, 140, 222–231. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zhu, Q.; Sun, S.; Peng, Y.; Shuai, Q. Suspect Screening and Target Quantification of Human Pharmaceutical Residues in the Surface Water of Wuhan, China, Using UHPLC-Q-Orbitrap HRMS. Sci. Total Environ. 2018, 635, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Rabbee, M.F.; Choi, J.; Baek, K.H. Biosynthesis, Molecular Regulation, and Application of Bacilysin Produced by Bacillus Species. Metabolites 2022, 12, 397. [Google Scholar] [CrossRef]
- Patel, A.; Kumar, A.; Sheoran, N.; Kumar, M.; Sahu, K.P.; Ganeshan, P.; Ashajyothi, M.; Gopalakrishnan, S.; Gogoi, R. Antifungal and Defense Elicitor Activities of Pyrazines Identified in Endophytic Pseudomonas Putida BP25 against Fungal Blast Incited by Magnaporthe Oryzae in Rice. J. Plant Dis. Prot. 2021, 128, 261–272. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Qin, L.; Li, Y.; Liao, X.; Han, P.; Yu, C.; Liao, X. Seed Treatment Containing Bacillus Subtilis BY-2 in Combination with Other Bacillus Isolates for Control of Sclerotinia sclerotiorum on Oilseed Rape. Biol. Control 2019, 133, 50–57. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, T.; Shen, A.; Yang, X.; Yu, Y.; Gao, C.; Li, Z.; Cheng, Y.; Chen, J.; Guo, L.; et al. Biocontrol Potential of Bacillus Subtilis IBFCBF-4 against Fusarium Wilt of Watermelon. J. Plant Pathol. 2020, 102, 433–441. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Rashid Khan, A.; Hanif, A.; Tahir, H.A.S.; Gao, X. Marker Assisted Detection and LC-MS Analysis of Antimicrobial Compounds in Different Bacillus Strains and Their Antifungal Effect on Sclerotinia sclerotiorum. Biol. Control 2019, 133, 91–102. [Google Scholar] [CrossRef]
- Fazle Rabbee, M.; Baek, K.H. Antimicrobial Activities of Lipopeptides and Polyketides of Bacillus velezensis for Agricultural Applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, P.; Zheng, L.; Li, Y.; Chen, Y.; Wen, S.; Yu, G. Comparative Genomics Analysis of Two Banana Fusarium Wilt Biocontrol Endophytes Bacillus Subtilis R31 and TR21 Provides Insights into Their Differences on Phytobeneficial Trait. Genomics 2021, 113, 900–909. [Google Scholar] [CrossRef]
- Jiao, R.; Cai, Y.; He, P.; Munir, S.; Li, X.; Wu, Y.; Wang, J.; Xia, M.; He, P.; Wang, G.; et al. Bacillus Amyloliquefaciens YN201732 Produces Lipopeptides with Promising Biocontrol Activity Against Fungal Pathogen Erysiphe Cichoracearum. Front. Cell. Infect. Microbiol. 2021, 11, 598999. [Google Scholar] [CrossRef]
- Badri Fariman, A.; Abbasiliasi, S.; Akmar Abdullah, S.N.; Mohd Saud, H.; Wong, M.Y. Stenotrophomonas Maltophilia Isolate UPMKH2 with the Abilities to Suppress Rice Blast Disease and Increase Yield a Promising Biocontrol Agent. Physiol. Mol. Plant Pathol. 2022, 121, 101872. [Google Scholar] [CrossRef]
- Azeem, S.; Agha, S.I.; Jamil, N.; Tabassum, B.; Ahmed, S.; Raheem, A.; Jahan, N.; Ali, N.; Khan, A. Characterization and Survival of Broad-Spectrum Biocontrol Agents against Phytopathogenic Fungi. Rev. Argent. Microbiol. 2022, 54, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus Thuringiensis Strains to Identify New Potential Biocontrol Agents against Sclerotinia Sclerotiorum and Plutella Xylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Chet, I.; Ordentlich, A.; Shapira, R.; Oppenheim, A. Mechanisms of Biocontrol of Soil-Borne Plant Pathogens by Rhizobacteria. Plant Soil 1990, 129, 85–92. [Google Scholar] [CrossRef]
- Gu, Q.; Qiao, J.; Wang, R.; Lu, J.; Wang, Z.; Li, P.; Zhang, L.; Ali, Q.; Khan, A.R.; Gao, X.; et al. The Role of Pyoluteorin from Pseudomonas Protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea Ananatis on Maize. Int. J. Mol. Sci. 2022, 23, 6431. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest Biocontrol Ability of Pseudomonas Synxantha against Monilinia Fructicola and Monilinia Fructigena on Stone Fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Liang, L.J.; Jeewon, R.; Dhandevi, P.; Durairajan, S.S.K.; Li, H.; Lin, F.C.; Wang, H.K. A Novel Species of Penicillium With Inhibitory Effects Against Pyricularia Oryzae and Fungal Pathogens Inducing Citrus Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 604504. [Google Scholar] [CrossRef]
- Eshel, D.; Regev, R.; Orenstein, J.; Droby, S.; Gan-Mor, S. Combining Physical, Chemical and Biological Methods for Synergistic Control of Postharvest Diseases: A Case Study of Black Root Rot of Carrot. Postharvest Biol. Technol. 2009, 54, 48–52. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma Asperellum Biocontrol Activity and Induction of Systemic Defenses against Sclerotium Cepivorum in Onion Plants under Tropical Climate Conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- de Azevedo Silva, F.; de Oliveira Vieira, V.; Correia da Silva, R.; Guariz Pinheiro, D.; Antônio Soares, M. Introduction of Trichoderma spp. Biocontrol Strains against Sclerotinia Sclerotiorum (Lib.) de Bary Change Soil Microbial Community Composition in Common Bean (Phaseolus vulgaris L.) Cultivation. Biol. Control 2021, 163, 104755. [Google Scholar] [CrossRef]
- Wonglom, P.; Daengsuwan, W.; Ito, S.; Sunpapao, A. Biological Control of Sclerotium Fruit Rot of Snake Fruit and Stem Rot of Lettuce by Trichoderma Sp. T76-12/2 and the Mechanisms Involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- He, A.; Sun, J.; Wang, X.; Zou, L.; Fu, B.; Chen, J. Reprogrammed Endophytic Microbial Community in Maize Stalk Induced by Trichoderma Asperellum Biocontrol Agent against Fusarium Diseases and Mycotoxin Accumulation. Fungal Biol. 2019, 123, 448–455. [Google Scholar] [CrossRef]
- Ji, S.; An, Y.B.; Zhang, H.; Wang, Y.; Liu, Z. Trichoderma Biofertilizer (MixTroTha) Mediates Malus Sieversii Resistance to Alternaria Alternata. Biol. Control 2021, 156, 104539. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Araújo, N.A.F.; Machado, N.B.; Costa Júnior, P.S.P.; Pasqual, M.; Alves, E.; Schwan-Estrada, K.R.F.; Dória, J. Yeasts and Bacillus Spp. as Potential Biocontrol Agents of Sclerotinia Sclerotiorum in Garlic. Sci. Hortic. 2020, 261, 108931. [Google Scholar] [CrossRef]
- Gajera, H.P.; Katakpara, Z.A.; Patel, S.V.; Golakiya, B.A. Antioxidant Defense Response Induced by Trichoderma Viride against Aspergillus Niger Van Tieghem Causing Collar Rot in Groundnut (Arachis hypogaea L.). Microb. Pathog. 2016, 91, 26–34. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Guzmán-Ortiz, D.A.; Gómez-Lim, M.A.; Délano-Frier, J.P.; de Folter, S.; Sánchez-García, P.; Peña-Cabriales, J.J. Potential Use of Trichoderma Asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a Biological Control Agent against Anthracnose in Mango (Mangifera indica L.). Biol. Control 2013, 64, 37–44. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological Control of Plant Diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological Control of Postharvest Diseases of Fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef]
- Djonović, S.; Vittone, G.; Mendoza-Herrera, A.; Kenerley, C.M. Enhanced Biocontrol Activity of Trichoderma Virens Transformants Constitutively Coexpressing β-1,3- and β-1,6-Glucanase Genes. Mol. Plant Pathol. 2007, 8, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yakoby, N.; Zhou, R.; Kobiler, I.; Dinoor, A.; Prusky, D. Development of Colletotrichum Gloeosporioides Restriction Enzyme-Mediated Integration Mutants as Biocontrol Agents Against Anthracnose Disease in Avocado Fruits. Phytopathology 2007, 91, 143–148. [Google Scholar] [CrossRef]
- Raman, N.M.; Easwaran, M.; Kaul, R.; Bharti, J.; Motelb, K.F.A.; Kaul, T.; Raman, N.M.; Easwaran, M.; Kaul, R.; Bharti, J.; et al. Antimicrobial Resistance with Special Emphasis on Pathogens in Agriculture. In Antimicrobial Resistance—A One Health Perspect; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Wubben, M.J.E.; Su, H.; Rodermel, S.R.; Baum, T.J. Susceptibility to the Sugar Beet Cyst Nematode Is Modulated by Ethylene Signal Transduction in Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2001, 14, 1206–1212. [Google Scholar] [CrossRef]
- Lumbreras, V.; Vilela, B.; Irar, S.; Solé, M.; Capellades, M.; Valls, M.; Coca, M.; Pagès, M. MAPK Phosphatase MKP2 Mediates Disease Responses in Arabidopsis and Functionally Interacts with MPK3 and MPK6. Plant J. 2010, 63, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Browse, J. Altered Rates of Protein Transport in Arabidopsis Mutants Deficient in Chloroplast Membrane Unsaturation. Phytochemistry 2006, 67, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.; Levings, I. The Texas Cytoplasm of Maize: Cytoplasmic Male Sterility and Disease Susceptibility. Science 1990, 250, 942–947. [Google Scholar] [CrossRef]
- Humphry, M.; Reinstädler, A.; Ivanov, S.; Bisseling, T.; Panstruga, R. Durable Broad-Spectrum Powdery Mildew Resistance in Pea Er1 Plants Is Conferred by Natural Loss-of-Function Mutations in PsMLO1. Mol. Plant Pathol. 2011, 12, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.A.; Arévalo-Soliz, L.M.; Jia, L.; Navarre, D.A.; Chen, Z.; Howe, G.A.; Meng, Q.W.; Smith, J.E.; Goggin, F.L. Loss of Function of FATTY ACID DESATURASE7 in Tomato Enhances Basal Aphid Resistance in a Salicylate-Dependent Manner. Plant Physiol. 2012, 158, 2028–2041. [Google Scholar] [CrossRef]
- Krasikov, V.; Dekker, H.L.; Rep, M.; Takken, F.L.W. The Tomato Xylem Sap Protein XSP10 Is Required for Full Susceptibility to Fusarium Wilt Disease. J. Exp. Bot. 2011, 62, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; Miyamoto, K.; Shimogawa, T.; Shimizu, T.; Otake, Y.; Yokotani, N.; Nishizawa, Y.; Shibuya, N.; Nojiri, H.; Yamane, H.; et al. OsWRKY28, a PAMP-Responsive Transrepressor, Negatively Regulates Innate Immune Responses in Rice against Rice Blast Fungus. Plant Mol. Biol. 2013, 82, 23–37. [Google Scholar] [CrossRef]
- Yoshii, M.; Shimizu, T.; Yamazaki, M.; Higashi, T.; Miyao, A.; Hirochika, H.; Omura, T. Disruption of a Novel Gene for a NAC-Domain Protein in Rice Confers Resistance to Rice Dwarf Virus. Plant J. 2009, 57, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, M.; Yamazaki, M.; Rakwal, R.; Kishi-Kaboshi, M.; Miyao, A.; Hirochika, H. The NAC Transcription Factor RIM1 of Rice Is a New Regulator of Jasmonate Signaling. Plant J. 2010, 61, 804–815. [Google Scholar] [CrossRef]
- Halterman, D.A.; Kramer, L.C.; Wielgus, S.; Jiang, J. Performance of Transgenic Potato Containing the Late Blight Resistance Gene RB. Plant Dis. 2008, 92, 339–343. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Witek, K.; Verweij, W.; Jupe, F.; Cooke, D.; Dorling, S.; Tomlinson, L.; Smoker, M.; Perkins, S.; Foster, S. Elevating Crop Disease Resistance with Cloned Genes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130087. [Google Scholar] [CrossRef]
- Bradeen, J.M.; Iorizzo, M.; Mollov, D.S.; Raasch, J.; Kramer, L.C.; Millett, B.P.; Austin-Phillips, S.; Jiang, J.; Carputo, D. Higher Copy Numbers of the Potato RB Transgene Correspond to Enhanced Transcript and Late Blight Resistance Levels. Mol. Plant. Microbe Interact. 2009, 22, 437–446. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic Engineering for Disease Resistance in Plants: Recent Progress and Future Perspectives. Plant Physiol. 2019, 180, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Y.; Vossen, J.H.; Visser, R.G.F.; Jacobsen, E. Functional Stacking of Three Resistance Genes against Phytophthora Infestans in Potato. Transgenic Res. 2012, 21, 89. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Vossen, J.H.; Visser, R.G.F. Durable Late Blight Resistance in Potato Through Dynamic Varieties Obtained by Cisgenesis: Scientific and Societal Advances in the DuRPh Project. Potato Res. 2016, 59, 35–66. [Google Scholar] [CrossRef]
- Halperin, S.O.; Tou, C.J.; Wong, E.B.; Modavi, C.; Schaffer, D.V.; Dueber, J.E. CRISPR-Guided DNA Polymerases Enable Diversification of All Nucleotides in a Tunable Window. Nature 2018, 560, 248–252. [Google Scholar] [CrossRef]
- Paul, N.C.; Park, S.W.; Liu, H.; Choi, S.; Ma, J.; MacCready, J.S.; Chilvers, M.I.; Sang, H. Plant and Fungal Genome Editing to Enhance Plant Disease Resistance Using the CRISPR/Cas9 System. Front. Plant Sci. 2021, 12, 700925. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, L.; Liu, D.; Wu, D.; Fang, Y.; Wu, J.; Wang, Y. CRISPR/Cas9-Mediated Multiplex Genome Editing of the BnWRKY11 and BnWRKY70 Genes in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2716. [Google Scholar] [CrossRef]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma Cacao. Front. Plant Sci. 2018, 9, 329023. [Google Scholar] [CrossRef]
- Li, L.; Kim, P.; Yu, L.; Cai, G.; Chen, S.; Alfano, J.R.; Zhou, J.M. Activation-Dependent Destruction of a Co-Receptor by a Pseudomonas Syringae Effector Dampens Plant Immunity. Cell Host Microbe 2016, 20, 504–514. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-Spectrum Resistance to Bacterial Blight in Rice Using Genome Editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid Generation of a Transgene-Free Powdery Mildew Resistant Tomato by Genome Deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Santillán Martínez, M.I.; Bracuto, V.; Koseoglou, E.; Appiano, M.; Jacobsen, E.; Visser, R.G.F.; Wolters, A.M.A.; Bai, Y. CRISPR/Cas9-Targeted Mutagenesis of the Tomato Susceptibility Gene PMR4 for Resistance against Powdery Mildew. BMC Plant Biol. 2020, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous Editing of Three Homoeoalleles in Hexaploid Bread Wheat Confers Heritable Resistance to Powdery Mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Grape in the First Generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Králová, M.; Bergougnoux, V.; Frébort, I. CRISPR/Cas9 Genome Editing in Ergot Fungus Claviceps Purpurea. J. Biotechnol. 2021, 325, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Wan, L.; Deng, Y.Z.; Yang, W.; Li, W.; Jiang, L.; Situ, J.; Xi, P.; Li, M.; Jiang, Z. Pectin Acetylesterase PAE5 Is Associated with the Virulence of Plant Pathogenic Oomycete Peronophythora Litchii. Physiol. Mol. Plant Pathol. 2019, 106, 16–22. [Google Scholar] [CrossRef]
- Qu, Z.; Fu, Y.; Lin, Y.; Zhao, Z.; Zhang, X.; Cheng, J.; Xie, J.; Chen, T.; Li, B.; Jiang, D. Transcriptional Responses of Sclerotinia Sclerotiorum to the Infection by SsHADV-1. J. Fungi 2021, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Liao, X.L.; Gao, B.; Lu, X.; Sun, D.; Gong, W.; Zhong, J.; Zhu, H.; Pan, X.; et al. Mycoviral Gene Integration Converts a Plant Pathogenic Fungus into a Biocontrol Agent. Proc. Natl. Acad. Sci. USA 2022, 119, e2214096119. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A Geminivirus-Related DNA Mycovirus That Confers Hypovirulence to a Plant Pathogenic Fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef]
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic Fungus Hosts a Plant Virus: A Naturally Occurring Cross-Kingdom Viral Infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef]
- Bian, R.; Andika, I.B.; Pang, T.; Lian, Z.; Wei, S.; Niu, E.; Wu, Y.; Kondo, H.; Liu, X.; Sun, L. Facilitative and Synergistic Interactions between Fungal and Plant Viruses. Proc. Natl. Acad. Sci. USA 2020, 117, 3779–3788. [Google Scholar] [CrossRef]
- Attai, H.; Rimbey, J.; Smith, G.P.; Brown, P.J.B. Expression of a Peptidoglycan Hydrolase from Lytic Bacteriophages Atu_ph02 and Atu_ph03 Triggers Lysis of Agrobacterium Tumefaciens. Appl. Environ. Microbiol. 2017, 83, e01498-17. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Sarafat Ali, M.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Bacillus Velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus Amyloliquefaciens S76-3 from Wheat Spikes against Fusarium Graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major Secondary Metabolites Produced by Two Commercial Trichoderma Strains Active against Different Phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Shanthiyaa, V.; Saravanakumar, D.; Rajendran, L.; Karthikeyan, G.; Prabakar, K.; Raguchander, T. Use of Chaetomium Globosum for Biocontrol of Potato Late Blight Disease. Crop Prot. 2013, 52, 33–38. [Google Scholar] [CrossRef]
- Chiu, T.; Poucet, T.; Li, Y. The Potential of Plant Proteins as Antifungal Agents for Agricultural Applications. Synth. Syst. Biotechnol. 2022, 7, 1075. [Google Scholar] [CrossRef]
- Rahman, M.; Wadud, M.; Islam, T.; Hussain, M.; Bristy, E.; Tuhin, A. Evaluation of Antibacterial Activity of Piper Betel Leaves and Nigella Sativa Seeds against Multidrug Resistant Food and Water Borne Pathogenic Bacteria: An in Vitro Study Model. Microbiol. Res. J. Int. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Shalahuddin Millat, M.; Islam, S.; Hussain, M.S.; Rahman Moghal, M.M.; Islam, T. Anti-Bacterial Profiling of Launaea Sarmentosa (Willd.) and Bruguiera cylindrical (L.): Two Distinct Ethno Medicinal Plants of Bangladesh. Eur. J. Exp. Biol. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Debnath, T.; Bhowmik, S.; Islam, T.; Chowdhury, M.M.H. Presence of Multidrug-Resistant Bacteria on Mobile Phones of Healthcare Workers Accelerates the Spread of Nosocomial Infection and Regarded as a Threat to Public Health in Bangladesh. J. Microsc. Ultrastruct. 2018, 6, 165. [Google Scholar] [CrossRef]
- Malik, A. Preety Purification and Properties of Plant Chitinases: A Review. J. Food Biochem. 2019, 43, e12762. [Google Scholar] [CrossRef]
- Shrestha, C.L.; Oña, I.; Muthukrishnan, S.; Mew, T.W. Chitinase Levels in Rice Cultivars Correlate with Resistance to the Sheath Blight Pathogen Rhizoctonia Solani. Eur. J. Plant Pathol. 2008, 120, 69–77. [Google Scholar] [CrossRef]
- Lay, F.T.; Anderson, M.A. Defensins–Components of the Innate Immune System in Plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Ng, T.B. Northeast Red Beans Produce a Thermostable and PH-Stable Defensin-like Peptide with Potent Antifungal Activity. Cell Biochem. Biophys. 2013, 66, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, S.C.; Kim, J.Y.; Lee, S.Y.; Lim, H.T.; Cheong, H.; Hahm, K.S.; Park, Y. Purification and Characterization of a Heat-Stable Serine Protease Inhibitor from the Tubers of New Potato Variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- Hermosa, M.R.; Turrà, D.; Fogliano, V.; Monte, E.; Lorito, M. Identification and Characterization of Potato Protease Inhibitors Able to Inhibit Pathogenicity and Growth of Botrytis Cinerea. Physiol. Mol. Plant Pathol. 2006, 68, 138–148. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and Antimicrobial Proteins and Peptides of Potato (Solanum tuberosum L.) Tubers and Their Applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B.; Rao, P.F. A Bowman-Birk-Type Trypsin-Chymotrypsin Inhibitor from Broad Beans. Biochem. Biophys. Res. Commun. 2001, 289, 91–96. [Google Scholar] [CrossRef]
- Narváez-Vásquez, J.; Ryan, C.A. The Cellular Localization of Prosystemin: A Functional Role for Phloem Parenchyma in Systemic Wound Signaling. Planta 2004, 218, 360–369. [Google Scholar] [CrossRef]
- Jones, R.W.; Ospina-Giraldo, M.; Clemente, T. Prosystemin-Antimicrobial-Peptide Fusion Reduces Tomato Late Blight Lesion Expansion. Mol. Breed. 2004, 14, 83–89. [Google Scholar] [CrossRef]
- Molisso, D.; Coppola, M.; Aprile, A.M.; Avitabile, C.; Natale, R.; Romanelli, A.; Chiaiese, P.; Rao, R. Colonization of Solanum Melongena and Vitis Vinifera Plants by Botrytis Cinerea Is Strongly Reduced by the Exogenous Application of Tomato Systemin. J. Fungi 2020, 7, 15. [Google Scholar] [CrossRef]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; García-Olmedo, F.; Molina, A. Snakin-2, an Antimicrobial Peptide from Potato Whose Gene Is Locally Induced by Wounding and Responds to Pathogen Infection. Plant Physiol. 2002, 128, 951–961. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Arafa, R.A.; Omara, R.I.; Kamel, S.M.; Ismail, W.; Ismail, S.; Derbalah, A. Developing Ag2O and Ag2O/TiO2 Nanostructures as a New Strategy for Control Late Blight of Potato Caused by Phytophthora Infestans. Physiol. Mol. Plant Pathol. 2022, 120, 101856. [Google Scholar] [CrossRef]
- Soleimani, P.; Mehrvar, A.; Michaud, J.P.; Vaez, N. Optimization of Silver Nanoparticle Biosynthesis by Entomopathogenic Fungi and Assays of Their Antimicrobial and Antifungal Properties. J. Invertebr. Pathol. 2022, 190, 107749. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc Nanoparticles: Mode of Action and Efficacy against Boscalid-Resistant Alternaria Alternata Isolates. Sci. Total Environ. 2022, 829, 154638. [Google Scholar] [CrossRef]
- Velmurugan, P.; Sivakumar, S.; Young-Chae, S.; Seong-Ho, J.; Pyoung-In, Y.; Jeong-Min, S.; Sung-Chul, H. Synthesis and Characterization Comparison of Peanut Shell Extract Silver Nanoparticles with Commercial Silver Nanoparticles and Their Antifungal Activity. J. Ind. Eng. Chem. 2015, 31, 51–54. [Google Scholar] [CrossRef]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice Leaf Extract Synthesized Silver Nanoparticles: An in Vitro Fungicidal Evaluation against Rhizoctonia Solani, the Causative Agent of Sheath Blight Disease in Rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of Silver Nanoparticles to Counter Fungicide-Resistance in Monilinia Fructicola. Sci. Total Environ. 2020, 747, 141287. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, E.M.; Jiménez, M.; Mateo, F. Antifungal Effect of Engineered Silver Nanoparticles on Phytopathogenic and Toxigenic Fusarium Spp. and Their Impact on Mycotoxin Accumulation. Int. J. Food Microbiol. 2019, 306, 108259. [Google Scholar] [CrossRef]
- Pal, S.; Singh, V.; Kumar, R.; Gogoi, R. Design and Development of 1,3,4-Thiadiazole Based Potent New Nano-Fungicides. J. Mol. Struct. 2020, 1219, 128507. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Muthukumar, S. Chitosan Guar Nanoparticle Preparation and Its in Vitro Antimicrobial Activity towards Phytopathogens of Rice. Int. J. Biol. Macromol. 2020, 153, 297–304. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Hwang, B.S.; Baek, K.H. Bacillus Velezensis: A Beneficial Biocontrol Agent or Facultative Phytopathogen for Sustainable Agriculture. Agronomy 2023, 13, 840. [Google Scholar] [CrossRef]

| Crop | Diseases | Causative Agent | Recommended Antibiotic | References |
|---|---|---|---|---|
| Maize | Banded leaf and sheath blight | R. solani | Azoxystrobin + Difenconazole, Flutolanil, Pencycuron | [50,51] |
| Southern corn leaf blight | Biopolaris maydis | Azoxystrobin + Difenconazole | [52] | |
| Sorghum downy mildew | Pernosclespora sorghi | Treat seeds with Metalaxyl + Mancozeb, Foliar spray Azoxystrobin + Difenconazole | [52] | |
| Rajasthan downy mildew | Pernosclespora heteropogoni | Treat seed with Apron 35 SD, foliar spray of Metalaxyl + Mancozeb | [52] | |
| Fusarium stalk rot | Fusarium verticillioides | Low dose of nitrogen and high dose of potassium | [52] | |
| Common rust | P. sorghi | Dithane M-45 | [52] | |
| Curvularia leaf spot | Curvularia lunata | Foliar spray with Carbendazim + Mancozeb or Zineb | [52] | |
| Turcicum leaf blight/Northern corn leaf blight | Exserohilum turcicum | Mancozeb 75 WP + Hexaconazole 5 EC + Azoxystrobin + Difenconazole | [52] | |
| Potato | Fusarium wilt | F. oxysporum | Fludioxonil | [53] |
| Late blight | Phytophthora infestans | Cyazofamid, Mandipropamid, Oxathiapiprolin + Benthiavalicarb | [54] | |
| Cercospora leaf spot | Cercospora spp. | Benzovindiflupyr + Difenoconazole, Pydiflumetofen + Difenoconazole | [55] | |
| Tomato | Fusarium wilt | F. oxysporum | Prochloraz, Bromuconazole, Benomyl, Carbendazim | [56,57] |
| Tomato leaf mold | Cladosporium fulvum | Trifloxystrobin | [58] | |
| Eggplant | Fusarium wilt | F. oxysporum | Trifloxystrobin + Tebuconazole, Difenconazole Carbendazim | [59] |
| Cercospora leaf spot | Cercospora spp. | Benzovindiflupyr + Difenoconazole, Pydiflumetofen + Difenoconazole | [55] | |
| Blackberry | Fusarium wilt | F. oxysporum | Prochloraz, Difenoconazole, Triaendazole | [60] |
| Onion | Neck rot | Botrytis allii | Fluopyram, Tebuconazole, Boscalid, Pyraclostrobin | [61] |
| Black mold | Aspergillus niger | Amphotericin B | [62] | |
| Pumpkin | Black rot | Didymella bryoniae | Chlorothalonil, Mancozeb | [63] |
| Sclerotinia rot | Sclerotinia sclerotiorum | Fludioxonil, Cyprodinil, Isopyrazam | [64,65] | |
| Sugarcane | Rhizoctonia root and crown rot | R. solani | Azoxystrobin + Difenoconazole | [50] |
| Sugarcane smut | Sporisorium scitamineum | Methoxy ethyl mercury Chloride, Zineb, Captafol, Triadimefon | [66] | |
| Wheat | Septoria leaf blotch | Z. tritici | Epoxiconazole + Metconazole, Prothioconazole + Tebuconazole | [67] |
| Yellow rust | P. striiformis | |||
| Brown rust | P. triticina | |||
| Eyespot | Oculimacula yallundae, O. acuformis | Prochloraz, Prothioconazole, Boscalid | [68] | |
| Rice | Rice blast | M. oryzae | Camptothecin | [69] |
| Soybean | Rust | P. pachyrhizi | Azoxystrobin + cyproconazole | [70] |
| Corn | Corn smut | U. maydis | There is no chemical control for this disease |
| Fungicide Class | Example | Mode of Action | References |
|---|---|---|---|
| Polyenes | Amphotericin B, Candicidin, Hamycin, Natamycin, Nystatin, Rimocidin | Binds with sterols in the fungal cell membrane, principally ergosterol, causing monovalent ion (K+, Na+, H+, and Cl−) and small organic molecule leaks | [71] |
| Allylamines | Butenafine, Naftifine, Terbinafine | Inhibits ergosterol biosynthesis by inhibiting squalene epoxidase | [45] |
| Morpholine | Aldomorph, Fenpropimorph, Dodemorph, Tridemorph | Inhibits ergosterol synthesis by blocking Erg2 and Erg24, catalyzing ∆14-reductase and ∆8-∆7-isomerase | [44,45] |
| Anilinopyrimidine | Cyprodinil, Mepanipyrim, Pyrimethanil | Inhibits methionine synthesis and secretion of hydrolytic enzymes | [72] |
| Azole | Abafungin, Bifonazole, Butoconazole, Clotrimazole, Econazole, Fenticonazole, Ketoconazole, Miconazole, Oxiconazole, Sulconazole, Albaconazole, Finaconazole, Epoxiconazole, Fluconazole, Cyproconazole, Prothioconazole, Tebuconazole, Metconazole, Propiconazole, Tebuconazole | Inhibits ergosterol biosynthesis by inhibiting 14α-demethylase | [44,45,46] |
| Echinocandins | Anidulafungin, Caspofungin, Micafungin | Inhibits 1,3-Beta-glucan synthase, thereby inhibiting the creation of glucan in the fungal cell wall | [48] |
| Others | Carboxin, Benodanil, Flutolanil, Fenfuran, Fluxapryroxad, Fluxypyram, Thifluzamide, Furametpyr | Inhibits succinate dehydrogenase and fungal respiration by binding to the ubiquinone-binding site in the complex II of mitochondria | [73] |
| Benomyl, Carbendazim, Flubendazole | Inhibits microtubule assembly | [74] | |
| Azoxystrobin, Mandestrobin, Pyraclostrobin, Kresoxim-Methyl, Dimoxystrobin, Famoxadone, Fluoxastrobin, Fenamidone, Pyribencarb | Inhibits mitochondrial respiration by binding to the quinol oxidation site of complex III | [75] |
| Type | Mechanism | Example |
|---|---|---|
| Direct Mechanisms | Apoptosis | Restrict the growth of biotrophs or hemibiotrophs in plants |
| Parasitism | Bacillus subtilis, Bacillus velezensis, Pasteuria penetrans, Trichoderma virens | |
| Cell wall degradation | Produce chitinases, glucanases, proteases | |
| Antibiotic biosynthesis | 2,4-diacetylphloroglucinol, phenazines, cyclic lipopeptides, and polyketides | |
| Competition | Exudates/leachates consumption, siderophore scavenging, physical niche occupation | |
| N2 fixation | Rhizobium (formerly Agrobacterium), Azospirillum, Azoarcus, Herbaspirillum, Cyanobacteria, Rhodobacter, Klebsiella | |
| Indirect Mechanisms | Induce hormone modulation | Cytokinin, gibberellin, salicylic acid, auxin, and ethylene |
| Growth promotion | Growth-promoting bacteria (PGPB), arbuscular mycorrhizal fungi (AMF), and rhizobia by N2 fixation, solubilizing phosphate and potassium | |
| Trigger immune system | Salicylic acid, jasmonic acid, and ethylene | |
| Adaptive Mechanisms | Drought stress | Microbial production of phytohormone, exopolysaccharides, and mineral uptake, induce stress response genes |
| Iron uptake via siderophore | Induce high-affinity Fe transport systems | |
| UV stress | Methylobacterium spp. mitigation of UV stress in mung bean | |
| Biofilm formation | Induce plant growth and protect plants from phytopathogens | |
| Carbohydrate utilization | Plant immunity and sugar recovery are activated by microbe-mediated sugar leakage | |
| Modulation in gene expression | Induce and regulate genes like CaPR-10, sHSP, P5CR, and Cadhn | |
| Induction of host resistance | Contact with fungal cell walls, detection of pathogen-associated molecular patterns, phytohormone-mediated induction |
| Plant | Pathogens | Bacterial BCAs | Mode of Action | References |
|---|---|---|---|---|
| Maize | S. sclerotiorum | B. velezensis VM11 | Produces volatile organic compounds (VOCs) reducing sclerotial production and mycelial growth | [18] |
| Tomato | S. sclerotiorum | B. amyloliquefaciens FZB42 | Produces VOCs, especially fengycin | [16] |
| Tobacco | E. cichoracearum | B. amyloliquefaciens YN201732 | Produces lipopeptides, especially bacillomycin D and fengycin | [146] |
| Banana | Fusarium spp. | B. subtilis | Biosynthesis of bioactive compounds like iturin A, bacillomycin, fengycin, surfactin, bacilysin, tasA, and mersacidin | [145] |
| Watermelon | F. oxysporum | B. subtilis IBFCBF-4 | Biosynthesis of bioactive compounds like iturin A, bacillomycin, fengycin, surfactin, bacilysin, tasA, and mersacidin | [142] |
| Tabaco | S. sclerotiorum | B. amyloliquefaciens EZ1509 | Produces lipopeptides, especially bacillomycin D and fengycin | [143] |
| Arabidopsis | A. alternata | B. amyloliquefaciens, B. subtilis | Produces lipopeptides, especially bacillomycin D and fengycin | [148] |
| Potato, Tomato | F. oxysporum | |||
| Maize | F. verticillioides | |||
| Mustard | S. sclerotiorum | B. thuringiensis | Produce various VOCs | [149] |
| Pumpkin | S. rolfsii | Serratia marcescens | Possesses chitinolytic activity by releasing chitinase | [150] |
| Maize | Pantoea ananatis DZ-12 | Pseudomonas protegens Pf-5 | Polyketide pyoluteorin-produced reactive oxygen species and lipopeptide orfamide A | [151] |
| Stone fruit | M. fructicola, M. fructigena | Pseudomonas synxantha | Product-diffusible toxic metabolites and VOCs | [152] |
| Rice | M. oryzae | P. putida BP25 | 2-methylpyrazine and 2-ethyl-3,6-dimethylpyrazine produced by BP-25 inhibits the growth of M. oryzae | [140] |
| Plant | Pathogens | Disease | Fungal BCAs | Mode of Action | References |
|---|---|---|---|---|---|
| Onion | Sclerotium cepivorum | White rot | T. asperellum | Activate salicylic acid-dependent defense pathways (1 and 7 days), and ethylene-dependent defense pathways (at 21 days) | [156] |
| Bean | S. sclerotiorum | White mold | T. harzianum ESALQ-1306, T. asperellum BRM-29104 | [157] | |
| Lettuce | Sclerotium sp. | Steam rot | Trichoderma sp. T76-12/2 | Produce β-1,3-glucanase | [158] |
| Maize | F. graminearum, F. verticillioides | Stalk rot, ear rot | T. asperellum | Accumulate of deoxynivalenol and fumonisin B1 decreased in the stem and ear of maize | [159] |
| Apple | A. alternata | Fusarium wilt | T. rossicum, T. harzianum | Increase jasmonic acid content and accelerate soil metabolic processes of plants | [160] |
| Fusarium spp. | |||||
| Cytospora mandshurica | |||||
| Garlic | S. sclerotiorum | White rot | Candida labiduridarum (Yeast) | Produce VOCs | [161] |
| Groundnut | A. niger | Collar rot | T. viride | Elevate activities of the antioxidant enzymes, viz., superoxide dismutases, guaiacol peroxidase, and ascorbate peroxidase, in response to pathogen infection in treated plants | [162] |
| Mango | Colletrotrichum gloeosporiodes | Anthracnose | T. asperellum T8a | Produce lytic enzymes cellulases, chitinase, and glucanase | [163] |
| Plant | Pathogens | Gene Product | Disease | References |
|---|---|---|---|---|
| Arabidopsis | Erysiphe orontii | Receptor-like kinase | Powdery mildew | [169] |
| Alternaria brassicicola, B. cinerea | Expansin | Gray mold/rot, leaf spot | [170] | |
| Golovinomyces orontii | Membrane-attached protein | Powdery mildew | [171] | |
| Hyaloperonospora arabidopsidis | ADP ribosylation factor—GTPase activating factor | Downy mildew | [172] | |
| Maize | Bipolaris maydis/Cochliobolus heterostrophus | Mitochondrial transmembrane protein | Southern corn leaf blight | [172] |
| B. graminis | Long-chain aldehyde synthesis | Powdery mildew | [173] | |
| Tomato | B. cinerea | Polygalacturonase and expansin | Gray mold/rot | [173] |
| ABA aldehyde oxidase | [174] | |||
| Leveillula taurica | Membrane-anchored protein | Powdery mildew | [175] | |
| F. oxysporum | Lipid transfer protein | Fusarium wilt | [176] | |
| Rice | M. oryzae | Transcription factor WRKY | Rice blast | [177,178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants 2024, 13, 2737. https://doi.org/10.3390/plants13192737
Islam T, Danishuddin, Tamanna NT, Matin MN, Barai HR, Haque MA. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants. 2024; 13(19):2737. https://doi.org/10.3390/plants13192737
Chicago/Turabian StyleIslam, Tarequl, Danishuddin, Noshin Tabassum Tamanna, Muhammad Nurul Matin, Hasi Rani Barai, and Md Azizul Haque. 2024. "Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions" Plants 13, no. 19: 2737. https://doi.org/10.3390/plants13192737









