Differences in Physicochemical Properties of Stems in Oat (Avena sativa L.) Varieties with Distinct Lodging Resistance and Their Regulation of Lodging at Different Planting Densities
Abstract
:1. Introduction
2. Results
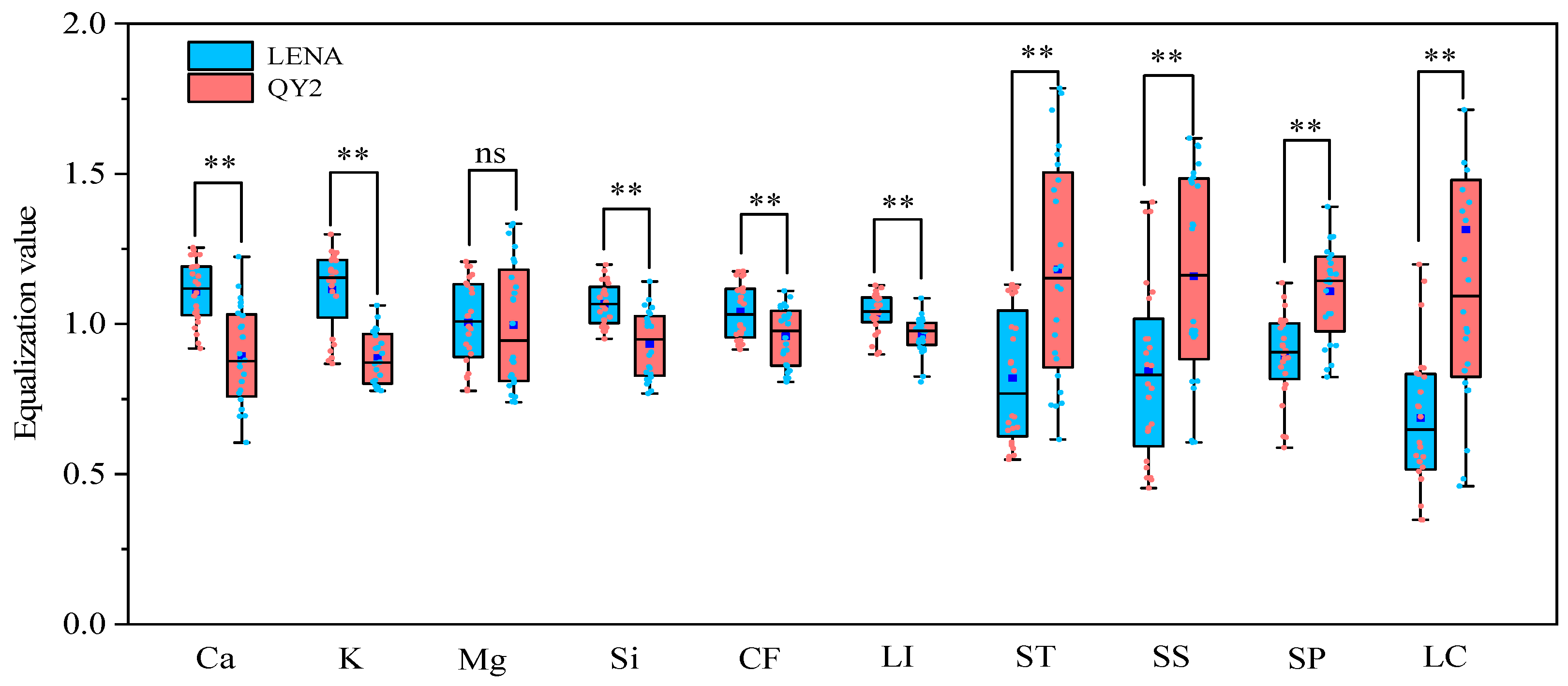
2.1. Differences Analysis between Two Varieties with Different Lodging Resistance
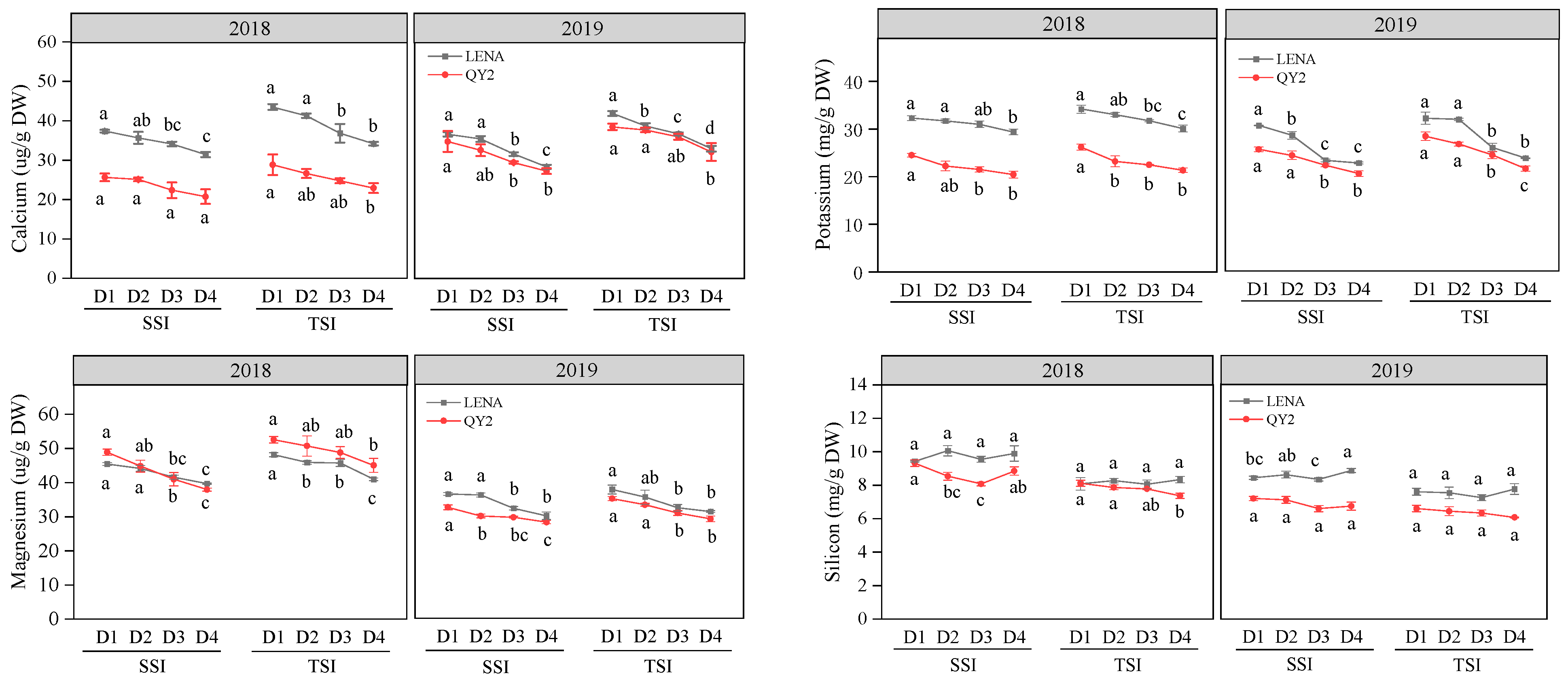
2.2. Changes of Oat Stem Physicochemical Properties and Lodging Coefficient under Different Planting Densities
2.3. Effect of Planting Density and Variety on Stem Physicochemical Properties and Lodging Coefficient
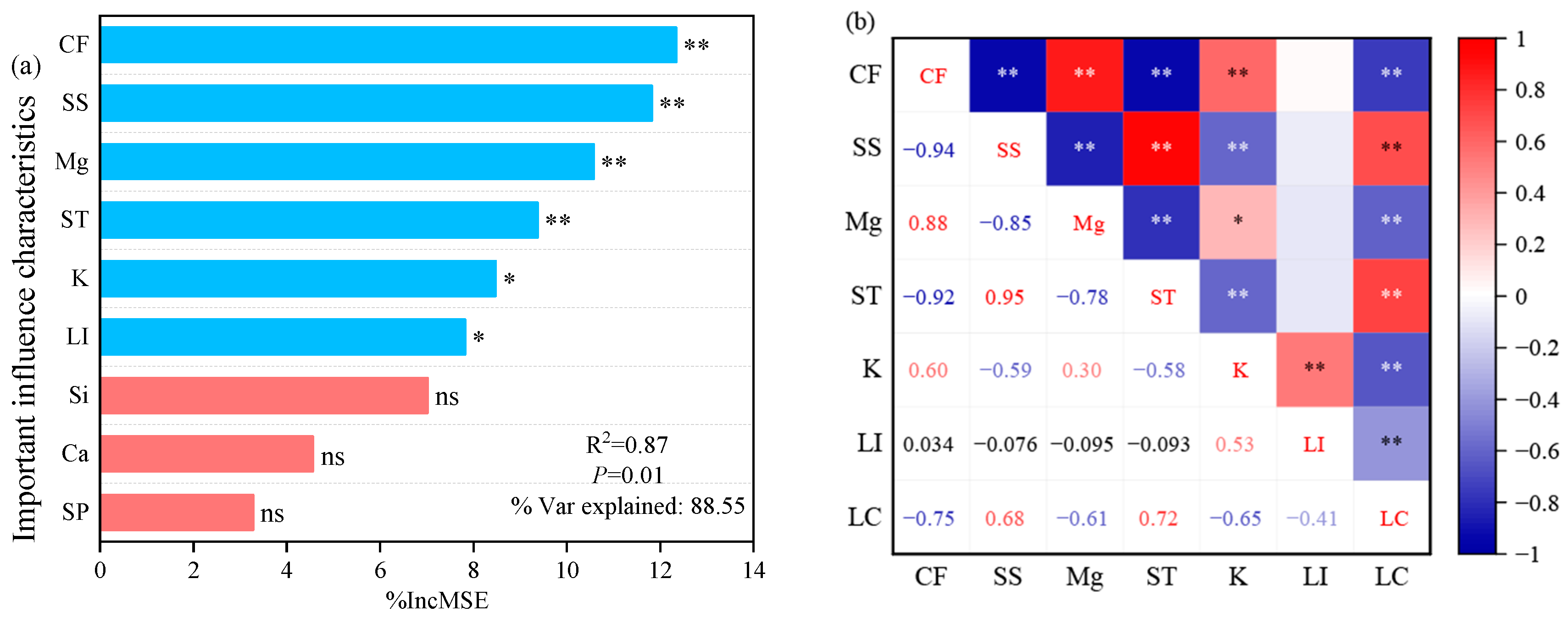
2.4. Effect of Stem Physicochemical Properties on Lodging Coefficient
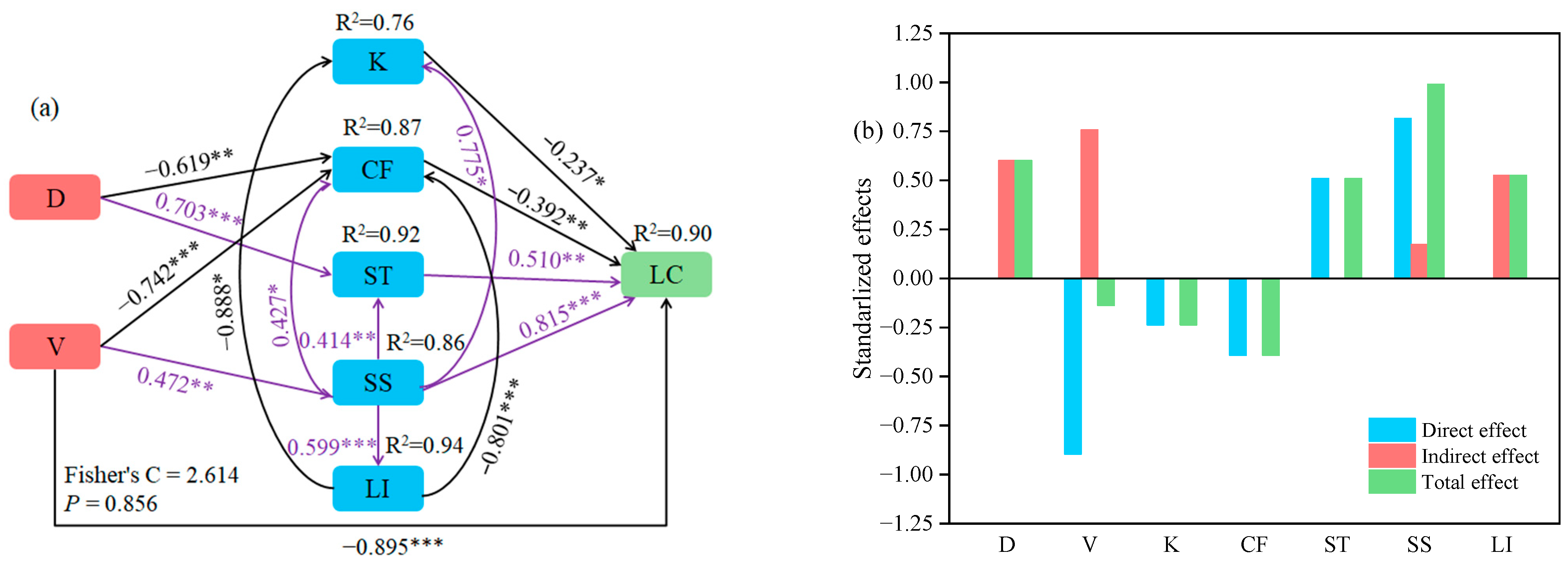
2.5. Relationships between Variety, Planting Density, Physicochemical Properties and Lodging Coefficient
2.6. Reasonable Planting Density for Oat Forage Production
3. Discussion
3.1. Variety Differences and Density Gradient Variations in Oat Stem Traits
3.2. Relationships among Variety, Planting Density, Stem Physicochemical Properties and Lodging Coefficient
3.3. Optimal Planting Density for Oat
4. Materials and Methods
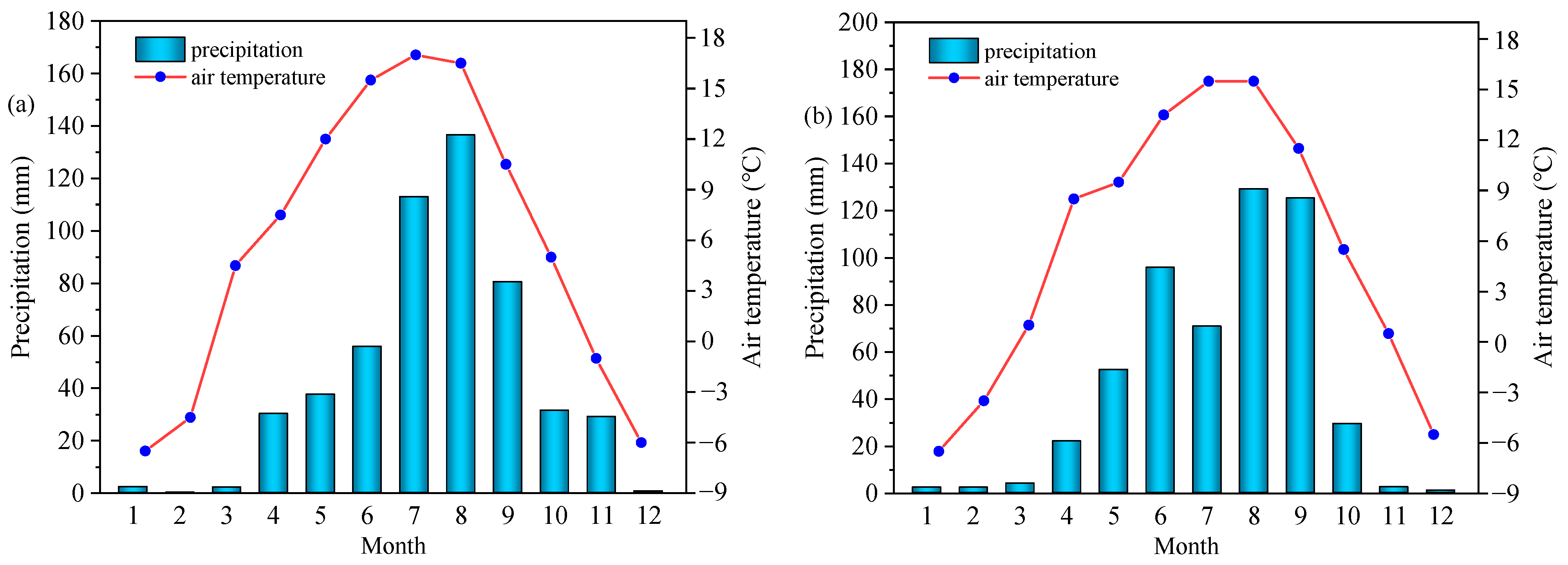
4.1. Experimental Site
4.2. Experimental Design and Field Management
4.3. Plant Sampling and Measurements
4.3.1. Main Agronomic Traits and Lodging Coefficient
4.3.2. Physicochemical Indicators
Determination of Mineral Element Content
Determination of Crude Fiber and Lignin Contents
Determination of Starch and Soluble Sugar Contents
Determination of Soluble Protein Content
4.4. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muhammad, U.H.; Gurmani, Z.A.; Khan, S.; Bakhsh, A. PARC Oat: A new Lodging-resistant, Late-maturing and High-yielding Variety of Oats (Avena sativa L.) for Pakistan. Pak. J. Agric. Res. 2020, 33, 601. [Google Scholar] [CrossRef]
- Mohammadi, M.; Finnan, J.; Sterling, M.; Baker, C. A calibrated oat lodging model compared with agronomic measurements. Field Crops Res. 2020, 255, 107784. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, T.; Zhao, C.; Zhou, M. Lodging prevention in cereals: Morphological, biochemical, anatomical traits and their molecular mechanisms, management and breeding strategies. Field Crops Res. 2022, 289, 108733. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Rehman, A.U.; Anjum, S.A.; Iqbal, J.; Ahmad, R. Lodging stress in cereal—Effects and management: An overview. Environ. Sci. Pollut. Res. 2017, 24, 5222–5237. [Google Scholar] [CrossRef] [PubMed]
- Weibel, R.O.; Pendleton, J.W. Effect of artificial lodging on winter wheat grain yield and quality. Agron. J. 1964, 56, 487–488. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L. Erect–leaf posture promotes lodging resistance in oat plants under high plant population. Eur. J. Agron. 2019, 103, 175–187. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, T.; Zhao, C.; Zhou, M. Improving crop lodging resistance by adjusting plant height and stem strength. Agronomy 2021, 11, 2421. [Google Scholar] [CrossRef]
- Welton, F.A. Lodging in oats and wheat. Bot. Gaz. 1928, 85, 121–151. [Google Scholar] [CrossRef]
- Liu, W.G.; Hussain, S.; Liu, T.; Zou, J.L.; Ren, M.L.; Zhou, T.; Yang, W.Y. Shade stress decreases stem strength of soybean through restraining lignin biosynthesis. J. Integr. Agric. 2019, 18, 43–53. [Google Scholar] [CrossRef]
- Zou, J.L.; Liu, W.G.; Yuan, J.; Jiang, T.; Yang, W.Y. Relationship between Lignin Synthesis and Lodging Resistance at Seedlings Stage in Soybean Intercropping System. Acta Agron. Sin. 2015, 41, 1098. [Google Scholar] [CrossRef]
- Liu, W.G.; Yang, W.Y.; Luo, L.; Yuan, X.Q.; Deng, Y.C. Relationship between cellulose accumulation and lodging resistance in the stem of relay intercropped soybean [Glycine max (L.) Men.]. Field Crops Res. 2016, 196, 261–267. [Google Scholar] [CrossRef]
- Ishimaru, K.; Togawa, E.; Ookawa, T.; Kashiwagi, T.; Madoka, Y.; Hirotsu, N. New target for rice lodging resistance and its effect in a typhoon. Planta 2008, 227, 601–609. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Madoka, Y.; Hirotsu, N.; Ishimaru, K. Locus prl5 improves lodging resistance of rice by delaying senescence and increasing carbohydrate reaccumulation. Plant Physiol. Biochem. 2006, 44, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-g.; Wen, B.-x.; Tao, Z.; Li, W.; Yang, G.; Li, S.-x.; Qin, S.-s.; Jiang, L.; Yang, W.-y. iTRAQ protein profile analysis of soybean stems reveals new aspects critical for lodging in intercropping systems. J. Integr. Agric. 2019, 18, 2029–2040. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Wang, F.; Liu, J.; Feng, B.; Si, J.; Zhang, B.; Li, S.; Li, H. Effects of high NH+4 on K+ uptake, culm mechanical strength and grain filling in wheat. Front. Plant Sci. 2014, 5, 703. [Google Scholar] [CrossRef] [PubMed]
- Demarty, M.; Morvan, C.; Thellier, M. Calcium and the cell wall. Plant Cell Environ. 1984, 7, 441–448. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Dorairaj, D.; Ismail, M.R.; Sinniah, U.R.; Kar Ban, T. Influence of silicon on growth, yield, and lodging resistance of MR219, a lowland rice of Malaysia. J. Plant Nutr. 2017, 40, 1111–1124. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Chen, L.; Wang, X.; Wang, R.; Ma, L.; Peng, S.; Luo, J.; Chen, Y. Combined drought and heat stress in Camellia oleifera cultivars: Leaf characteristics, soluble sugar and protein contents, and Rubisco gene expression. Trees 2015, 29, 1483–1492. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Yang, X.N.; Meng, X.P.; Yang, B.P.; Liu, T.; Han, Q.F. Effect of uniconazole and nitrogen level on lodging resistance and yield potential of maize under medium and high plant density. Plant Biol. 2021, 23, 485–496. [Google Scholar] [CrossRef]
- Sparkes, D.; Berry, P.; King, M. Effects of shade on root characters associated with lodging in wheat (Triticum aestivum). Ann. Appl. Biol. 2008, 152, 389–395. [Google Scholar] [CrossRef]
- Xiang, D.B.; Zhao, G.; Wan, Y.; Tan, M.L.; Song, C.; Song, Y. Effect of planting density on lodging-related morphology, lodging rate, and yield of tartary buckwheat (Fagopyrum tataricum). Plant Prod. Sci. 2016, 19, 479–488. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Zhou, G.S. Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.M.; Li, Y.S.; Nie, J.; Wang, C.; Huang, K.H.; Zhang, Y.K.; Ruan, R.W. Effects of nitrogen fertilizer and planting density on the leaf photosynthetic characteristics, agronomic traits and grain yield in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2018, 219, 160–168. [Google Scholar] [CrossRef]
- Williams, M.; Hausman, N.E.; Dhaliwal, D.S.; Bohn, M. Economic optimum plant density of sweet corn does not increase root lodging incidence. Crop Sci. 2021, 61, 3637–3646. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, Y.; Jiao, F.; Wang, M.; Zhang, J.; Zhang, Z.; Huang, Y.; Jia, X.; Zhu, L.; Zhao, Y. Identification of quantitative trait loci for related traits of stalk lodging resistance using genome-wide association studies in maize (Zea mays L.). BMC Genom. Data 2022, 23, 76. [Google Scholar] [CrossRef]
- Bhagat, K.P.; Sairam, R.; Deshmukh, P.; Kushwaha, S. Biochemical analysis of stem in lodging tolerant and susceptible wheat (Triticum aestivum L.) genotypes under normal and late sown conditions. Indian J. Plant Physiol. 2011, 16, 68–74. [Google Scholar]
- Deng, Y.; Liu, W.; Yuan, X.; Yuan, J.; Zou, J.; Du, J.; Yang, W. Relationship between cellulose synthesis metabolism and lodging resistance in intercropping soybean at seedling stage. J. Appl. Ecol. 2016, 27, 469–476. [Google Scholar] [PubMed]
- Ruuska, S.A.; Rebetzke, G.J.; Van Herwaarden, A.F.; Richards, R.A.; Fettell, N.A.; Tabe, L.; Jenkins, C.L. Genotypic variation in water-soluble carbohydrate accumulation in wheat. Funct. Plant Biol. 2006, 33, 799–809. [Google Scholar] [CrossRef]
- Slewinski, T.L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Croy, L.I.; Hageman, R. Relationship of Nitrate Reductase Activity to Grain Protein Production in Wheat. Crop Sci. 1970, 10, 280–285. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Ding, Y.; Yao, X.; Wu, X.; Weng, F.; Li, G.; Liu, Z.; Tang, S.; Ding, C. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Liu, H.; Ahmad, A.; Xiang, L.; Ali, W.; Khan, A.; Kamran, M.; Ahmad, S.; Li, J. Impact of nitrogen regimes and planting densities on stem physiology, lignin biosynthesis and grain yield in relation to lodging resistance in winter wheat (Triticum aestivum L.). Cereal Res. Commun. 2019, 47, 566–579. [Google Scholar] [CrossRef]
- Xue, J.; Gou, L.; Zhao, Y.; Yao, M.; Yao, H.; Tian, J.; Zhang, W. Effects of light intensity within the canopy on maize lodging. Field Crops Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Cheng, Y.; Feng, L.; Wu, X.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chang, Y.; Luo, Y.; Li, W.; Jin, M.; Wang, Y.; Cui, H.; Sun, S.; Li, Y.; Wang, Z. Nitrogen regulates stem lodging resistance by breaking the balance of photosynthetic carbon allocation in wheat. Field Crops Res. 2023, 296, 108908. [Google Scholar] [CrossRef]
- Knapp, J.S.; Harms, C.L.; Volenec, J.J. Growth Regulator Effects on Wheat Culm Nonstructural and Structural Carbohydrates and Lignin. Crop Sci. 1987, 27, 1201–1205. [Google Scholar] [CrossRef]
- Luo, Y.L.; Chang, Y.L.; Li, C.H.; Wang, Y.Y.; Cui, H.X.; Jin, M.; Li, Y. Shading decreases lodging resistance of wheat under different planting densities by altering lignin monomer composition of stems. Front. Plant Sci. 2022, 13, 1056193. [Google Scholar] [CrossRef]
- Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Liu, W.G. Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem. Agronomy 2020, 10, 1177. [Google Scholar] [CrossRef]
- Marafon, A.C.; Endres, L. Silicon: Fertilization and nutrition in higher plants. Amaz. J. Agric. Environ. Sci. 2013, 56, 380–388. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, K.W.; Park, E.W.; Choi, D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathology 2002, 92, 1095–1103. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Li, S.; Chu, X.; Ye, Y.J. Improving the growth, lodging and yield of different densityresistance maize by optimising planting density and nitrogen fertilisation. Plant Soil Environ. 2020, 66, 453–460. [Google Scholar] [CrossRef]
- Shi, D.; Li, Y.; Zhang, J.; Liu, P.; Zhao, B.; Dong, S. Effects of plant density and nitrogen rate on lodging-related stalk traits of summer maize. Plant Soil. Environ. 2016, 62, 299–306. [Google Scholar] [CrossRef]
- Liu, X.M.; Gu, W.R.; Li, C.F.; Jing, L.; Shi, W. Effects of nitrogen fertilizer and chemical regulation on spring maize lodging characteristics, grain filling and yield formation under high planting density in Heilongjiang Province, China. J. Integr. Agric. 2021, 20, 511–526. [Google Scholar] [CrossRef]
- Tian, B.; Wang, J.; Zhang, L.; Li, Y.; Wang, S.; Li, H. Assessment of resistance to lodging of landrace and improved cultivars in foxtail millet. Euphytica 2010, 172, 295–302. [Google Scholar] [CrossRef]
- Dos Santos, É.J.; Baika, L.M.; Herrmann, A.B.; Kulik, S.; Sato, C.S.; Santos, A.B.d.; Curtius, A.J. Fast assessment of mineral constituents in grass by inductively coupled plasma optical emission spectrometry. Braz. Arch. Biol. Technol. 2012, 55, 457–464. [Google Scholar] [CrossRef]
- Barros, J.A.; de Souza, P.F.; Schiavo, D.; Nóbrega, J.A. Microwave-assisted digestion using diluted acid and base solutions for plant analysis by ICP OES. J. Anal. At. Spectrom. 2016, 31, 337–343. [Google Scholar] [CrossRef]
- Zakirullah, M.; Ali, N.; Jan, T.; Aka Khil, H.; Ikramullah, M. Effect of different nitrogen levels and cutting stages on crude protein, crude fiber, dry matter and green fodder yield of oat (Avena sativa L.). Pure Appl. Biol. 2017, 6, 448–453. [Google Scholar] [CrossRef]
- Brinkmann, K.; Blaschke, L.; Polle, A. Comparison of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J. Chem. Ecol. 2002, 28, 2483–2501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jia, Z.; Ma, X.; Ma, H.; Zhao, Y. Characterising the morphological characters and carbohydrate metabolism of oat culms and their association with lodging resistance. Plant Biol. 2020, 22, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Delaplace, P.; van der Wal, F.; Dierick, J.F.; Cordewener, J.H.; Fauconnier, M.L.; Du Jardin, P.; America, A.H. Potato tuber proteomics: Comparison of two complementary extraction methods designed for 2-DE of acidic proteins. Proteomics 2006, 6, 6494–6497. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

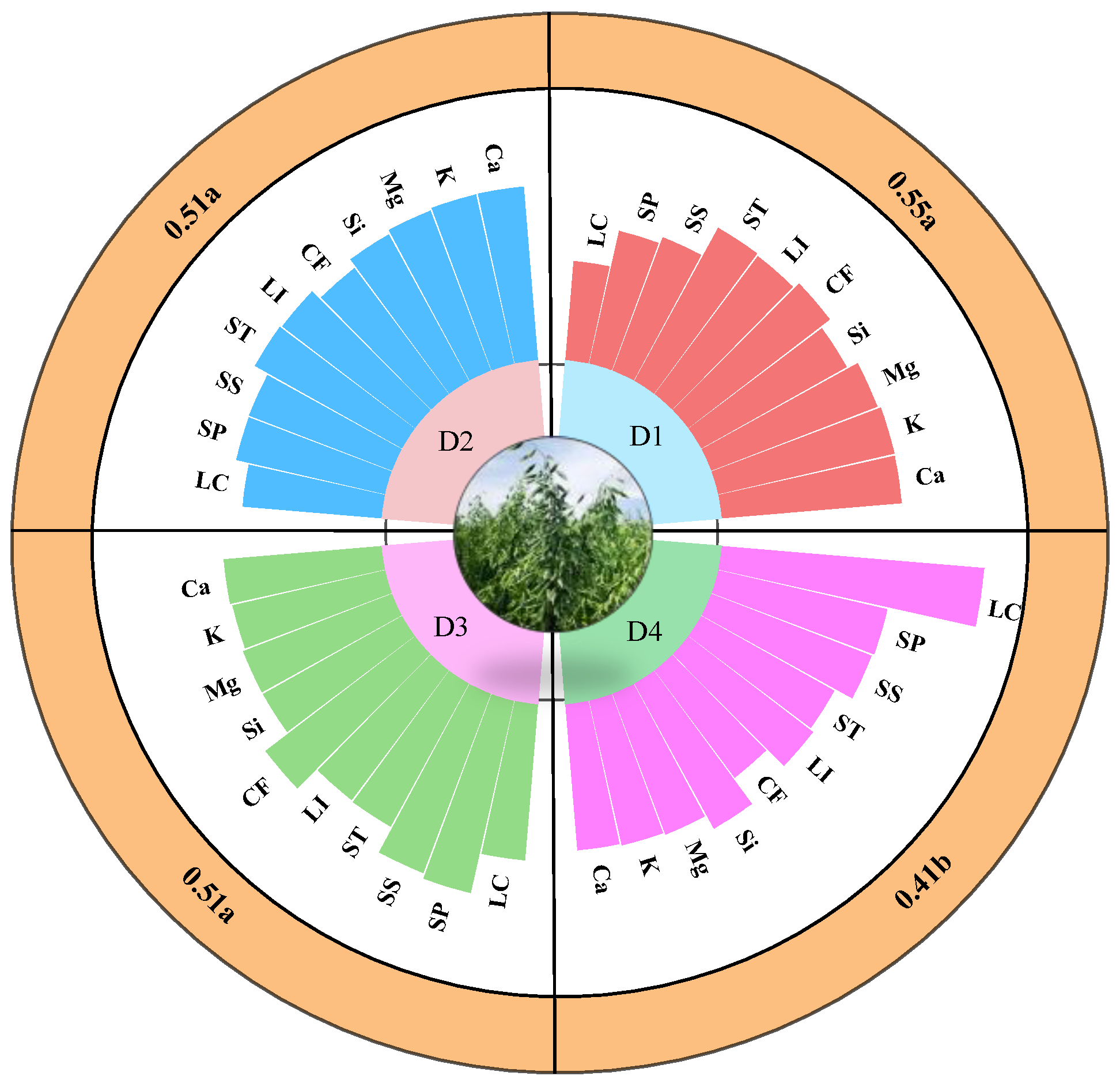
| Characteristics | Variety Main Effect | Density Main Effect | Variety * Density | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Partial η2 | F | p | Partial η2 | F | p | Partial η2 | |
| Ca | 277.674 | <0.001 | 0.897 | 55.920 | <0.001 | 0.840 | 0.981 | 0.414 | 0.084 |
| K | 629.901 | <0.001 | 0.952 | 103.738 | <0.001 | 0.907 | 3.005 | 0.045 | 0.220 |
| Mg | 0.589 | 0.449 | 0.018 | 45.487 | <0.001 | 0.810 | 0.266 | 0.849 | 0.024 |
| Si | 176.040 | <0.001 | 0.846 | 3.720 | 0.021 | 0.259 | 5.151 | 0.005 | 0.326 |
| CF | 347.593 | <0.001 | 0.916 | 75.573 | <0.001 | 0.876 | 0.978 | 0.415 | 0.084 |
| LI | 132.930 | <0.001 | 0.806 | 75.938 | <0.001 | 0.877 | 0.288 | 0.834 | 0.026 |
| ST | 1067.410 | <0.001 | 0.971 | 137.716 | <0.001 | 0.928 | 37.316 | <0.001 | 0.778 |
| SS | 3826.877 | <0.001 | 0.992 | 965.997 | <0.001 | 0.989 | 67.080 | <0.001 | 0.863 |
| SP | 436.853 | <0.001 | 0.932 | 206.239 | <0.001 | 0.951 | 6.483 | 0.001 | 0.378 |
| LC | 571.908 | <0.001 | 0.947 | 267.687 | <0.001 | 0.962 | 91.805 | <0.001 | 0.896 |
| D | Simple Effects of Variety | V | Simple Effects of Density | ||||
|---|---|---|---|---|---|---|---|
| F | p | Partial η2 | F | p | Partial η2 | ||
| D1 | 1.269 | 0.267 | 0.031 | LENA | 1.382 | 0.262 | 0.094 |
| D2 | 2.874 | 0.098 | 0.067 | QY2 | 18.747 | <0.001 | 0.584 |
| D3 | 5.519 | 0.024 | 0.121 | ||||
| D4 | 37.784 | <0.001 | 0.486 | ||||
| Variety | Characteristics |
|---|---|
| LENA | Plant height 100–115 cm, medium-late maturity, panicle type is scattered, 2–5 tillers |
| QY2 | Plant height 120–140 cm, early maturity, panicle type is scattered, 2–3 tillers |
| Parameter | Condition |
|---|---|
| Forward power | 1.2 kW |
| Plasma gas flow rate | 10 L/min |
| Auxiliary gas flow rate | 0.6 L/min |
| Carrier gas flow rate | 0.7 L/min |
| Instrument stabilization delay | 15 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liang, G.; Liu, W. Differences in Physicochemical Properties of Stems in Oat (Avena sativa L.) Varieties with Distinct Lodging Resistance and Their Regulation of Lodging at Different Planting Densities. Plants 2024, 13, 2739. https://doi.org/10.3390/plants13192739
Liu L, Liang G, Liu W. Differences in Physicochemical Properties of Stems in Oat (Avena sativa L.) Varieties with Distinct Lodging Resistance and Their Regulation of Lodging at Different Planting Densities. Plants. 2024; 13(19):2739. https://doi.org/10.3390/plants13192739
Chicago/Turabian StyleLiu, Lingling, Guoling Liang, and Wenhui Liu. 2024. "Differences in Physicochemical Properties of Stems in Oat (Avena sativa L.) Varieties with Distinct Lodging Resistance and Their Regulation of Lodging at Different Planting Densities" Plants 13, no. 19: 2739. https://doi.org/10.3390/plants13192739






