Bioactive Compounds and Valorization of Coffee By-Products from the Origin: A Circular Economy Model from Local Practices in Zongolica, Mexico
Abstract
1. Introduction
2. Results and Discussion
2.1. Traditional and Local Practices of Reuse of By-Products in Sierra de Zongolica
2.2. Soluble Bioactive Compounds in Coffee Cherry Cascara by HPLC-ESI-HRMS
2.3. Rapid Analysis of Caffeine and Other Compounds in Coffee By-Products by DART-MS
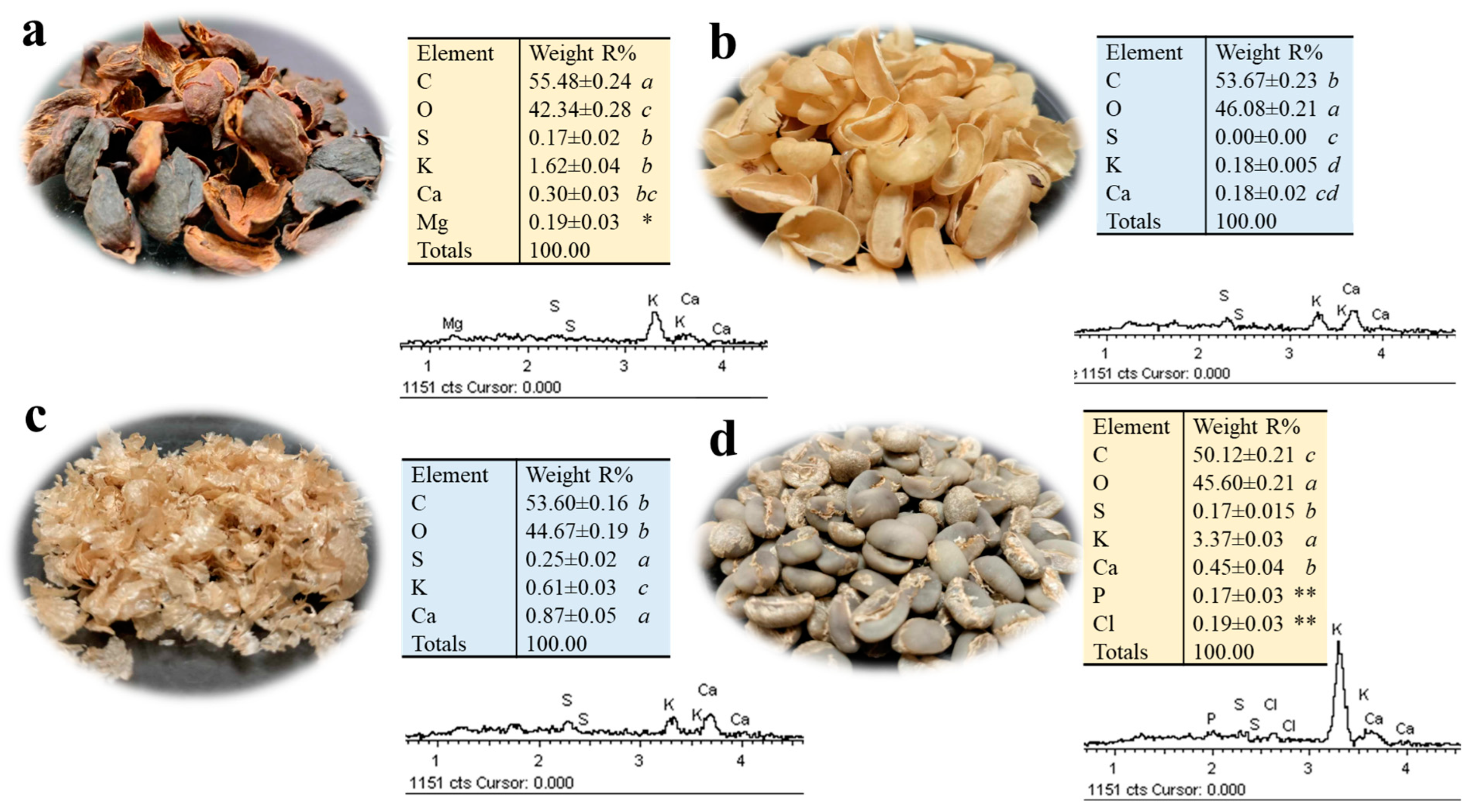
2.4. Mineral Content of Green Coffee and By-Products of Primary Coffee Processing
3. Materials and Methods
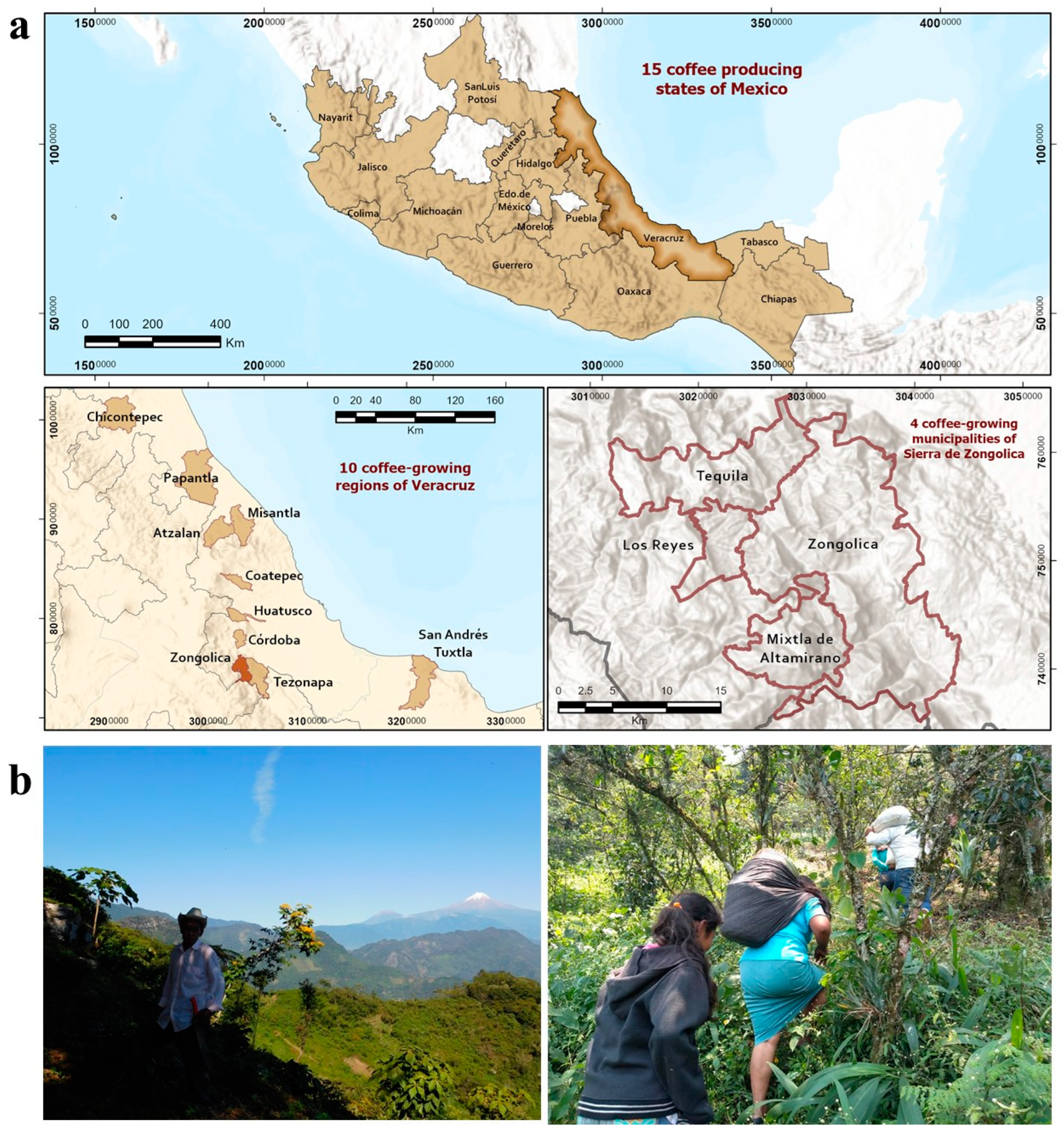
3.1. Ethnographic Study Area
3.2. Plant Material
3.3. Sample Preparation
3.4. Extraction and Analysis of Polar and Semi-Polar Metabolome of Cascara by HPLC-ESI (+/−)-HRMS
3.5. Aqueous Extraction of Coffee By-Products and Green Coffee
3.6. DART Mass Spectrometry of Aqueous Extractions of By-Products and Green Coffee
3.7. SEM-EDS Mineral Microanalysis Method
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xotlanihua-Flores, D.; Crespo-Stupková, L. Exportaciones del café mexicano a los mercados estadounidense y alemán. RIVAR 2024, 11, 150–169. [Google Scholar] [CrossRef]
- FAO. Food Outlook—Biannual Report on Global Food Markets; Rome, Italy, 2023. [Google Scholar] [CrossRef]
- International Coffee Organization (ICO). December 2023 Coffee Report Outlook; London, UK, 2023; pp. 1–43. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 13 September 2024).
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silverskin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of compounds from coffee processing by-products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Serna-Jiménez, J.A.; Siles, J.A.; de los Ángeles Martín, M.; Chica, A.F. A Review on the applications of coffee waste derived from primary processing: Strategies for revalorization. Processes 2022, 10, 2436. [Google Scholar] [CrossRef]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-added metabolites from agricultural waste and application of green extraction techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Oliveira, M.B.P.P.; Ferreira, H.; Alves, R.C. Bioactive potential and chemical composition of coffee by-products: From pulp to silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef]
- Valdés, A.; Álvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Foodomics: Analytical opportunities and challenges. Anal. Chem. 2022, 94, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Heeger, A.; Kosinka-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilization for production of cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Machado, M.; Ferreira, H.; Oliveira, M.B.P.P.; Alves, R.C. Coffee by-products: An underexplored source of prebiotic ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 7181–7200. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The potential of spent coffee grounds in functional food development. Nutrients 2023, 15, 994. [Google Scholar] [CrossRef]
- Pua, A.; Desmond Choo, W.X.; Vivian Goh, R.M.; Liu, S.Q.; Cornuz, M.; Ee, K.-H.; Sun, J.; Lassabliere, B.; Yu, B. A systematic study of key odourants, non-volatile compounds, and antioxidant capacity of cascara (dried Coffea arabica pulp). LWT-Food Sci. Technol. 2021, 138, 110630. [Google Scholar] [CrossRef]
- DePaula, J.; Cunha, S.C.; Cruz, A.; Sales, A.L.; Revi, I.; Fernandes, J.; Ferreira, I.M.; Miguel, M.A.; Farah, A. Volatile fingerprinting and sensory profiles of coffee cascara teas produced in Latin American countries. Foods 2022, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-G.; Cho, E.-J.; Maskey, S.; Nguyen, D.-T.; Bae, H.-J. Value-added products from coffee waste: A review. Molecules 2023, 28, 3562. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Gil-Ramírez, A.; Martin-Trueba, M.; Benítez, V.; Aguilera, Y.; Martín-Cabrejas, M.A. Valorization of coffee pulp as bioactive food ingredient by sustainable extraction methodologies. Curr. Res. Food Sci. 2023, 6, 100475. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee by-products and their suitability for developing active food packaging materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Barbero-López, A.; Monzó-Beltrán, J.; Virjamo, V.; Akkanen, J.; Antti Haapala, A. Revalorization of coffee silverskin as a potential feedstock for antifungal chemicals in wood preservation. Int. Biodeterior. Biodegrad. 2020, 152, 105011. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green coffee infusion as a source of caffeine and chlorogenic acid. J. Food Compos. Anal. 2019, 84, 103307. [Google Scholar] [CrossRef]
- Al-Rooqi, M.M.; Mughal, E.U.; Raja, Q.A.; Obaid, R.J.; Sadiq, A.; Naeem, N.; Qurban, J.; Asghar, B.H.; Moussa, Z.; Ahmed, S.A. Recent advancements on the synthesis and biological significance of pipecolic acid and its derivatives. J. Mol. Struct. 2022, 1268, 133719. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; del Castillo, M.D. Assessment of healthy and harmful maillard reaction products in a novel coffee cascara beverage: Melanoidins and acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.N.; Singh, R.B.; Buttar, H.S. Biochemical and dietary functions of tryptophan and its metabolites in human health. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press, 2022; pp. 783–798. [Google Scholar] [CrossRef]
- Cardoso, W.S.; Rodrigues Dias, S.; Coelho, V.S.; Louzada Pereira, L.; Bassini Fioresi, D.; Flávia de Abreu Pinheiro, F. Maillard reaction precursors and arabica coffee (Coffea arabica L.) beverage quality. Food Humanit. 2023, 1, 1–7. [Google Scholar] [CrossRef]
- Farah, A. Coffee constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.-F., Ed.; Wiley: Chichester, UK, 2012; pp. 21–58. [Google Scholar] [CrossRef]
- Hall, R.D.; Trevisan, F.; de Vos, R.C.H. Coffee berry and green bean chemistry—Opportunities for improving cup quality and crop circularity. Food Res. Int. 2022, 151, 110825. [Google Scholar] [CrossRef]
- Silva, A.C.R.; Garrett, R.; Rezende, C.M. A workflow for llipid annotation in coffee samples by liquid chromatography-mass spectrometry. In Mass Spectrometry for Food Analysis. Methods and Protocols in Food Science; Koolen, H., Ed.; Humana: New York, NY, USA, 2022; Garrett, R.; pp. 71–87. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, L.; Zhao, M.; Wei, F.; Fu, H.; Marchioni, E. Reveling the dynamic changes of lipids in coffee beans during roasting based on UHPLC-QE-HR-AM/MS/MS. Food Res. Int. 2023, 174, 113507. [Google Scholar] [CrossRef] [PubMed]
- Górnas, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef]
- Sun, N.; Chen, J.; Di, W.; Lin, S. Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities. Trends Food Sci. Technol. 2018, 80, 199–211. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 15, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Yeager, S.E.; Batali, M.E.; Guinard, J.X.; Ristenpart, W.D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2023, 63, 1010–1036. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Yang, X.; Hong, J.; Wang, L.; Cai, C.; Mo, H.; Wang, J.; Fang, X.; Liao, Z. Effect of lactic acid bacteria fermentation on plant-based products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, T.; Tsai, T. Lactic acid bacteria-fermented product of green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. J. Food Drug Anal. 2018, 26, 12. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- Martínez, S.J.; Bressani, A.P.P.; Dias, D.R.; Simão, J.B.P.; Schwan, R.F. Effect of bacterial and yeast starters on the formation of volatile and organic acid compounds in coffee beans and selection of flavors markers precursors during wet fermentation. Front. Microbiol. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Appelblad, P.; Gazzani, G.; Papetti, A. Determination of free quinic acid in food matrices by hydrophilic interaction liquid chromatography with UV detection. J. Food Compos. Anal. 2015, 44, 80–85. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Carazzone, C.; Stauder, M.; Spratt, D.A.; Wilson, M.; Pratten, J.; Ciric, L.; Lingström, P.; Zaura, E.; et al. Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria. Food Chem. 2013, 138, 1706–1712. [Google Scholar] [CrossRef]
- Heena; Kaushal, S.; Kaur, V.; Panwar, H.; Sharma, P.; Jangra, R. Isolation of quinic acid from dropped Citrus reticulata Blanco fruits: Its derivatization, antibacterial potential, docking studies, and ADMET profiling. Front. Chem. 2024, 12, 1372560. [Google Scholar] [CrossRef] [PubMed]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of polyphenolic fraction from Arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Ahmad, H.; Maher, M.; Fang, C.; Luo, J. Benefiting others and self: Production of vitamins in plants. J. Integr. Plant. Biol. 2021, 63, 210–227. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Domínguez, L.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.A. Review of foods of plant origin as sources of vitamins with proven activity in oxidative stress prevention according to EFSA scientific evidence. Molecules 2023, 28, 7269. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 15, 131188. [Google Scholar] [CrossRef] [PubMed]
- Rungraung, N.; Muangpracha, N.; Trachootham, D. Twelve-week safety and potential lipid control efficacy of coffee cherry pulp juice concentrate in healthy volunteers. Nutrients 2023, 15, 1602. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Laramee, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Riddellova, K.; Tomaniova, M.; Hajslova, J. Ambient mass spectrometry employing a DART ion source for metabolomic fingerprinting/profiling: A powerful tool for beer origin recognition. Metabolomics 2011, 7, 500–508. [Google Scholar] [CrossRef]
- Danhelova, H.; Hradecky, J.; Prinosilova, S.; Cajka, T.; Riddellova, K.; Vaclavik, L.; Hajslova, J. Rapid analysis of cafeeine in varios coffee samples employing direct analysis in real time ionization-high-resolution mass spectrometry. Anal. Bional. Chem. 2012, 403, 2883–2889. [Google Scholar] [CrossRef]
- López-Parra, M.B.; Gómez-Domínguez, I.; Iriondo-DeHond, M.; Villamediana Merino, E.; Sánchez-Martín, V.; Mendiola, J.A.; Iriondo-DeHond, A.; Del Castillo, M.D. The impact of the drying process on the antioxidant and anti-inflammatory potential of dried ripe coffee cherry pulp soluble powder. Foods 2024, 13, 1114. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.; Khamis, M.M.; Saeid, W.M.; Purves, R.W.; Katselis, G.; Low, N.; El-Aneed, A. Analyses of a series of chlorogenic scid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, L.; Liu, S. Identification of saccharides by using direct analysis in real time (DART) mass spectrometry. Int. J. Mass Spectrom. 2014, 357, 51–57. [Google Scholar] [CrossRef]
- Procacci, S.; Bojórquez-Quintal, E.; Platamone, G.; Maccioni, O.; Lo Vecchio, V.; Morreale, V.; Alisi, C.; Balducchi, R.; Bacchetta, L. Opuntia ficus-indica pruning waste recycling: Recovery and characterization of mucilage from cladodes. Nat. Resour. 2021, 12, 91–107. [Google Scholar] [CrossRef]
- Scigelova, M.; Hornshaw, M.; Giannakopulos, A.; Makarov, A. Fourier transform mass spectrometry. Mol. Cell. Proteom. 2011, 10, M111.009431. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. Coffee Silverskin as a potential bio-based antioxidant for polymer materials: Brief review. Proceedings 2021, 69, 20. [Google Scholar] [CrossRef]
- Carlotto, J.; da Silva, L.M.; Dartora, N.; Maria-Ferreira, D.; de A. Sabry, D.; Filho, A.P.; de Paula Werner, M.F.; Sassaki, G.L.; Gorin, P.A.; Iacomini, M.; et al. Identification of a dicaffeoylquinic acid isomer from Arctium lappa with a potent anti-ulcer activity. Talanta 2015, 135, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martin-Cabrejas, M. Response surface methodology to optimize the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef] [PubMed]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of coffee silver skin as potential food-safe ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef]
- Nasti, R.; Galeazzi, A.; Marzorati, S.; Zaccheria, F.; Ravasio, N.; Bozzano, L.; Manenti, F.; Verotta, L. Valorisation of coffee roasting by-products: Recovery of silverskin fat by supercritical CO2 extraction. Waste Biomass Valor. 2021, 12, 6021–6033. [Google Scholar] [CrossRef]
- Brzezicha, J.; Blazejewicz, D.; Brzezinska, J.; Grembecka, M. Green coffee vs. dietary supplements: A comparative analysis of bioactive compounds and antioxidant activity. Food Chem. Toxicol. 2021, 155, 112377. [Google Scholar] [CrossRef] [PubMed]
- Yusufoğlu, B.; Kezer, G.; Wang, Y.; Ziora, Z.M.; Esatbeyoglu, T. Bio-recycling of spent coffee grounds: Recent advances and potential applications. Curr. Opin. Food Sci. 2024, 55, 101111. [Google Scholar] [CrossRef]
- Van der Ent, A.; Echevarria, G.; Pollard, A.J.; Erskine, P.D. X-ray fluorescence ionomics of herbarium collections. Sci. Rep. 2019, 9, 4746. [Google Scholar] [CrossRef] [PubMed]
- Kasongo, R.K.; Verdoodt, A.; Kanyankogote, P.; Baert, G.; Van Ranst, E. Response of italian ryegrass (Lolium multiflorum Lam.) to coffee waste application on a humid tropical sandy soil. Soil Use Manag. 2012, 29, 22–29. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Cervera-Mata, A.; Fernández-Arteaga, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Delgado, G. Why should we be concerned with the use of spent coffee grounds as an organic amendment of Soils? A narrative review. Agronomy 2022, 12, 2771. [Google Scholar] [CrossRef]
- Vitale, E.; Motta, C.M.; Avallone, B.; Amoresano, A.; Fontanarosa, C.; Battaglia, G.; Spinelli, M.; Fogliano, C.; Paradiso, R.; Arena, C. Sustainable reuse of expresso coffee by-products as a natural fertilizer to improve growth and photosynthesis in cucumber (Cucumis sativus L.) plants. Waste Biomass Valor. 2024, 15, 543–559. [Google Scholar] [CrossRef]
- Schmidt Rivera, X.C.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life cycle environmental sustainability of valorisation routes for spent coffee grounds: From waste to resources. Resour. Conserv. Recycl. 2020, 157, 104751. [Google Scholar] [CrossRef]
- Stufano, P.; Perrotta, A.; Labarile, R.; Labarile, R.; Trotta, M. The second life of coffee can be even more energizing: Circularity of materials for bio-based electrochemical energy storage devices. MRS Energy Sustain. 2022, 9, 443–460. [Google Scholar] [CrossRef]
- Santato, A.; Bertoldi, D.; Perini, M.; Camin, F.; Larcher, R. Using elemental profiles and stable isotopes to trace the origin of green coffee beans on the global market. J. Mass Spectrom. 2012, 47, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Awwad, S.; Issa, R.; Alnsour, L.; Albals, D.; Al-Momani, I. Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules 2021, 26, 7502. [Google Scholar] [CrossRef] [PubMed]
- Carcea, M.; Danesi, I.; De Gara, L.; Diretto, G.; Fanali, C.; Raffo, A.; Sinesio, F.; Della Posta, S.; Frusciante, S.; Moneta, E.; et al. Chemical composition and sensory profile of the Italian espresso coffee powder and beverage under different roasting conditions. Eur. Food Res. Technol. 2023, 249, 1287–1301. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, D. A la Sombra de los Cafetales. Dinámica de un Paisaje Agrícola en Tlecuaxco, Tequila, Veracruz. Master’s Thesis, El Colegio de Michoacán, Zamora, Michoacán, México, 2019. [Google Scholar]
- Benincasa, P.; D’Amato, R.; Falcinelli, B.; Troni, E.; Fontanella, M.C.; Frusciante, S.; Guiducci, M.; Beone, G.M.; Businelli, D.; Diretto, G. Grain endogenous selenium and moderate salt stress work as synergic elicitors in the enrichment of bioactive compounds in maize sprouts. Agronomy 2020, 10, 735. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]

| Metabolite Class | Cascara Arabica (A) | Cascara Garnica (B) | AVG A/B | p Value A/B |
|---|---|---|---|---|
| Organic acids | ||||
| Citraconic acid | 0.16 ± 0.03 | 0.10 ± 0.02 | 1.61 | 0.0113 |
| Glutaric acid | 9.88 ± 1.01 | 1.91 ± 0.23 | 5.18 | 0 |
| Glycolic acid | 0.51 ± 0.04 | 0.47 ± 0.06 | 1.09 | 0.2916 |
| Itaconic acid | 0.39 ± 0.09 | 0.43 ± 0.04 | 0.92 | 0.521 |
| Lactic acid | 125.77 ± 7.31 | 174.81 ± 8.81 | 0.72 | 0.0001 |
| Malic acid | 0.21 ± 0.06 | 0.13 ± 0.08 | 1.67 | 0.1461 |
| Oxalic acid | 0.04 ± 0.04 | 0.18 ± 0.06 | 0.21 | 0.0068 |
| Pyruvic acid | 15.73 ± 0.71 | 18.63 ± 2.97 | 0.84 | 0.1068 |
| Quinic acid | 1001.62 ± 97.4 | 799.13 ± 54.55 | 1.25 | 0.011 |
| Shikimic acid | 2.71 ± 0.22 | 0.90 ± 0.13 | 3 | 0 |
| Succinic acid | 1.85 ± 0.26 | 1.16 ± 0.14 | 1.6 | 0.0032 |
| Tartaric acid | 1.84 ± 0.29 | 0.37 ± 0.15 | 4.99 | 0.0001 |
| Alkaloids | ||||
| Caffeine | 4637.42 ± 248.28 | 5084.88 ± 151.08 | 0.91 | 0.0217 |
| Serotonin | 0.04 ± 0.02 | 0.25 ± 0.05 | 0.15 | 0.0004 |
| Theobromine | 9.50 ± 1.05 | 7.43 ± 0.52 | 1.28 | 0.0126 |
| Theophylline | 10.25 ± 0.66 | 2.41 ± 0.14 | 4.25 | 0 |
| Trigonelline | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.54 | 0.2815 |
| Amino acids | ||||
| 4-Hydroxyproline | 0.85 ± 0.13 | 0.91 ± 0.12 | 0.93 | 0.5246 |
| Arginine | 0.38 ± 0.10 | 0.16 ± 0.06 | 2.43 | 0.0072 |
| Asparagine | 12.86 ± 0.92 | 6.96 ± 0.15 | 1.85 | 0 |
| Aspartic acid | 6.92 ± 0.66 | 14.50 ± 3.14 | 0.48 | 0.0033 |
| Glutamic acid | 4.18 ± 2.30 | 5.25 ± 0.42 | 0.8 | 0.3964 |
| Glutamine | 0.42 ± 0.03 | 0.36 ± 0.03 | 1.17 | 0.0376 |
| Histidine | 0.07 ± 0.04 | 0.12 ± 0.01 | 0.55 | 0.0306 |
| Leucine-Isoleucine | 1.50 ± 0.07 | 2.95 ± 0.40 | 0.51 | 0.0004 |
| Lysine | 0.12 ± 0.06 | 0.22 ± 0.06 | 0.52 | 0.0376 |
| Phenylalanine | 4.50 ± 0.32 | 7.31 ± 0.78 | 0.62 | 0.0006 |
| Pipecolic acid | 27.43 ± 0.82 | 27.04 ± 4.92 | 1.01 | 0.8808 |
| Proline | 8.96 ± 12.79 | 17.82 ± 11.39 | 0.5 | 0.341 |
| Serine | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.88 | 0.7755 |
| Threonine | 0.11 ± 0.01 | 0.10 ± 0.02 | 1.1 | 0.4729 |
| Tryptophan | 2.25 ± 0.66 | 0.11 ± 0.02 | 21.26 | 0.0006 |
| Tyrosine | 4.55 ± 0.36 | 3.35 ± 0.44 | 1.36 | 0.0057 |
| Valine | 37.86 ± 6.90 | 13.63 ± 2.01 | 2.78 | 0.0005 |
| Fatty acids | ||||
| Adipic acid | 0.72 ± 0.29 | 0.62 ± 0.25 | 1.16 | 0.6113 |
| Arachidic acid | 23.05 ± 1.17 | 24.05 ± 2.57 | 0.96 | 0.5063 |
| Diglyceride | ||||
| DG 18:3, 18:3 | 4.66 ± 1.31 | 3.27 ± 1.41 | 1.43 | 0.1988 |
| Diterperne | ||||
| Cafestol | 1.43 ± 0.13 | 0.16 ± 0.10 | 8.84 | 0 |
| Flavan-3-ol | ||||
| Catechin | 65.10 ± 18.64 | 188.20 ± 9.69 | 0.35 | 0 |
| Flavonoids | ||||
| Hyperoside | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.88 | 0.4182 |
| Isogentisin | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.71 | 0.1892 |
| Kaempferol | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.89 | 0.4122 |
| Naringin | 0.01 ± 0.00 | 0.03 ± 0.03 | 0.38 | 0.1868 |
| Quercetin | 0.03 ± 0.01 | 0.01 ± 0.00 | 3.43 | 0.0269 |
| Quercetin-3-O-glucoside | 0.56 ± 0.07 | 1.40 ± 0.06 | 0.4 | 0 |
| Quercitrin | 24.33 ± 1.60 | 11.91 ± 0.96 | 2.04 | 0 |
| Rutin | 0.08 ± 0.04 | 0.15 ± 0.04 | 0.58 | 0.0616 |
| Phenolic acids | ||||
| 3,4,5-Trihydroxycinnamic acid | 0.13 ± 0.06 | 0.04 ± 0.02 | 3.55 | 0.0241 |
| 3,4-Dimethoxycinnamic acid | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.92 | 0.5788 |
| 3,4,5-Trimethoxycinnamic acid | 0.05 ± 0.02 | 0.03 ± 0.01 | 1.84 | 0.0791 |
| Caffeic acid | 0.16 ± 0.05 | 0.14 ± 0.05 | 1.16 | 0.539 |
| Cinnamic acid | 0.41 ± 0.05 | 0.68 ± 0.05 | 0.61 | 0.0002 |
| Coumaric acid | 0.04 ± 0.01 | 0.03 ± 0.01 | 1.18 | 0.2813 |
| Ferulic acid | 0.19 ± 0.05 | 0.42 ± 0.05 | 0.46 | 0.0008 |
| Gallic acid | 0.03 ± 0.01 | 0.00 ± 0.00 | 0 | 0 |
| Sinapic acid | 0.04 ± 0.01 | 0.06 ± 0.03 | 0.7 | 0.2638 |
| Syringic acid | 0.05 ± 0.02 | 0.00 ± 0.00 | 0 | 0 |
| Vanillic acid | 0.15 ± 0.03 | 0.20 ± 0.04 | 0.75 | 0.0894 |
| Phospholipids | ||||
| PC 16:0 | 53.61 ± 4.42 | 0.00 ± 0.00 | 0 | 0 |
| PC 18 2 | 61.63 ± 8.12 | 127.55 ± 9.26 | 0.48 | 0 |
| PC 18:1 | 7.35 ± 0.91 | 10.02 ± 1.18 | 0.73 | 0.0114 |
| PE 18:0 | 81.71 ± 8.04 | 91.67 ± 3.10 | 0.89 | 0.0602 |
| PI 16:0 | 57.55 ± 5.08 | 66.38 ± 4.41 | 0.87 | 0.0394 |
| PS 17:1 | 13.17 ± 1.93 | 0.00 ± 0.00 | 0 | 0 |
| PS 21:0 | 9.79 ± 0.46 | 12.73 ± 0.55 | 0.77 | 0.0002 |
| Chlorogenic acids | ||||
| 1,4-Dicaffeoylquinic acid | 0.47 ± 0.07 | 0.69 ± 0.01 | 0.68 | 0.0009 |
| 3-Caffeoyl-4-feruloylquinic acid | 6.44 ± 0.36 | 16.37 ± 0.96 | 0.39 | 0 |
| 3-O-Caffeoyl-γ-quinide | 14.12 ± 3.51 | 39.74 ± 3.02 | 0.36 | 0 |
| 3-O-Caffeoylquinic acid | 46.20 ± 5.10 | 95.10 ± 2.78 | 0.49 | 0 |
| 3-O-Dimethoxycinnamoyl-4-O-quinic acid | 0.16 ± 0.02 | 0.26 ± 0.04 | 0.62 | 0.0046 |
| 3-O-Dimethoxycinnamoylquinic acid | 0.31 ± 0.19 | 0.28 ± 0.03 | 1.12 | 0.7396 |
| 3-O-Feruloylquinic acid | 1.18 ± 0.23 | 2.95 ± 0.26 | 0.4 | 0.0001 |
| 3-O-p-Coumaroyl-4-O-caffeoylquinic acid | 0.22 ± 0.01 | 0.94 ± 0.18 | 0.24 | 0.0002 |
| 3,5-Dicaffeoylquinic acid | 247.07 ± 12.43 | 474.24 ± 68.40 | 0.52 | 0.0006 |
| 4-Caffeoyl-5-feruloylquinic acid | 2.55 ± 0.10 | 6.76 ± 0.40 | 0.38 | 0 |
| 4-O-Caffeoylquinic acid | 966.22 ± 63.02 | 1343.81 ± 68.85 | 0.72 | 0.0002 |
| 4-O-Feruloylquinic acid | 67.48 ± 5.45 | 104.13 ± 10.47 | 0.65 | 0.0008 |
| 4,5-Dicaffeoylquinic acid | 247.21 ± 12.30 | 518.56 ± 22.23 | 0.48 | 0 |
| 5-O-Caffeoyl-muco-γ-quinide | 1.11 ± 0.06 | 0.79 ± 0.03 | 1.41 | 0.0001 |
| 5-O-Caffeoylquinic acid | 4.96 ± 0.45 | 5.03 ± 0.29 | 0.99 | 0.8145 |
| 5-O-Coumaroylquinic acid | 61.29 ± 3.29 | 83.67 ± 6.64 | 0.73 | 0.0009 |
| Feruloyl-1,5-quinide lactone | 0.09 ± 0.03 | 0.06 ± 0.01 | 1.64 | 0.0973 |
| Sugars | ||||
| Arabinose | 2.06 ± 0.56 | 3.18 ± 0.80 | 0.65 | 0.0617 |
| Glucose | 122.05 ± 7.17 | 166.72 ± 12.13 | 0.73 | 0.0007 |
| Raffinose | 1.37 ± 0.28 | 1.45 ± 0.44 | 0.95 | 0.7821 |
| Stachyose | 0.00 ± 0.00 | 0.17 ± 0.06 | 0 | 0 |
| Sucrose | 30.05 ± 3.47 | 98.30 ± 8.03 | 0.31 | 0 |
| Vitamins | ||||
| α-tocopherol | 0.08 ± 0.04 | 0.06 ± 0.02 | 1.36 | 0.3491 |
| Nicotinic acid | 5.23 ± 0.30 | 5.02 ± 0.39 | 1.04 | 0.4296 |
| Pyridoxine | 0.96 ± 0.17 | 1.60 ± 0.14 | 0.6 | 0.0012 |
| Riboflavin | 0.33 ± 0.04 | 0.30 ± 0.03 | 1.13 | 0.1755 |
| Thiamine | 0.36 ± 0.06 | 0.17 ± 0.03 | 2.19 | 0.001 |
| Putative Molecule | Plant Material | Mode | m/z | Precursor Ion/Adduct | R.I. (%) | Others m/z [53,54,55] | Molecule Weight | Molecule Formula (M) | Chemical Class |
|---|---|---|---|---|---|---|---|---|---|
| Caffeine | Cascara Silverskin Parchment Green coffee | (+) | 195.14 195.08 195.08 195.08 | [M + H]+ | 100 100 100 100 | 285, 171, 117 | 194.16 | C8H10N4O2 | Alkaloid |
| Caffeoylquinic acid | Silverskin Parchment | (+) | 355.20 | [M + H]+ | -- -- -- -- | 193, 181, 175, 163, 157, 147, 145, 139, 135, 129, 117 | 354.31 | C16H18O9 | Chlorogenic acid |
| Caffeic acid | Cascara Silverskin Parchment Green coffee | (+) | 181.14 181.08 181.08 181.08 | [M + H]+ | 14–3 15–5 16–6 2.5–1 | 163, 145, 135, 117 | 180.16 | C9H8O4 | Phenolic acid |
| Quinic acid | Cascara Silverskin Parchment | (+) | 193.13 193.08 193.08 | [M + H]+ | 5.6–2 7–3 11.5–8 | 175, 157, 147, 139, 129 | 192.17 | C7H12O6 | Carboxylic acid |
| Glucose | Cascara Silverskin Parchment Green coffee | (+) | 198.14 198.09 198.09 198.09 | [M + NH4]+ | 18–1 55–39 80–38 0.95 | 180, 163, 145, 127 | 180.16 | C6H12O6 | Sugar |
| Quercetin | Parchment | (+) | 303.12 | [M + H]+ | 10–26 | 285 | 302.23 | C15H10O7 | Flavonoid |
| Caffeine | Cascara Silverskin Parchment Green coffee | (−) | 179.06 179.05 179.05 179.05 | [M-CH3]− | 13–10 20–41 100–38 100–48 | 387, 283, 255, 237, 209, 193 | 194.16 | C8H10N4O2 | Alkaloid |
| Caffeoylquinic acid | Cascara Silverskin Parchment Green coffee | (−) | 191.06 191.05 191.05 191.05 | [M-Caffeoyl]− | 31–15 14–3 7–2 100–60 | 353, 179, 173, 135 | 354.31 | C16H18O9 | Chlorogenic acid |
| Dicaffeoylquinic acid | Green coffee | (−) | 533.18 | [M + OH]− | 18–16 | 489, 371, 353, 335, 191 | 516.4 | C25H24O12 | Chlorogenic acid |
| Glucose | Cascara Silverskin Parchment Green coffee | (−) | 215.04 215.03 215.03 215.03 | [M + Cl]− | 100–80 100 100 58–30 | 359 | 180.16 | C6H12O6 | Sugar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojórquez-Quintal, E.; Xotlanihua-Flores, D.; Bacchetta, L.; Diretto, G.; Maccioni, O.; Frusciante, S.; Rojas-Abarca, L.M.; Sánchez-Rodríguez, E. Bioactive Compounds and Valorization of Coffee By-Products from the Origin: A Circular Economy Model from Local Practices in Zongolica, Mexico. Plants 2024, 13, 2741. https://doi.org/10.3390/plants13192741
Bojórquez-Quintal E, Xotlanihua-Flores D, Bacchetta L, Diretto G, Maccioni O, Frusciante S, Rojas-Abarca LM, Sánchez-Rodríguez E. Bioactive Compounds and Valorization of Coffee By-Products from the Origin: A Circular Economy Model from Local Practices in Zongolica, Mexico. Plants. 2024; 13(19):2741. https://doi.org/10.3390/plants13192741
Chicago/Turabian StyleBojórquez-Quintal, Emanuel, Damián Xotlanihua-Flores, Loretta Bacchetta, Gianfranco Diretto, Oliviero Maccioni, Sarah Frusciante, Luis M. Rojas-Abarca, and Esteban Sánchez-Rodríguez. 2024. "Bioactive Compounds and Valorization of Coffee By-Products from the Origin: A Circular Economy Model from Local Practices in Zongolica, Mexico" Plants 13, no. 19: 2741. https://doi.org/10.3390/plants13192741
APA StyleBojórquez-Quintal, E., Xotlanihua-Flores, D., Bacchetta, L., Diretto, G., Maccioni, O., Frusciante, S., Rojas-Abarca, L. M., & Sánchez-Rodríguez, E. (2024). Bioactive Compounds and Valorization of Coffee By-Products from the Origin: A Circular Economy Model from Local Practices in Zongolica, Mexico. Plants, 13(19), 2741. https://doi.org/10.3390/plants13192741











