Light-Regulated Growth, Anatomical, Metabolites Biosynthesis and Transcriptional Changes in Angelica sinensis
Abstract
:1. Introduction
2. Results
2.1. Changes in Growth Parameters in Response to White-Light (WL) and UV-B Radiation
2.2. Changes in Cell Micro-Structure and Leaf Stomata in Response to WL and UV-B Radiation
2.3. Changes in Cell Ultra-Structure in Response to WL and UV-B Radiation
2.4. Changes in Contents of Z-Ligustilide and Ferulic Acid in Response to WL and UV-B Radiation
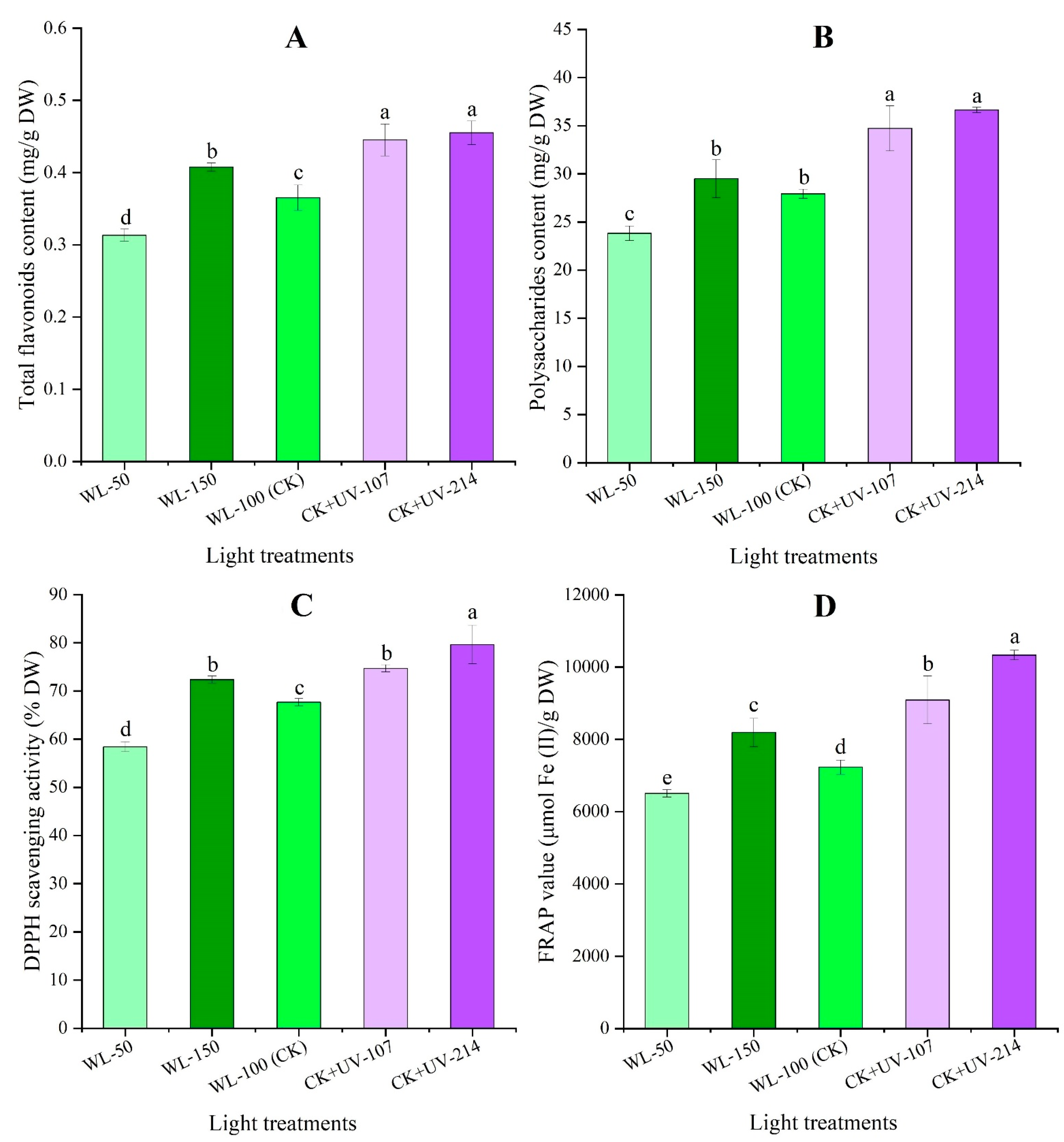
2.5. Changes in Contents of Bioactive Metabolites and Antioxidant Capacity in Response to WL and UV-B Radiation
2.6. Changes in Gene Expression in Response to WL and UV-B Radiation
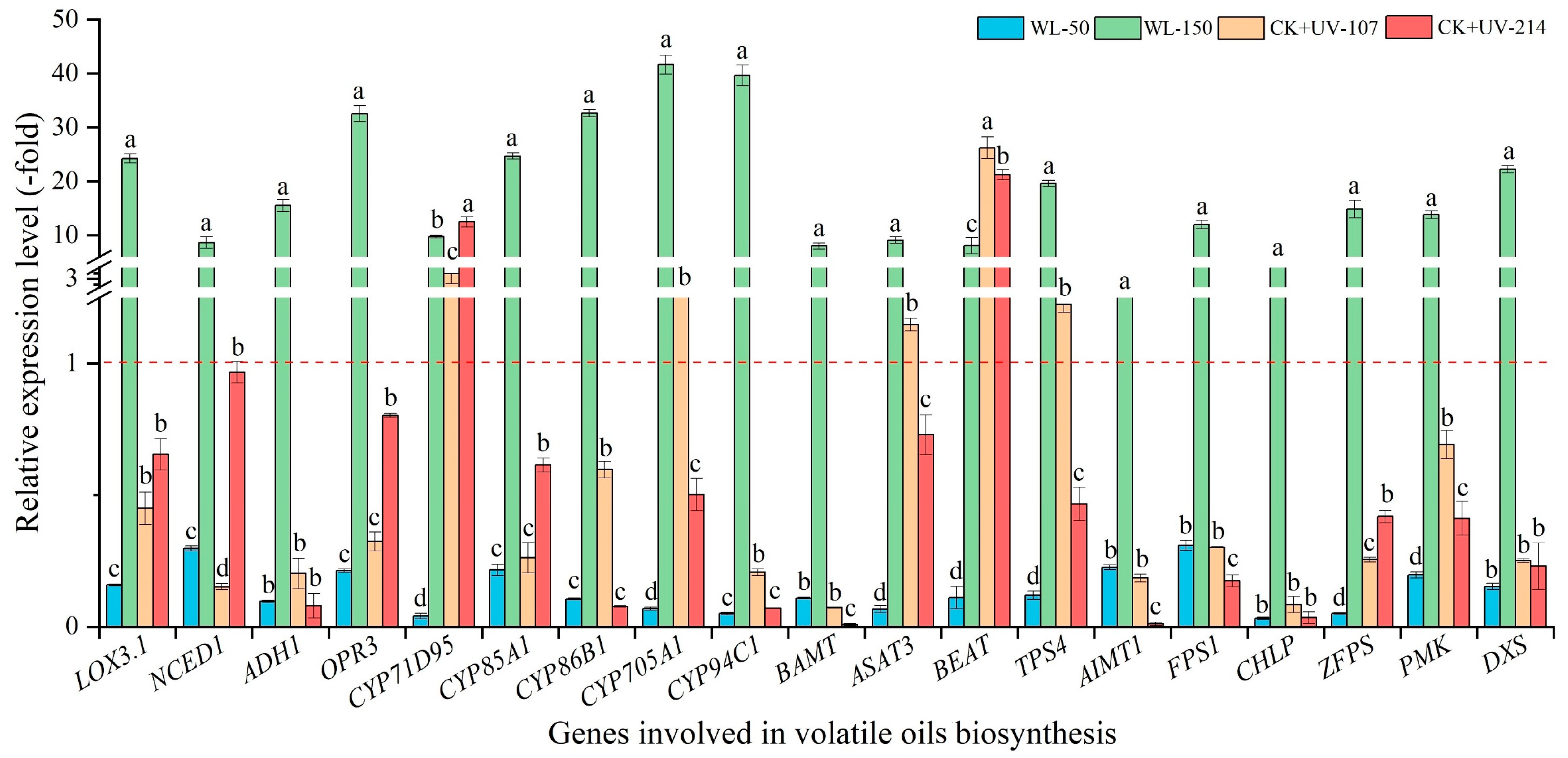
2.6.1. Changes in Expression Level of Genes Related to Volatile Oil Biosynthesis
2.6.2. Changes in Expression Level of Genes Related to Ferulic Acid Biosynthesis
2.6.3. Changes in Expression Level of Genes Related to Flavonoids Biosynthesis
2.6.4. Changes in Expression Level of Genes Related to Polysaccharides Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Chlorophyll Content, Leaf Stomata, and Cell Structure
4.3. Determination of Bioactive Metabolites Content and Antioxidant Capacity
4.4. Quantification of Gene Expression
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Upton, R. American Herbal Pharmacopoeia and Therapeutic Compendium: Dang Gui Root-Angelica sinensis (Oliv.); American Herbal Pharmacopoeia: Scotts Valley, CA, USA, 2003; pp. 1–41. [Google Scholar]
- Zhang, H.Y.; Bi, W.G.; Yu, Y.; Liao, W.B. Angelica sinensis (Oliv.) Diels in China: Distribution, cultivation, utilization and variation. Genet. Resour. Crop Evol. 2012, 59, 607–613. [Google Scholar] [CrossRef]
- Ma, J.P.; Guo, Z.B.; Jin, L.; Li, Y.D. Phytochemical progress made in investigations of Angelica sinensis (Oliv.) Diels. Chin. J. Nat. Med. 2015, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef]
- Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; p. 139.
- Turi, C.E.; Murch, S.J. Targeted and untargeted phytochemistry of Ligusticum canbyi: Indoleamines, phthalides, antioxidant potential, and use of metabolomics as a hypothesis-generating technique for compound discovery. Planta Med. 2013, 79, 1370–1379. [Google Scholar] [CrossRef]
- Xu, X.Q.; Zhu, T.T.; Shi, T.T.; Chen, J.; Jin, L. Quality suitability regionalization analysis of Angelica sinensis in Gansu, China. PLoS ONE 2020, 15, 0243750. [Google Scholar] [CrossRef]
- Xu, R.; Xu, J.; Li, Y.C.; Dai, Y.T.; Zhang, S.P.; Wang, G.; Liu, Z.G.; Dong, L.L.; Chen, S.L. Integrated chemical and transcriptomic analyses unveils synthetic characteristics of different medicinal root parts of Angelica sinensis. Chin. Herb. Med. 2020, 12, 19–28. [Google Scholar] [CrossRef]
- Li, M.F.; Li, J.; Wei, J.H.; Paré, P.W. Transcriptional controls for early bolting and flowering in Angelica sinensis. Plants 2021, 10, 1931. [Google Scholar] [CrossRef]
- Li, J.; Li, M.L.; Zhu, T.T.; Zhang, X.N.; Li, M.F.; Wei, J.H. Integrated transcriptomics and metabolites at different growth stages reveals the regulation mechanism of bolting and flowering of Angelica sinensis. Plant Biol. 2021, 23, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.M.; Liu, X.X.; Su, H.Y.; Li, M.L.; Li, M.F.; Wei, J.H. Regulatory networks of flowering genes in Angelica sinensis during vernalization. Plants 2022, 11, 1355. [Google Scholar] [CrossRef]
- Koeduka, T.; Baiga, T.J.; Noel, J.P.; Pichersky, E. Biosynthesis of t-anethole in anise: Characterization of t-anol/isoeugenol synthase and an O-methyltransferase specific for a C7–C8 propenyl side chain. Plant Physiol. 2009, 149, 384–394. [Google Scholar] [CrossRef]
- Nam, K.H.; Dudareva, N.; Pichersky, E. Characterization of benzylalcohol acetyltransferases in scented and non-scented Clarkia species. Plant Cell Physiol. 1999, 40, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, R.; Huh, J.H.; Badieyan, S.; Rakotondraibe, L.H.; Kliebenstein, D.J.; Sobrado, P.; Tholl, D. In planta variation of volatile biosynthesis: An alternative biosynthetic route to the formation of the pathogen-induced volatile homoterpene DMNT via triterpene degradation in Arabidopsis roots. Plant Cell 2015, 27, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, M.L.; Jin, L.; Xie, X.R.; Li, M.F.; Wei, J.H. Cool temperature enhances growth, ferulic acid and flavonoid biosynthesis while inhibiting polysaccharide biosynthesis in Angelica sinensis. Molecules 2022, 27, 320. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Cui, X.W.; Jin, L.; Li, M.F.; Wei, J.H. Bolting reduces ferulic acid and flavonoid biosynthesis and induces root lignification in Angelica sinensis. Plant Physiol. Biochem. 2022, 170, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhang, Y.Y.; Chen, H.P.; Lin, X.Y.; Xia, P.F.; Sun, Y.J.; Zhao, L.; Luo, J.; Yang, F.X.; Li, S.H. Influence of ecological factors on the quality of Angelica sinensis based on OPLS model. Chin. Tradit. Pat. Med. 2021, 43, 1226–1230. [Google Scholar]
- Li, M.S.; Hao, Y.R.; Zhang, Z.G. Influence of the high altitude on the yield of Angelica sinensis and principal components of essential oils. Acta Bot. Bor. Occ. Sin. 1985, 5, 155–160. [Google Scholar]
- Ma, Y.; Jin, L.; Wang, Z.H.; Cui, Z.J.; Zhang, Y.L.; Li, Y.D. Comparative study of cultivated radix Angelica sinensis polysaccharide content from different altitude in Min county of Gansu province. J. Trad. Chin. Vet. Med. 2013, 32, 7–9. [Google Scholar]
- Jin, L.; Jin, Y.; Wang, H.Z.; Zhang, X.F.; Zhang, P.S.; Li, Y.D. Effects of altitude on the yield and content of ferulic acid in Angelica sinensis. J. Trad. Chin. Vet. Med. 2014, 33, 47–49. [Google Scholar]
- Li, J.; Gao, S.F.; Li, Y.D.; Jin, L.; Wang, H.Z. Response study of ferulic and ligustilide in the root of Angelica sinensis on altitude. J. Gansu Coll. Tradit. Chin. Med. 2014, 31, 23–26. [Google Scholar]
- Wang, H.Z.; Jin, L.; Zhang, E.H. Effect of altitude on the photosynthate accumulation and distribution pattern of Angelica sinensis. J. Chin. Medic. Mater. 2012, 35, 1191–1194. [Google Scholar]
- Wang, H.Z.; Jin, L.; Zhang, E.H. Effect of altitude on ferulic acid in Angelica sinensis and analysis in key factors. Chin. Trad. Herb. Drugs 2013, 44, 219–223. [Google Scholar]
- Jacobson, M.Z. Fundamentals of Atmospheric Modeling, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Liu, B.; Rao, B.Y.; Qian, C.; Wu, Z.J. Effect on different irrigation treatments on growth, yield and quality of Angelica sinensis. Mod. Agric. Sci. Technol. 2013, 2, 77–80+82. [Google Scholar]
- Lin, H.M.; Wu, Y.A.; Cao, Z.F.; Lv, S.L.; Mao, X.J. Influence of sun shade cultivation on premature bolting in Angelica sinensis and growth environment factors. Chin. J. Exp. Tradit. Med. For. 2010, 16, 79–83. [Google Scholar]
- Xiang, H.; Li, Y.; Jin, Y. Effects of light intensity on yield and ferulic acid content in Angelica sinensis. J. Tradit. Chin. Vet. Med. 2015, 2, 53–54. [Google Scholar]
- Wang, Z.H.; Wang, Y.Q.; Fan, Q.; Yang, X.; Luo, J.; Li, S.N.; Jiao, M.T. Effects of UV-B radiation on the content of phthalides in Angelica sinensis. J. Gansu Univ. Chin. Med. 2019, 36, 31–34. [Google Scholar]
- Miao, N.; Yun, C.; Shi, Y.T.; Gao, Y.; Wu, S.; Zhang, Z.H.; Han, S.L.; Wang, H.M.; Wang, W.J. Enhancement of flavonoid synthesis and antioxidant activity in Scutellaria baicalensis aerial parts by UV-A radiation. Ind. Crops Prod. 2022, 187, 115532. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, J.; Yi, T.Y.; Li, Y.; Li, M.Y.; Liu, H.D.; Zheng, L.J.; Chen, Z.H.; Hao, J.; Xu, M.J.; et al. Integrated mRNA and miRNA omics reveal the regulatory role of UV-B radiation in active ingredient biosynthesis of Chrysanthemum morifolium Ramat. Ind. Crops Prod. 2023, 197, 116657. [Google Scholar] [CrossRef]
- Ri, I.; Pak, S.; Pak, U.; Yun, C.; Tang, Z.H. How does UV-B radiation influence the photosynthesis and secondary metabolism of Schisandra chinensis leaves? Ind. Crops Prod. 2024, 208, 117832. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Chapter two-light-regulated plant growth and development. In Timmermans, Current Topics in Developmental Biology; Marja, C.P., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 91, pp. 29–66. [Google Scholar]
- Fang, L.Z.; Ma, Z.Y.; Wang, Q.B.; Nian, H.; Ma, Q.B.; Huang, Q.L.; Mu, Y.H. Plant growth and photosynthetic characteristics of soybean seedlings under different LED lighting quality conditions. J. Plant Growth Regul. 2021, 40, 668–678. [Google Scholar] [CrossRef]
- Kong, D.X.; Li, Y.Q.; Wang, M.L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant. 2016, 38, 120. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Li, H.E.; Gao, C.; Yang, R. Leaf traits and photosynthetic characteristics of endangered Sinopodophyllum hexandrum (Royle) Ying under different light regimes in Southeastern Tibet Plateau. Photosynthetica 2019, 57, 548–555. [Google Scholar] [CrossRef]
- Zuk-Golaszewska, K.; Upadhyaya, M.K.; Golaszewski, J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2003, 49, 135–140. [Google Scholar] [CrossRef]
- Haliapas, S.; Yupsanis, T.A.; Syros, T.D.; Kofidis, G.; Economou, A.S. Petunia × hybrida during transition to flowering as affected by light intensity and quality treatments. Acta Physiol. Plant. 2008, 30, 807–815. [Google Scholar] [CrossRef]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Arellano, A.; Latsague, M.; Acevedo, P.; Loyola, R.; Arce-Johnson, P.; Alberdi, M. Effects of UV-B radiation on anatomical characteristics, phenolic compounds and gene expression of the phenylpropanoid pathway in highbush blueberry leaves. Plant Physiol. Biochem. 2014, 85, 85–95. [Google Scholar] [CrossRef] [PubMed]
- An, L.Z.; Wang, J.H.; Liu, Y.H.; Chen, T.; Xu, S.J.; Feng, H.Y.; Wang, X.L. The effects of enhanced UV-B radiation on growth, stomata, flavonoid, and ABA content in cucumber leaves. In Proceedings of the Third International Asia-Pacific Environmental Remote Sensing Remote Sensing of the Atmosphere, Ocean, Environment, and Space, Hangzhou, China, 23–27 October 2002; Volume 4896. [Google Scholar]
- Wang, W.X.; Wang, Y.J.; Fu, Q.S.; Zhao, B.; Guo, Y.D. Effects of low light stress on morphological trait physiological characters and leaf ultrastructure of Tomato (Lycopersicon esculentum L.) seedlings. Acta Agric. Bor. Sin. 2009, 24, 144–149. [Google Scholar]
- Chen, M.H.; Huang, Y.Y.; Liu, G.; Qin, F.; Yang, S.A.; Xu, X. Effects of enhanced UV-B radiation on morphology, physiology, biomass, leaf anatomy and ultrastructure in male and female mulberry (Morus alba) saplings. Environ. Exp. Bot. 2016, 129, 85–93. [Google Scholar] [CrossRef]
- Whitmarsh, J.; Govindjee, G. The Photosynthetic Process. Concepts in Photobiology: Photosynthesis and Photomorphogenesis; Springer: Dordrecht, The Netherlands, 1999; pp. 11–51. [Google Scholar]
- Chen, H.X.; Qu, Z.S.; Fu, L.L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Zhao, Q.Z.; Dong, M.Y.; Li, M.F.; Jin, L.; Pare, P.W. Light-induced flavonoid biosynthesis in Sinopodophyllum hexandrum with high-altitude adaptation. Plants 2023, 12, 575. [Google Scholar] [CrossRef]
- Sakalauskaitė, J.; Viskelis, P.; Dambrauskienė, E.; Sakalauskienė, S.; Samuolienė, G.; Brazaitytė, A.; Duchovskis, P.; Urbonavicien, D. The effects of different UV-B radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. J. Sci. Food Agric. 2013, 93, 1266–1271. [Google Scholar] [CrossRef]
- Lv, M.; Su, H.Y.; Li, M.L.; Yang, D.L.; Yao, R.Y.; Li, M.F.; Wei, J.H. Effect of UV-B radiation on growth, flavonoid and podophyllotoxin accumulation, and related gene expression in Sinopodophyllum hexandrum. Plant Biol. 2021, 23, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Royo, J.; Vancanneyt, G.; Pérez, A.G.; Sanz, C.; Störmann, K.; Rosahl, S.; Sánchez-Serrano, J.J. Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J. Biol. Chem. 1996, 271, 21012–21019. [Google Scholar] [CrossRef]
- Sallaud, C.; Rontein, D.; Onillon, S.; Jabès, F.; Duffé, P.; Giacalone, C.; Thoraval, S.; Escoffier, C.; Herbette, G.; Leonhardt, N.; et al. A novel pathway for sesquiterpene biosynthesis from Z, Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell 2009, 21, 301–317. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A myth or a reality for cancer therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Sunnadeniya, R.; Bean, A.; Brown, M.; Akhavan, N.; Hatlestad, G.; Gonzalez, A.; Symonds, V.V.; Lloyd, A. Tyrosine hydroxylation in betalain pigment biosynthesis is performed by Cytochrome P450 enzymes in Beets (Beta vulgaris). PLoS ONE 2016, 11, e0149417. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Liu, J.Y.; Xu, T.; Noman, M.; Jameel, A.; Yao, N.; Dong, Y.Y.; Wang, N.; Li, X.W.; Wang, F.W.; et al. Overexpression of a novel Cytochrome P450 promotes flavonoid biosynthesis and osmotic stress tolerance in transgenic Arabidopsis. Genes 2019, 10, 756. [Google Scholar] [CrossRef]
- Griesser, M.; Vitzthum, F.; Fink, B.; Bellido, M.L.; Raasch, C.; Munoz-Blanco, J.; Schwab, W. Multi-substrate flavonol O-glucosyltransferases from strawberry (Fragaria × ananassa) achene and receptacle. J. Exp. Bot. 2008, 59, 2611–2625. [Google Scholar] [CrossRef]
- Panikulangara, T.J.; Eggers-Schumacher, G.; Wunderlich, M.; Stransky, H.; Schöffl, F. Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol. 2004, 136, 3148–3158. [Google Scholar] [CrossRef]
- Xiang, L.; Le Roy, K.; Bolouri-Moghaddam, M.R.; Vanhaecke, M.; Lammens, W.; Rolland, F.; Van den Ende, W. Exploring the neutral invertase-oxidative stress defense connection in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 3849–3862. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; Fiehn, O.; Jean-Richard-dit-Bressel, L.; Boller, T.; Wiemken, A.; Aeschbacher, R.A.; Wingler, A. Trehalose metabolism in Arabidopsis: Occurrence of trehalose and molecular cloning and characterization of trehalose-6-phosphate synthase homologues. J. Exp. Bot. 2001, 52, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Luo, M.M.; Li, M.F.; Wei, J.H. Depicting precise temperature and duration of vernalization and inhibiting early bolting and flowering of Angelica sinensis by freezing storage. Front Plant Sci. 2022, 13, 853444. [Google Scholar] [CrossRef]
- Li, L.; Li, N.H.; Jiang, S.M. Experimental Guidance of Plant Physiology Module; Science Press: Beijing, China, 2009; pp. 22–39. [Google Scholar]
- Hu, C.H.; Xu, Q.; Sun, X.; Yu, H.; Yuan, Y.Q. Several biological typical samples preparation methods of scanning electron microscope. Hubei Agric. Sci. 2016, 55, 5389–5392. [Google Scholar]
- Li, Y.; Huang, H.P.; Deng, R.; Zhang, X.C.; Cui, Y.M.; Lu, X.L.; Lin, P.Q. The improvement of the sample preparation technique for TEM of plant. Chin. J. Trop. Crop. 2016, 37, 2100–2105. [Google Scholar]
- Deng, X.Q.; Guan, X.J.; Huang, N.N.; Li, J. Simultaneous determination of eight constituents in Angelica sinensis by HPLC. Chin. Tradit. Pat. Med. 2020, 42, 2075–2079. [Google Scholar]
- Ma, M.L.; Karsani, S.A.; Mohajer, S.; Malek, S.N.A. Phytochemical constituents, nutritional values, phenolics, flavonols, flavonoids, antioxidant and cytotoxicity studies on Phaleria macrocarpa (Scheff.) Boerl fruits. BMC Complement. Altern. Med. 2014, 14, 1187–1198. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nencini, C.; Menchiari, A.; Franchi, G.G.; Micheli, L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum. Nutr. 2011, 66, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef] [PubMed]







| Growth Parameters | WL-50 | WL-150 | WL-100 (CK) | CK+UV-107 | CK+UV-214 |
|---|---|---|---|---|---|
| Plant height (cm) | 20.34 ± 0.83 d | 23.34 ± 1.69 a | 22.62 ± 1.31 b | 21.46 ± 1.29 c | 20.13 ± 0.79 d |
| Root length (cm) | 10.81 ± 0.55 c | 12.08 ± 0.73 a | 11.42 ± 0.88 b | 11.30 ± 0.65 b | 10.45 ± 0.45 d |
| Aerial parts dry weight (g) | 0.22 ± 0.02 c | 0.26 ± 0.02 a | 0.24 ± 0.02 b | 0.22 ± 0.02 c | 0.20 ± 0.02 d |
| Root dry weight (g) | 0.20 ± 0.02 c | 0.24 ± 0.03 a | 0.23 ± 0.03 a | 0.21 ± 0.02 b | 0.19 ± 0.02 c |
| Chlorophyll (a + b) (mg/g FW) | 1.54 ± 0.03 c | 1.77 ± 0.02 a | 1.69 ± 0.01 b | 1.38 ± 0.03 d | 1.04 ± 0.02 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Cui, X.; Zhao, Y.; Li, M.; Wei, J.; Paré, P.W. Light-Regulated Growth, Anatomical, Metabolites Biosynthesis and Transcriptional Changes in Angelica sinensis. Plants 2024, 13, 2744. https://doi.org/10.3390/plants13192744
Su H, Cui X, Zhao Y, Li M, Wei J, Paré PW. Light-Regulated Growth, Anatomical, Metabolites Biosynthesis and Transcriptional Changes in Angelica sinensis. Plants. 2024; 13(19):2744. https://doi.org/10.3390/plants13192744
Chicago/Turabian StyleSu, Hongyan, Xiuwen Cui, Yan Zhao, Mengfei Li, Jianhe Wei, and Paul W. Paré. 2024. "Light-Regulated Growth, Anatomical, Metabolites Biosynthesis and Transcriptional Changes in Angelica sinensis" Plants 13, no. 19: 2744. https://doi.org/10.3390/plants13192744









