Agricultural Pest Management: The Role of Microorganisms in Biopesticides and Soil Bioremediation
Abstract
1. Introduction
2. Major Pest Crop
2.1. Weeds
2.2. Ticks (Miticides/Acaricides)
2.3. Plant Microbial Diseases
2.3.1. Fungi
2.3.2. Phytopathogenic Bacteria
2.3.3. Virus
2.3.4. Microalgae
2.4. Insects
2.4.1. Termites
2.4.2. Other Insects
2.5. Nematodes
2.6. Rodents
2.7. Mollusks
3. Pesticides and Biopesticides
3.1. Challenges and Limitations of Biopesticides
3.2. Circular Economy and Production of Biopesticides
4. Botanical Biopesticides—Phytopesticides
5. Microbial Biopesticides
6. Producing Microorganisms
6.1. Bacteria
6.2. Virus
6.3. Microalgae
6.4. Fungal Biopesticides
6.4.1. Entomopathogenic Fungi (EFP)
6.4.2. Mechanisms of Action
6.5. Entomopathogenic Nematodes and Their Bacterial Symbionts
6.6. Microsporidia
- Nosema locustae: The only commercially available species of microsporidium has a vast host range, infecting 121 orthopteran species. This microorganism has been used successfully to control grasshopper populations. N. locustae is a promising biopesticide for locust control due to its potential for ultralow-volume production and dissemination [257]. The use of microsporidia Nosema locustae and Paranosema locustae, in combination with the fungi Metarhizium anisopliae var. acridum, were found to be the best biological control agents (BCA) [258,259].
- Vairimorpha necatrix: This microsporidium has commercial potential in a broad host range among caterpillar pests, including corn earworms. It can be more virulent than other species, with infected insects potentially dying within six days of infection [260]. It was tested as a microbial insecticide for the tobacco budworm Heliothis virescens. It can be more virulent than other species, with infected insects potentially dying within six days of infection [260].
6.7. Apicomplexa
7. Biopesticides Specificity
8. Delivery System of Biological Control Agents after Production
8.1. Soil Application
8.2. Seed Treatment
8.3. Spraying Techniques
8.4. Endotherapy
9. Adjuvants Used for Microbial Pesticide
9.1. Surfactants and Carriers
9.2. Protective Agents
10. Production of Biological Control Agents
10.1. Solid-State Fermentation (SSF)
10.2. Submerged Fermentation (SmF)
10.3. Biphasic Fermentation
11. Formulation of Biological Agents
11.1. Formulation
- 4.
- 5.
- 6.
11.1.1. Liquid Formulation
11.1.2. Solid Formulation
12. Pesticides and the Environment
Environmental Residues
13. Bioremediation of Pesticides by Microorganisms
14. The Biopesticide Market
15. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tefera, M.W. Review on Valuation of Environmental Amenity and Pollution. J. Environ. Sci. Econ. 2024, 3, 1–16. [Google Scholar] [CrossRef]
- Awewomom, J.; Dzeble, F.; Takyi, Y.D.; Ashie, W.B.; Ettey, E.N.Y.O.; Afua, P.E.; Sackey, L.N.A.; Opoku, F.; Akoto, O. Addressing Global Environmental Pollution Using Environmental Control Techniques: A Focus on Environmental Policy and Preventive Environmental Management. Discov. Environ. 2024, 2, 8. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Alternative Pathways to 2050. In Food and Agriculture Organization of the United Nations Rome; FAO: Rome, Italy, 2018; p. 228. [Google Scholar]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do Pesticides Promote or Hinder Sustainability in Agriculture? The Challenge of Sustainable Use of Pesticides in Modern Agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef] [PubMed]
- Merrill, B.F.; Lu, N.; Yamaguchi, T.; Takagaki, M.; Maruo, T.; Kozai, T.; Yamori, W. Next Evolution of Agriculture. In Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018; pp. 723–740. ISBN 978-1-315-37213-6. [Google Scholar]
- Möhring, N.; Ingold, K.; Kudsk, P.; Martin-Laurent, F.; Niggli, U.; Siegrist, M.; Studer, B.; Walter, A.; Finger, R. Pathways for Advancing Pesticide Policies. Nat. Food 2020, 1, 535–540. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide Toxicity: A Mechanistic Approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- Chauhan, B.S. Grand Challenges in Weed Management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Horvath, D.P.; Clay, S.A.; Swanton, C.J.; Anderson, J.V.; Chao, W.S. Weed-Induced Crop Yield Loss: A New Paradigm and New Challenges. Trends Plant Sci. 2023, 28, 567–582. [Google Scholar] [CrossRef]
- Heap, I. International Herbicide-Resistant Weed Database. Available online: https://weedscience.org/Home.aspx (accessed on 15 May 2024).
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Vilà, M.; Beaury, E.M.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Laginhas, B.B.; Trillo, A.; Dukes, J.S.; Sorte, C.J.B.; Ibáñez, I. Understanding the Combined Impacts of Weeds and Climate Change on Crops. Environ. Res. Lett. 2021, 16, 034043. [Google Scholar] [CrossRef]
- Mossie, T.; Yirdaw, B. Documentation of Major Poisonous Plants and Their Toxic Effects on Livestock: A Review. Am. J. Biosci. Bioeng. 2023, 11, 47–55. [Google Scholar] [CrossRef]
- Aneja, K.R. Non-Chemical Management of Weeds through Bioherbicides: Current Status, Market, Development, Constraints and Future Prospects. Braz. J. Dev. 2024, 10, e67432. [Google Scholar] [CrossRef]
- Haq, S.M.; Lone, F.A.; Kumar, M.; Calixto, E.S.; Waheed, M.; Casini, R.; Mahmoud, E.A.; Elansary, H.O. Phenology and Diversity of Weeds in the Agriculture and Horticulture Cropping Systems of Indian Western Himalayas: Understanding Implications for Agro-Ecosystems. Plants 2023, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A. Mites. In Polyphagous Pests of Crops; Springer: Singapore, 2021; pp. 409–455. ISBN 9789811580758. [Google Scholar]
- De Rouck, S.; İnak, E.; Dermauw, W.; Van Leeuwen, T. A Review of the Molecular Mechanisms of Acaricide Resistance in Mites and Ticks. Insect Biochem. Mol. Biol. 2023, 159, 103981. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Partida, V.L.; Otero-Colina, G.; Guzmán-Franco, A.W.; Lomeli-Flores, J.R.; Olmos-Zepeda, J.R.; Soto-Rojas, L.; Carrillo-Benítez, G.; Díaz-Martínez, V. Susceptibility of Tetranychus cinnabarinus and Tetranychus urticae (Trombidiformes: Tetranychidae) to Neozygites floridana (Entomophthorales: Neozygitaceae). J. Entomol. Sci. 2022, 57, 502–515. [Google Scholar] [CrossRef]
- Lindsey, A.P.J.; Murugan, S.; Renitta, R.E. Microbial Disease Management in Agriculture: Current Status and Future Prospects. Biocatal. Agric. Biotechnol. 2020, 23, 101468. [Google Scholar] [CrossRef]
- Liu, B.; Stevens-Green, R.; Johal, D.; Buchanan, R.; Geddes-McAlister, J. Fungal Pathogens of Cereal Crops: Proteomic Insights into Fungal Pathogenesis, Host Defense, and Resistance. J. Plant Physiol. 2022, 269, 153593. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Maurya, S.; Pandey, K.K.; Behera, T.K. Global Scenario of Vegetable Fungal Diseases. Veg. Sci. 2024, 51, 54–65. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.-S.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the Pathogenomic Features of a Global Pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Ukladov, E.O.; Golubeva, T.S. Phytophthora infestans: An Overview of Methods and Attempts to Combat Late Blight. J. Fungi 2021, 7, 1071. [Google Scholar] [CrossRef]
- Salotti, I.; Ji, T.; Rossi, V. Temperature Requirements of Colletotrichum spp. Belonging to Different Clades. Front. Plant Sci. 2022, 13, 953760. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.T.T.; de Neergaard, E.; Jørgensen, H.J.L. Infection Biology of Stagonosporopsis cucurbitacearum in Watermelon and Defence Responses in the Host. Agriculture 2024, 14, 380. [Google Scholar] [CrossRef]
- Rohini, M.; Jayapala, N.; Pushpalatha, H.G.; Gavirangappa, H.; Puttaswamy, H.; Ramachandrappa, N.S. Biochemical, Pathological and Molecular Characterisation of Phomopsis vexans: A Causative of Leaf Blight and Fruit Rot in Brinjal. Microb. Pathog. 2023, 179, 106114. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Mubeen, M.; Ateeq, M.; Shad, M.A.; Atiq, M.N.; Kaleem, M.M.; Iqbal, S.; Shaikh, A.A.; Ullah, I.; Khan, M.; et al. Etiology, Epidemiology And Management of Citrus Black Rot Caused by Alternaria citri—An Outlook. Plant Prot. 2021, 5, 105–115. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, J.S.; Choi, Y.-J. Co-Occurrence of Two Phylogenetic Clades of Pseudoperonospora cubensis, the Causal Agent of Downy Mildew Disease, on Oriental Pickling Melon. Mycobiology 2021, 49, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Díaz, M.; Bernal-Cabrera, A.; Trapero, A.; Medina-Marrero, R.; Sifontes-Rodríguez, S.; Cupull-Santana, R.D.; García-Bernal, M.; Agustí-Brisach, C. Characterization of Actinobacterial Strains as Potential Biocontrol Agents against Macrophomina phaseolina and Rhizoctonia solani, the Main Soil-Borne Pathogens of Phaseolus vulgaris in Cuba. Plants 2022, 11, 645. [Google Scholar] [CrossRef]
- Ferraz, P.; Brandão, R.L.; Cássio, F.; Lucas, C. Moniliophthora perniciosa, the Causal Agent of Cacao Witches’ Broom Disease Is Killed in Vitro by Saccharomyces cerevisiae and Wickerhamomyces anomalus Yeasts. Front. Microbiol. 2021, 12, 706675. [Google Scholar] [CrossRef]
- Foresto, E.; Carezzano, M.E.; Giordano, W.; Bogino, P. Ascochyta Blight in Chickpea: An Update. J. Fungi 2023, 9, 203. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Orfei, B.; Moretti, C.; Scian, A.; Paglialunga, M.; Loreti, S.; Tatulli, G.; Scotti, L.; Aceto, A.; Buonaurio, R. Combat Phytopathogenic Bacteria Employing Argirium-SUNCs: Limits and Perspectives. Appl. Microbiol. Biotechnol. 2024, 108, 357. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in Phytopathogenic Bacteria: Do We Know Enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Prgomet, I.; Godena, S. Microbial and Plant-Based Compounds as Alternatives for the Control of Phytopathogenic Bacteria. Horticulturae 2023, 9, 1124. [Google Scholar] [CrossRef]
- Okiro, L.A.; Mulwa, R.M.; Oyoo, M.E.; Nyalala, S. The Danger of the Spread of Ralstonia solanacearum on Potato Crops Worldwide and Potential Mitigation Options. CABI Rev. 2024. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, W.; Cheng, S.; Zhang, H.; Zong, J.; Zhang, Z. Ralstonia solanacearum—A Soil Borne Hidden Enemy of Plants: Research Development in Management Strategies, Their Action Mechanism and Challenges. Front. Plant Sci. 2023, 14, 1141902. [Google Scholar] [CrossRef] [PubMed]
- Robic, K.; Munier, E.; Effantin, G.; Lachat, J.; Naquin, D.; Gueguen, E.; Faure, D. Dissimilar Gene Repertoires of Dickeya Solani Involved in the Colonization of Lesions and Roots of Solanum Tuberosum. Front. Plant Sci. 2023, 14, 1154110. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Devi, B.M.; Guruprasath, S.; Balu, P.; Chattopadhyay, A.; Thilagar, S.S.; Dhanabalan, K.V.; Choudhary, M.; Moparthi, S.; Jailani, A.A.K. Dissecting Diagnostic and Management Strategies for Plant Viral Diseases: What Next? Agriculture 2024, 14, 284. [Google Scholar] [CrossRef]
- Sun, K.; Fu, K.; Hu, T.; Shentu, X.; Yu, X. Leveraging Insect Viruses and Genetic Manipulation for Sustainable Agricultural Pest Control. Pest Manag. Sci. 2023, 80, 2515–2527. [Google Scholar] [CrossRef]
- Jangra, S.; Chinnaiah, S.; Patil, S.R.; Shukla, B.; Devendran, R.; Kumar, M. Deciphering the Role of Virus Receptors in Plant–Virus–Vector Interactions. Receptors 2024, 3, 255–279. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.U.; Khanal, S.; Gyawali, S. Insect Pests and Diseases of Cinnamon (Cinnamomum verum Presi.) and Their Management in Agroforestry System: A Review. Acta Entomol. Zool. 2020, 1, 51–59. [Google Scholar] [CrossRef]
- Rahman, H.; Ahmad, I.; Jon, P.H.; Salam, A.; Rabbi, M.F. Automated Detection of Selected Tea Leaf Diseases in Bangladesh with Convolutional Neural Network. Sci. Rep. 2024, 14, 14097. [Google Scholar] [CrossRef] [PubMed]
- Chouvenc, T.; Šobotník, J.; Engel, M.S.; Bourguignon, T. Termite Evolution: Mutualistic Associations, Key Innovations, and the Rise of Termitidae. Cell. Mol. Life Sci. 2021, 78, 2749–2769. [Google Scholar] [CrossRef] [PubMed]
- Kusumawardhani, D.T.; Almulqu, A.A. A Review of Termite for Sustainable Green Building. Al-Hayat J. Biol. Appl. Biol. 2024, 7, 57–70. [Google Scholar]
- Rana, A.; Chandel, R.S.; Verma, K.S.; Joshi, M.J. Termites in Important Crops and Their Management. Indian J. Entomol. 2021, 83, 486–504. [Google Scholar] [CrossRef]
- Paul, B.; Khan, M.A.; Paul, S.; Shankarganesh, K.; Chakravorty, S. Termites and Indian Agriculture. In Termites and Sustainable Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 51–96. ISBN 978-3-319-68726-1. [Google Scholar]
- Ahmad, F.; Fouad, H.; Liang, S.; Hu, Y.; Mo, J. Termites and Chinese Agricultural System: Applications and Advances in Integrated Termite Management and Chemical Control. Insect Sci. 2019, 28, 2–20. [Google Scholar] [CrossRef]
- Bayen, S.; Modak, D.; Roy, S.; Chakraborti, D.; Babu, A. Non-Chemical Management of Termite Pests: An Overview. Int. J. Trop. Insect Sci. 2024, 44, 995–1011. [Google Scholar] [CrossRef]
- Sharma, S.; Kooner, R.; Arora, R. Insect Pests and Crop Losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Springer: Singapore, 2017; pp. 45–66. ISBN 978-981-10-6056-4. [Google Scholar]
- Finlay, K.J.; Luck, J.E. Response of the Bird Cherry-Oat Aphid (Rhopalosiphum padi) to Climate Change in Relation to Its Pest Status, Vectoring Potential and Function in a Crop–Vector–Virus Pathosystem. Agric. Ecosyst. Environ. 2011, 144, 405–421. [Google Scholar] [CrossRef]
- Bateman, R. The Role of Pesticides in SE Asian Rice IPM: A View from the Mekong Delta. Outlooks Pest Manag. 2016, 27, 53–60. [Google Scholar] [CrossRef][Green Version]
- Koo, H.-N.; An, J.-J.; Park, S.-E.; Kim, J.-I.; Kim, G.-H. Regional Susceptibilities to 12 Insecticides of Melon and Cotton Aphid, Aphis gossypii (Hemiptera: Aphididae) and a Point Mutation Associated with Imidacloprid Resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Houndété, T.A.; Kétoh, G.K.; Hema, O.S.; Brévault, T.; Glitho, I.A.; Martin, T. Insecticide Resistance in Field Populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in West Africa. Pest Manag. Sci. 2010, 66, 1181–1185. [Google Scholar] [CrossRef]
- Veres, A.; Wyckhuys, K.A.G.; Kiss, J.; Tóth, F.; Burgio, G.; Pons, X.; Avilla, C.; Vidal, S.; Razinger, J.; Bazok, R.; et al. An Update of the Worldwide Integrated Assessment (WIA) on Systemic Pesticides. Part 4: Alternatives in Major Cropping Systems. Environ. Sci. Pollut. Res. 2020, 27, 29867–29899. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wu, L.; Zhao, Y.; Yun, Y.; Peng, Y. Tea Saponin Reduces the Damage of Ectropis obliqua to Tea Crops, and Exerts Reduced Effects on the Spiders Ebrechtella tricuspidata and Evarcha albaria Compared to Chemical Insecticides. PeerJ 2018, 6, e4534. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Gowda, M.T.; Dutta, T.K. Grafting Vegetable Crops to Manage Plant-Parasitic Nematodes: A Review. J. Pest Sci. 2023, 97, 539–560. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, M.R.; Walia, R.K. Crop Loss Estimations Due to Plant-Parasitic Nematodes in Major Crops in India. Natl. Acad. Sci. Lett. 2020, 43, 409–412. [Google Scholar] [CrossRef]
- Mesa-Valle, C.M.; Garrido-Cardenas, J.A.; Cebrian-Carmona, J.; Talavera, M.; Manzano-Agugliaro, F. Global Research on Plant Nematodes. Agronomy 2020, 10, 1148. [Google Scholar] [CrossRef]
- Witmer, G. Rodents in Agriculture: A Broad Perspective. Agronomy 2022, 12, 1458. [Google Scholar] [CrossRef]
- Aulicky, R. Rodents in Crop Production Agricultural Systems—Special Issue. Agronomy 2022, 12, 2813. [Google Scholar] [CrossRef]
- Salmon, P. Terrell Rodents, Rodent Control, and Food Safety. Proc. Vertebr. Pest Conf. 2008, 23, 16–19. [Google Scholar] [CrossRef][Green Version]
- Sallam, A.; El-Wakeil, N. Biological and Ecological Studies on Land Snails and Their Control. In Integrated Pest Management and Pest Control—Current and Future Tactics; Soloneski, S., Ed.; InTech: Vienna, Austria, 2012; ISBN 978-953-51-0050-8. [Google Scholar]
- Le Gall, M.; Tooker, J.F. Developing Ecologically Based Pest Management Programs for Terrestrial Molluscs in Field and Forage Crops. J. Pest Sci. 2017, 90, 825–838. [Google Scholar] [CrossRef]
- Raut, S.K.; Barker, G.M. Achatina fulica Bowdich and Other Achatinidae as Pests in Tropical Agriculture. In Molluscs as Crop Pests; Barker, G.M., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 55–114. ISBN 978-0-85199-320-1. [Google Scholar]
- Barua, A.; Williams, C.D.; Ross, J.L. A Literature Review of Biological and Bio-Rational Control Strategies for Slugs: Current Research and Future Prospects. Insects 2021, 12, 541. [Google Scholar] [CrossRef]
- Kumar, P. A Review—On Molluscs as an Agricultural Pest and Their Control. Int. J. Food Sci. Agric. 2020, 4, 383–389. [Google Scholar] [CrossRef]
- Makgoba, L.; Abrams, A.; Röösli, M.; Cissé, G.; Dalvie, M.A. DDT Contamination in Water Resources of Some African Countries and Its Impact on Water Quality and Human Health. Heliyon 2024, 10, e28054. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Boucaud-Maitre, D.; Rambourg, M.-O.; Sinno-Tellier, S.; Puskarczyk, E.; Pineau, X.; Kammerer, M.; Bloch, J.; Langrand, J. Human Exposure to Banned Pesticides Reported to the French Poison Control Centers: 2012–2016. Environ. Toxicol. Pharmacol. 2019, 69, 51–56. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a Promising Alternative to Synthetic Pesticides: A Case for Microbial Pesticides, Phytopesticides, and Nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Eddleston, M. Poisoning by Pesticides. Medicine 2024, 52, 390–393. [Google Scholar] [CrossRef]
- Bharti, V.; Ibrahim, S. Biopesticides: Production, Formulation and Application Systems. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3931–3946. [Google Scholar] [CrossRef]
- Sudakin, D.L. Biopesticides. Toxicol. Rev. 2003, 22, 83–90. [Google Scholar] [CrossRef]
- Marrone, P.G. Status of the Biopesticide Market and Prospects for New Bioherbicides. Pest Manag. Sci. 2023, 80, 81–86. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, N.; Rajput, V.D.; Mandzhieva, S.; Minkina, T.; Saharan, B.S.; Kumar, D.; Sadh, P.K.; Duhan, J.S. Advances in Biopolymeric Nanopesticides: A New Eco-Friendly/Eco-Protective Perspective in Precision Agriculture. Nanomaterials 2022, 12, 3964. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Corrigendum: Biopesticides as an Alternative to Synthetic Pesticides: A Case for Nanopesticides, Phytopesticides and Microbial Pesticides. Front. Microbiol. 2024, 14, 1258968. [Google Scholar] [CrossRef]
- Chia, X.K.; Hadibarata, T.; Kristanti, R.A.; Jusoh, M.N.H.; Tan, I.S.; Foo, H.C.Y. The Function of Microbial Enzymes in Breaking down Soil Contaminated with Pesticides: A Review. Bioprocess Biosyst. Eng. 2024, 47, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, M.K.; Singh, H.K.; Singh, K.N. Fungal Biopesticides and Their Uses for Control of Insect Pest and Diseases. In Biofertilizers and Biopesticides in Sustainable Agriculture; Apple Academic Press: Waretown, NJ, USA, 2019; pp. 43–70. ISBN 978-0-429-05938-4. [Google Scholar]
- Rodrigo, S.; García-Latorre, C.; Santamaria, O. Metabolites Produced by Fungi against Fungal Phytopathogens: Review, Implementation and Perspectives. Plants 2021, 11, 81. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Šunjka, D.; Mechora, Š. An Alternative Source of Biopesticides and Improvement in Their Formulation—Recent Advances. Plants 2022, 11, 3172. [Google Scholar] [CrossRef]
- Cappa, F.; De Fazi, L.; Baracchi, D.; Cervo, R. Adverse Effects of the Fungal Biopesticide Beauveria bassiana on a Predatory Social Wasp. Sci. Total Environ. 2024, 908, 168202. [Google Scholar] [CrossRef]
- Dar, S.A.; Khan, Z.; Khan, A.A.; Ahmad, S.B. Biopesticides–Its Prospects and Limitations: An Overview. In Perspective in Animal Ecology and Reproduction; Astral International (P) Ltd.: New Delhi, India, 2019; pp. 296–314. [Google Scholar]
- Coffin, R.H.; Borza, T.; Alam, M.Z.; Liu, Y.; Desai, F.; Xi, Y.; Zhang, Z.; Beaton, B.; Goyer, C.; Coffin, J.; et al. Assessing the Suppressive Effects of Biopesticides and Phosphite on Common Scab Development in Potatoes. Biocontrol Sci. Technol. 2020, 30, 1133–1149. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Bock, C.; Mai, K.; Boykin, D.; Wells, L.; Hudson, W.G.; Mizell, R.F. Control of Pecan Weevil with Microbial Biopesticides. Environ. Entomol. 2017, 46, 1299–1304. [Google Scholar] [CrossRef]
- Aniwanou, C.T.S.; Sinzogan, A.A.C.; Deguenon, J.M.; Sikirou, R.; Stewart, D.A.; Ahanchede, A. Bio-Efficacy of Diatomaceous Earth, Household Soaps, and Neem Oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae in Benin. Insects 2020, 12, 18. [Google Scholar] [CrossRef]
- Ataide, L.M.S.; Vargas, G.; Velazquez-Hernandez, Y.; Reyes-Arauz, I.; Villamarin, P.; Canon, M.A.; Yang, X.; Riley, S.S.; Revynthi, A.M. Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions. Insects 2024, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Alagumalai, A.; Balaji, D.; Song, H. Bio-Based Agricultural Products: A Sustainable Alternative to Agrochemicals for Promoting a Circular Economy. RSC Sustain. 2023, 1, 746–762. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Valorisation of Agri-Food Waste to Fertilisers Is a Challenge in Implementing the Circular Economy Concept in Practice. Environ. Pollut. 2022, 312, 119906. [Google Scholar] [CrossRef] [PubMed]
- Cong, R.-G.; Thomsen, M. Review of Ecosystem Services in a Bio-Based Circular Economy and Governance Mechanisms. Ecosyst. Serv. 2021, 50, 101298. [Google Scholar] [CrossRef]
- Khan, A.W.; Zohora, U.S.; Rahman, M.S.; Okanami, M.; Ano, T. Production of Iturin A through Glass Column Reactor (GCR) from Soybean Curd Residue (Okara) by Bacillus subtilis RB14-CS under Solid State Fermentation (SSF). Adv. Biosci. Biotechnol. 2012, 3, 143–148. [Google Scholar] [CrossRef]
- Sakdapetsiri, C.; Fukuta, Y.; Aramsirirujiwet, Y.; Shirasaka, N.; Tokuyama, S.; Kitpreechavanich, V. Solid State Fermentation, Storage and Viability of Streptomyces similanensis 9X166 Using Agro-Industrial Substrates against Phytophthora palmivora -Induced Black Rot Disease in Orchids. Biocontrol Sci. Technol. 2019, 29, 276–292. [Google Scholar] [CrossRef]
- Chaparro, M.L.; Sanabria, P.J.; Jiménez, A.M.; Gómez, M.I.; Bautista, E.J.; Mesa, L. A Circular Economy Approach for Producing a Fungal-Based Biopesticide Employing Pearl Millet as a Substrate and Its Economic Evaluation. Bioresour. Technol. Rep. 2021, 16, 100869. [Google Scholar] [CrossRef]
- Camargo, A.F.; Bonatto, C.; Scapini, T.; Klanovicz, N.; Tadioto, V.; Cadamuro, R.D.; Bazoti, S.F.; Kubeneck, S.; Michelon, W.; Reichert Júnior, F.W.; et al. Fungus-Based Bioherbicides on Circular Economy. Bioprocess Biosyst. Eng. 2023, 46, 1729–1754. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Botanical Pesticides for Eco-Friendly Pest Management: Drawbacks and Limitations. In Pesticides in Crop Production: Physiological and Biochemical Action; Wiley: Hoboken, NJ, USA, 2020; pp. 181–193. [Google Scholar]
- Rajput, S.B.; Tonge, M.B.; Karuppayil, S.M. An Overview on Traditional Uses and Pharmacological Profile of Acorus calamus Linn. (Sweet Flag) and Other Acorus Species. Phytomedicine 2014, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, W.; Yang, C.; Hua, H. Supercritical Fluid CO2 Extraction of Acorus calamus L. (Arales: Araceae) and Its Contact Toxicity to Sitophilus zeamais Motschusky (Coleoptera: Curculionidae). Nat. Prod. Res. 2012, 26, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Yuan, H.; He, W.; Deng, Y.; Sun, R.; Zhong, G. Synergistic Effects of Botanical Curcumin-Induced Programmed Cell Death on the Management of Spodoptera litura Fabricius with Avermectin. Ecotoxicol. Environ. Saf. 2022, 229, 113097. [Google Scholar] [CrossRef]
- Veeran, S.; Cui, G.; Shu, B.; Yi, X.; Zhong, G. Curcumin-induced Autophagy and Nucleophagy in Spodoptera frugiperda Sf9 Insect Cells Occur via PI3K/AKT/TOR Pathways. J. Cell. Biochem. 2019, 120, 2119–2137. [Google Scholar] [CrossRef]
- Koma, S. Plants as Potential Sources of Pesticidal Agents: A Review. In Pesticides—Advances in Chemical and Botanical Pesticides; Soundararajan, R.P., Ed.; InTech: Vienna, Austria, 2012; ISBN 978-953-51-0680-7. [Google Scholar]
- Zhang, J.-W.; Li, S.-K.; Wu, W.-J. The Main Chemical Composition and in Vitro Antifungal Activity of the Essential Oils of Ocimum basilicum Linn. Var. Pilosum (Willd.) Benth. Molecules 2009, 14, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Chengala, L.; Singh, N. Botanical Pesticides—A Major Alternative to Chemical Pesticides: A Review. Int. J. Life Sci. 2017, 5, 722–729. [Google Scholar]
- Aimad, A.; Bourhia, M.; Hana, H.; Sanae, R.; Salamatullah, A.M.; Soufan, W.; Rihan, H.Z.; Ouahmane, L.; Youness, E.A.; Noureddine, E.; et al. Essential Oils from Artemisia herba alba Asso., Maticaria recutita L., and Dittrichia viscosa L. (Asteraceae): A Promising Source of Eco-Friendly Agents to Control Callosobruchus maculatus Fab. Warehouse Pest. J. Chem. 2022, 2022, 2373460. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides and Innovative Nano-Based Application Strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Bhadra, P. Medicinal Plants as Bio-Pesticides. In Advanced Agriculture; New Delhi Publishers: Delhi, India, 2020; ISBN 978-93-88879-99-6. [Google Scholar]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical Activity and Role of Botanical Pesticides in Pest Management for Sustainable Agricultural Crop Production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Perello, A.E.; Noll, U.; Slusarenko, A.J. In Vitro Efficacy of Garlic Extract to Control Fungal Pathogens of Wheat. Med. Res. Rev. 2013, 7, 1809–1817. [Google Scholar]
- Vero, S.; Garmendia, G.; Allori, E.; Sanz, J.M.; Gonda, M.; Alconada, T.; Cavello, I.; Dib, J.R.; Diaz, M.A.; Nally, C.; et al. Microbial Biopesticides: Diversity, Scope, and Mechanisms Involved in Plant Disease Control. Diversity 2023, 15, 457. [Google Scholar] [CrossRef]
- Glare, T.R.; O’Callaghan, M. Microbial Biopesticides for Control of Invertebrates: Progress from New Zealand. J. Invertebr. Pathol. 2019, 165, 82–88. [Google Scholar] [CrossRef]
- Karnwal, A.; Kapoor, D. Soil Microbes as Biopesticides: Agricultural Applications and Future Prospects. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Springer: Singapore, 2020; pp. 499–524. ISBN 9789811569494. [Google Scholar]
- Ali, S.; Ahmad, N.; Dar, M.A.; Manan, S.; Rani, A.; Alghanem, S.M.S.; Khan, K.A.; Sethupathy, S.; Elboughdiri, N.; Mostafa, Y.S.; et al. Nano-Agrochemicals as Substitutes for Pesticides: Prospects and Risks. Plants 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Hamedi, J. Biopesticides: Microbes for Agricultural Sustainability. In Soil Microbiomes for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2021; pp. 471–501. ISBN 978-3-030-73507-4. [Google Scholar]
- Wend, K.; Zorrilla, L.; Freimoser, F.M.; Gallet, A. Microbial Pesticides—Challenges and Future Perspectives for Testing and Safety Assessment with Respect to Human Health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Kaur, K.; Vyas, P. Biofungicides and Plant Growth Promoters: Advantages and Opportunities in Entrepreneurship. In Entrepreneurship with Microorganisms; Elsevier: Amsterdam, The Netherlands, 2024; pp. 259–277. ISBN 978-0-443-19049-0. [Google Scholar]
- Steen, A.D.; Crits-Christoph, A.; Carini, P.; DeAngelis, K.M.; Fierer, N.; Lloyd, K.G.; Thrash, J.C. High Proportions of Bacteria and Archaea across Most Biomes Remain Uncultured. ISME J. 2019, 13, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.; Schenk, P.M.; Mirzaee, H. Microbial Biopesticides against Bacterial, Fungal and Oomycete Pathogens of Tomato, Cabbage and Chickpea. Appl. Microbiol. 2022, 2, 288–301. [Google Scholar] [CrossRef]
- Oberhardt, M.A.; Zarecki, R.; Gronow, S.; Lang, E.; Klenk, H.-P.; Gophna, U.; Ruppin, E. Harnessing the Landscape of Microbial Culture Media to Predict New Organism–Media Pairings. Nat. Commun. 2015, 6, 8493. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Negi, R.; Sharma, B.; Kaur, S.; Kaur, T.; Khan, S.S.; Kumar, S.; Ramniwas, S.; Rustagi, S.; Singh, S.; Rai, A.K.; et al. Microbial Antagonists: Diversity, Formulation and Applications for Management of Pest–Pathogens. Egypt. J. Biol. Pest Control 2023, 33, 105. [Google Scholar] [CrossRef]
- Chamberlain, S.; Arendsee, Z.; Stirling, T.; Boettiger, C.; James, T.D.; Salmon, M.; Li, G.; Grenié, M. Ropensci/Taxizedb: Taxizedb v0.3.1 2023. Available online: https://zenodo.org/records/7797082 (accessed on 2 May 2024).
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread Adoption of Bt Cotton and Insecticide Decrease Promotes Biocontrol Services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Keyhani, N.O.; Wang, C.; Li, Y.; Pu, H.; Li, J.; Liu, S.; Lai, P.; Zhu, M.; et al. Isolation of a Highly Virulent Metarhizium Strain Targeting the Tea Pest, Ectropis obliqua. Front. Microbiol. 2023, 14, 1164511. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Cao, F.; Guo, X.; Wang, Y.; Cui, Z.; Huang, T.; Hou, Y.; Guan, X. Development of a Safe and Effective Bacillus thuringiensis-Based Nanobiopesticide for Controlling Tea Pests. J. Agric. Food Chem. 2024, 72, 7807–7817. [Google Scholar] [CrossRef]
- Buisson, C.; Gohar, M.; Huillet, E.; Nielsen-LeRoux, C. Bacillus thuringiensis Spores and Vegetative Bacteria: Infection Capacity and Role of the Virulence Regulon PlcR Following Intrahaemocoel Injection of Galleria mellonella. Insects 2019, 10, 129. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Griko, N.; Junker, M.; Bulla, L.A. Bacillus Thuringiensis: A Genomics and Proteomics Perspective. Bioeng. Bugs 2010, 1, 31–50. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2019, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kumar, H.; Kaur, S. Vegetative Insecticidal Protein (Vip): A Potential Contender From Bacillus thuringiensis for Efficient Management of Various Detrimental Agricultural Pests. Front. Microbiol. 2021, 12, 659736. [Google Scholar] [CrossRef]
- Hemthanon, T.; Promdonkoy, B.; Boonserm, P. Screening and Characterization of Bacillus thuringiensis Isolates for High Production of Vip3A and Cry Proteins and High Thermostability to Control Spodoptera spp. J. Invertebr. Pathol. 2023, 201, 108020. [Google Scholar] [CrossRef]
- Konecka, E.; Kaznowski, A.; Grzesiek, W.; Nowicki, P.; Czarniewska, E.; Baranek, J. Synergistic Interaction between Carvacrol and Bacillus thuringiensis Crystalline Proteins against Cydia pomonella and Spodoptera exigua. BioControl 2020, 65, 447–460. [Google Scholar] [CrossRef]
- Santos, V.S.V.; Pereira, B.B. Properties, Toxicity and Current Applications of the Biolarvicide Spinosad. J. Toxicol. Environ. Health Part B 2019, 23, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.D.; Dutton, R.; Sparks, T.C. Spinosad–A Case Study: An Example from a Natural Products Discovery Programme. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 696–702. [Google Scholar] [CrossRef]
- Yao, S.; Yang, Y.; Xue, Y.; Zhao, W.; Liu, X.; Du, M.; Yin, X.; Guan, R.; Wei, J.; An, S. New Insights on the Effects of Spinosad on the Development of Helicoverpa armigera. Ecotoxicol. Environ. Saf. 2021, 221, 112452. [Google Scholar] [CrossRef]
- Amaral, I.; Antunes, S.C.; Rebelo, D.; Carvalho, A.P.; Rodrigues, S. Biopesticide Spinosad: Unraveling Ecotoxicological Effects on Zebrafish, Danio rerio. Environ. Toxicol. Pharmacol. 2024, 108, 104458. [Google Scholar] [CrossRef]
- Kljajić, P.; Andrić, G.; Pražić Golić, M. Evaluation of Long-Term Residual Activity of Insecticides against Acanthoscelides obtectus (Say) on Common Bean in Laboratory Tests. J. Stored Prod. Res. 2023, 103, 102156. [Google Scholar] [CrossRef]
- Gelaye, Y.; Negash, B. The Role of Baculoviruses in Controlling Insect Pests: A Review. Cogent Food Agric. 2023, 9, 2254139. [Google Scholar] [CrossRef]
- Sajid, Z. A Review on Nucleopolyhydroviruses (NPV) as Biological Control of Army Worm, Spodoptera litura. Curr. Res. Agric. Farming 2021, 2, 30–39. [Google Scholar] [CrossRef]
- Bahadur, A.B. Entomopathogens: Role of Insect Pest Management in Crops. Trends Hortic. 2018, 1, 833. [Google Scholar] [CrossRef]
- Irsad; Shahid, M.; Haq, E.; Mohamed, A.; Rizvi, P.Q.; Kolanthasamy, E. Entomopathogen-Based Biopesticides: Insights into Unraveling Their Potential in Insect Pest Management. Front. Microbiol. 2023, 14, 1208237. [Google Scholar] [CrossRef]
- Moscardi, F.; de Souza, M.L.; de Castro, M.E.B.; Lara Moscardi, M.; Szewczyk, B. Baculovirus Pesticides: Present State and Future Perspectives. In Microbes and Microbial Technology; Springer: New York, NY, USA, 2011; pp. 415–445. ISBN 978-1-4419-7931-5. [Google Scholar]
- Agboola, A.R.; Okonkwo, C.O.; Agwupuye, E.I.; Mbeh, G. Biopesticides and Conventional Pesticides: Comparative Review of Mechanism of Action and Future Perspectives. AROC Agric. 2022, 1, 14–32. [Google Scholar] [CrossRef]
- Nidhi; Mushtaq, S.; Saji, G.; Gupta, V.K. Mode of Action of Biopesticides against Pests and Future Prospects of Biopesticides and Nanobiopesticides. J. Adv. Sci. Res. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Wilson, K.; Grzywacz, D.; Curcic, I.; Scoates, F.; Harper, K.; Rice, A.; Paul, N.; Dillon, A. A Novel Formulation Technology for Baculoviruses Protects Biopesticide from Degradation by Ultraviolet Radiation. Sci. Rep. 2020, 10, 13301. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Prasad, R. (Eds.) Microbial Biotechnology in Crop Protection; Springer: Singapore, 2021; ISBN 9789811600487. [Google Scholar]
- De Clercq, P.; Coudron, T.A.; Riddick, E.W. Production of Heteropteran Predators. In Mass Production of Beneficial Organisms; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–69. ISBN 978-0-12-822106-8. [Google Scholar]
- El-Wakeil, N.; Saleh, M.; Abu-hashim, M. (Eds.) Cottage Industry of Biocontrol Agents and Their Applications: Practical Aspects to Deal Biologically with Pests and Stresses Facing Strategic Crops; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-33160-3. [Google Scholar]
- Ignoffo, C.M.; Garcia, C. Host Spectrum and Relative Virulence of an Ecuadoran and a Mississippian Biotype of Nomuraea rileyi. J. Invertebr. Pathol. 1985, 45, 346–352. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Zarmakoupi, C.; Kitsiou, F.; Eliopoulos, P.A.; Patakioutas, G. Evaluation of Commercial Virus Biopesticides for the Control of Moth Pests in Laboratory Conditions: The Cases of Thaumetopoea pityocampa and Helicoverpa armigera. Appl. Sci. 2024, 14, 506. [Google Scholar] [CrossRef]
- Scholz, B.C.G.; Monsour, C.J.; Zalucki, M.P. An Evaluation of Selective Helicoverpa armigera Control Options in Sweet Corn. Aust. J. Exp. Agric. 1998, 38, 601. [Google Scholar] [CrossRef]
- Kutinkova, H.; Samietz, J.; Dzhuvinov, V.; Zingg, D.; Kessler, P. Successful Application of the Baculovirus Product Madex® for Control of Cydia pomonella (L.) in Bulgaria. J. Plant Prot. Res. 2012, 52, 205–213. [Google Scholar] [CrossRef]
- Kalawate, A.S. Microbial Viral Insecticides. In Basic and Applied Aspects of Biopesticides; Sahayaraj, K., Ed.; Springer: New Delhi, India, 2014; pp. 47–68. ISBN 978-81-322-1876-0. [Google Scholar]
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus Biopesticides: An Overview. JAPS J. Anim. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Haase, S.; Sciocco-Cap, A.; Romanowski, V. Baculovirus Insecticides in Latin America: Historical Overview, Current Status and Future Perspectives. Viruses 2015, 7, 2230–2267. [Google Scholar] [CrossRef]
- Inceoglu, A.B.; Kamita, S.G.; Hinton, A.C.; Huang, Q.; Severson, T.F.; Kang, K.; Hammock, B.D. Recombinant Baculoviruses for Insect Control. Pest Manag. Sci. 2001, 57, 981–987. [Google Scholar] [CrossRef]
- Vail, P.V.; Jay, D.L.; Hink, W.F. Replication and Infectivity of the Nuclear Polyhedrosis Virus of the Alfalfa Looper, Autographa californica, Produced in Cells Grown in Vitro. J. Invertebr. Pathol. 1973, 22, 231–237. [Google Scholar] [CrossRef]
- Szewczyk, B.; Rabalski, L.; Krol, E.; Sihler, W.; de Souza, M.L. Baculovirus Biopesticides—A Safe Alternative to Chemical Protection of Plants. J. Biopestic. 2009, 2, 209–216. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Adoxophyes Orana Granulovirus. EFSA J. 2012, 10, 2654. [Google Scholar] [CrossRef]
- Moscardi, F. Assessment of the application of baculoviruses for control of lepidoptera. Annu. Rev. Entomol. 1999, 44, 257–289. [Google Scholar] [CrossRef]
- Vail, P.V.; Barnett, W.; Cowan, D.C.; Sibbett, S.; Beede, R.; Tebbets, J.S. Codling Moth (Lepidoptera: Tortricidae) Control on Commercial Walnuts with a Granulosis Virus. J. Econ. Entomol. 1991, 84, 1448–1453. [Google Scholar] [CrossRef]
- Cisneros, J.; Pérez, J.A.; Penagos, D.I.; Ruiz, V.J.; Goulson, D.; Caballero, P.; Cave, R.D.; Williams, T. Formulation of a Nucleopolyhedrovirus with Boric Acid for Control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Maize. Biol. Control 2002, 23, 87–95. [Google Scholar] [CrossRef]
- Zhang, J.; Lapointe, R.; Thumbi, D.; Morin, B.; Lucarotti, C.J. Molecular Comparisons of Alphabaculovirus-Based Products: Gypchek with Disparvirus (Lymantria Dispar) and TM BioControl-1 with Virtuss (Orgyia Pseudotsugata). Can. Entomol. 2010, 142, 546–556. [Google Scholar] [CrossRef]
- Rowley, D.L.; Popham, H.J.R.; Harrison, R.L. Genetic Variation and Virulence of Nucleopolyhedroviruses Isolated Worldwide from the Heliothine Pests Helicoverpa armigera, Helicoverpa zea, and Heliothis virescens. J. Invertebr. Pathol. 2011, 107, 112–126. [Google Scholar] [CrossRef]
- Grzywacz, D.; Moore, S. Production, Formulation, and Bioassay of Baculoviruses for Pest Control. In Microbial Control of Insect and Mite Pests; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–124. ISBN 978-0-12-803527-6. [Google Scholar]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Erlandson, M. Insect Pest Control by Viruses. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 125–133. ISBN 978-0-12-374410-4. [Google Scholar]
- Podgwaite, J.D.; Rush, P.; Hall, D.; Walton, G.S. Efficacy of the Neodiprion sertifer (Hymenoptera: Diprionidae) Nucleopolyhedrosis Virus (Baculovirus) Product, Neochek-S. J. Econ. Entomol. 1984, 77, 525–528. [Google Scholar] [CrossRef]
- Casanova, L.M.; Macrae, A.; de Souza, J.E.; Neves Junior, A.; Vermelho, A.B. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants 2023, 12, 1896. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of Microalgae as Biopesticides to Contribute to Sustainable Agriculture and Environmental Development. J. Environ. Sci. Health Part B 2019, 54, 366–375. [Google Scholar] [CrossRef]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New Insights into the Biodiversity and Applications of Cyanobacteria (Blue-Green Algae)—Prospects and Challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Mol, I.; Purushothaman, T. Micro-Algae as Bio-Pesticides for the Development of Sustainable Agriculture. Wide Spectr. 2020, 8, 5–22. [Google Scholar]
- Pratt, R.; Daniels, T.C.; Eiler, J.J.; Gunnison, J.B.; Kumler, W.D.; Oneto, J.F.; Strait, L.A.; Spoehr, H.A.; Hardin, G.J.; Milner, H.W.; et al. Chlorellin, an Antibacterial Substance from Chlorella. Science 1944, 99, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Asthana, R.K.; Srivastava, A.; Singh, A.P.; Deepali; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Identification of an Antimicrobial Entity from the Cyanobacterium Fischerella sp. Isolated from Bark of Azadirachta indica (Neem) Tree. J. Appl. Phycol. 2006, 18, 33–39. [Google Scholar] [CrossRef]
- Kajiyama, S.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an Antifungal Lipopeptide from the Field-Grown Terrestrial Blue-Green Alga Nostoc Commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A Fatty Acid from the Diatom Phaeodactylum tricornutum Is Antibacterial Against Diverse Bacteria Including Multi-Resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2008, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Seel, C.J.; Ji, X.; Gulder, T.; Gulder, T.A.M. Biosynthesis of Cyanobacterin, a Paradigm for Furanolide Core Structure Assembly. Nat. Chem. Biol. 2022, 18, 652–658. [Google Scholar] [CrossRef]
- Gleason, F.K.; Thoma, W.J.; Carlson, J.L. Cyanobacterin and Analogs: Structure and Activity Relationships of a Natural Herbicide. In Progress in Photosynthesis Research; Springer: Dordrecht, The Netherlands, 1987; pp. 763–766. ISBN 978-94-017-0516-5. [Google Scholar]
- Hassan, M.E.; Mohafrash, S.M.M.; Fallatah, S.A.; El-Sayed, A.E.-K.B.; Mossa, A.-T.H. Eco-Friendly Larvicide of Amphora Coffeaeformis and Scenedesmus Obliquus Microalgae Extracts against Culex pipiens. J. Appl. Phycol. 2021, 33, 2683–2693. [Google Scholar] [CrossRef]
- Viaggi, D. Exploring the Economics of the Circular Bioeconomy. In Sustainable Bioeconomy; Springer: Singapore, 2020; pp. 1–10. ISBN 9789811573217. [Google Scholar]
- Ferreira, J.M.; Soares, F.E.d.F. Entomopathogenic Fungi Hydrolytic Enzymes: A New Approach to Biocontrol? J. Nat. Pestic. Res. 2023, 3, 100020. [Google Scholar] [CrossRef]
- Rajamani, M.; Negi, A. Biopesticides for Pest Management. In Sustainable Bioeconomy; Springer: Singapore, 2020; pp. 239–266. ISBN 9789811573217. [Google Scholar]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, X.-Q.; Zhao, D.-L.; Zhang, P. Antifungal Secondary Metabolites Produced by the Fungal Endophytes: Chemical Diversity and Potential Use in the Development of Biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, D.-L.; Liu, G.-H.; Fu, X.; Gu, Y.-C.; Ding, L.-S.; Zhou, Y. Antifungal Halogenated Cyclopentenones from the Endophytic Fungus Saccharicola bicolor of Bergenia purpurascens by the One Strain-Many Compounds Strategy. J. Agric. Food Chem. 2019, 68, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.W.; Jiao, R.H.; Cheng, A.B.; Tan, S.H.; Song, Y.C. Antimicrobial Potentials of Endophytic Fungi Residing in Quercus variabilis and Brefeldin A Obtained from Cladosporium sp. World J. Microbiol. Biotechnol. 2006, 23, 79–83. [Google Scholar] [CrossRef]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrügge, M.; Müller, W.E.G.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine Derivatives from Endophytic Fungi Trichoderma harzianum and Epicoccum Nigrum. Nat. Prod. Res. 2019, 35, 257–265. [Google Scholar] [CrossRef]
- Qin, J.; Lyu, A.; Zhang, Q.; Yang, L.; Zhang, J.; Wu, M.; Li, G. Strain Identification and Metabolites Isolation of Aspergillus capensis CanS-34A from Brassica napus. Mol. Biol. Rep. 2019, 46, 3451–3460. [Google Scholar] [CrossRef] [PubMed]
- Shentu, X.; Zhan, X.; Ma, Z.; Yu, X.; Zhang, C. Antifungal Activity of Metabolites of the Endophytic Fungus Trichoderma brevicompactum from Garlic. Braz. J. Microbiol. 2014, 45, 248–254. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Liu, Z. Antifungal Activity of an Endophytic Fungus Aspergillus versicolor DYSJ3 from Aphanamixis grandifolia Blume against Colletotrichum musae. Mycobiology 2021, 49, 498–506. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-Fungal-Interactions: A Detailed Review on Entomopathogenic Fungi Pathogenicity to Combat Insect Pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Moreno-Gavíra, A.; Huertas, V.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces and Its Importance in the Biological Control of Agricultural Pests and Diseases. Plants 2020, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Li, J.; Zhang, Y.; Guo, Y.; Fang, W.; Valverde, B.E.; Yin, J.; Qiang, S.; Chen, S. A Fungal Bipolaris Bicolor Strain as a Potential Bioherbicide for Goosegrass (Eleusine indica) Control. Pest Manag. Sci. 2021, 78, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Konvalina, P.; Bohatá, A.; Kavková, M.; Nguyen, T.G.; Hoang, T.N. Relevance of Entomopathogenic Fungi in Soil–Plant Systems. Plant Soil 2024, 495, 287–310. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; González-Mas, N.; Yousef-Yousef, M.; Garrido-Jurado, I.; Fernández-Bravo, M. Key Role of Environmental Competence in Successful Use of Entomopathogenic Fungi in Microbial Pest Control. J. Pest Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- Karthi, S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Han, Y.S.; Shivakumar, M.S.; Murali-Baskaran, R.K.; Kalaivani, K.; Radhakrishnan, N.; Park, K.B.; Malafaia, G. Entomopathogenic Fungi Promising Biocontrol Agents for Managing Lepidopteran Pests: Review of Current Knowledge. Biocatal. Agric. Biotechnol. 2024, 58, 103146. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Leather, S.R. Virulence of Verticillium lecanii (Z.) against Cereal Aphids; Does Timing of Infection Affect the Performance of Parasitoids and Predators? Pest Manag. Sci. 2013, 69, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Huang, Y.; Keyhani, N.O.; Huang, Z. Lack of Resistance Development in Bemisia tabaci to Isaria fumosorosea after Multiple Generations of Selection. Sci. Rep. 2017, 7, 42727. [Google Scholar] [CrossRef]
- Mesquita, E.; Hu, S.; Lima, T.B.; Golo, P.S.; Bidochka, M.J. Utilization of Metarhizium as an Insect Biocontrol Agent and a Plant Bioinoculant with Special Reference to Brazil. Front. Fungal Biol. 2023, 4, 1276287. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Q.; Wang, D.; Zou, W.-Q.; Tang, D.-X.; Hongthong, P.; Yu, H. Species Diversity and Virulence Potential of the Beauveria bassiana Complex and Beauveria scarabaeidicola Complex. Front. Microbiol. 2022, 13, 841604. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Sarmah, S.R.; Roy, S.; Sarma, B.; Nath, B.C.; Bhattacharyya, L.H. Perspectives of Beauveria bassiana, an Entomopathogenic Fungus for the Control of Insect-Pests in Tea [Camellia Sinensis (L.) O. Kuntze]: Opportunities and Challenges. Int. J. Trop. Insect Sci. 2022, 43, 1–19. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Huang, S.; Deng, J.; Li, X.; Luo, Z.; Zhang, Y. Pest Management via Endophytic Colonization of Tobacco Seedlings by the Insect Fungal Pathogen Beauveria bassiana. Pest Manag. Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Sinno, M.; Ranesi, M.; Di Lelio, I.; Iacomino, G.; Becchimanzi, A.; Barra, E.; Molisso, D.; Pennacchio, F.; Digilio, M.C.; Vitale, S.; et al. Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests. Pathogens 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Higashi, Y.; Nishi, O.; Kouda, M.; Maeda, K.; Yoshida, K.; Asano, S.; Kawakami, T.; Nakajima, K.; Kuroda, K.; et al. Entomopathogenic Fungus Beauveria bassiana–Based Bioinsecticide Suppresses Severity of Powdery Mildews of Vegetables by Inducing the Plant Defense Responses. Front. Plant Sci. 2023, 14, 1211825. [Google Scholar] [CrossRef] [PubMed]
- Sarven, M.S.; Hao, Q.; Deng, J.; Yang, F.; Wang, G.; Xiao, Y.; Xiao, X. Biological Control of Tomato Gray Mold Caused by Botrytis cinerea with the Entomopathogenic Fungus Metarhizium anisopliae. Pathogens 2020, 9, 213. [Google Scholar] [CrossRef]
- Hao, Q.; Albaghdady, D.M.D.; Xiao, Y.; Xiao, X.; Mo, C.; Tian, T.; Wang, G. Endophytic Metarhizium anisopliae Is a Potential Biocontrol Agent against Wheat Fusarium Head Blight Caused by Fusarium graminearum. J. Plant Pathol. 2021, 103, 875–885. [Google Scholar] [CrossRef]
- Kim, H.M.; Jeong, S.-G.; Choi, I.S.; Yang, J.E.; Lee, K.H.; Kim, J.; Kim, J.C.; Kim, J.S.; Park, H.W. Mechanisms of Insecticidal Action of Metarhizium anisopliae on Adult Japanese Pine Sawyer Beetles (Monochamus Alternatus). ACS Omega 2020, 5, 25312–25318. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; He, Y.; Zheng, L.; Fu, J.; Shi, M. Infection of Metarhizium anisopliae Ma6 and Defense Responses of Host Phyllotreta striolata Adults. Arch. Insect Biochem. Physiol. 2022, 110, e21908. [Google Scholar] [CrossRef]
- Hausrao Thube, S.; Thava Prakasa Pandian, R.; Babu, M.; Josephrajkumar, A.; Mhatre, P.H.; Santhosh Kumar, P.; Nirmal Kumar, B.J.; Hegde, V.; Namdeo Chavan, S. Evaluation of a Native Isolate of Metarhizium anisopliae (Metschn.) Sorokin TMBMA1 against Tea Mosquito Bug, Helopeltis theivora Infesting Cocoa (Theobroma cacao L.). Biol. Control 2022, 170, 104909. [Google Scholar] [CrossRef]
- Samal, I.; Bhoi, T.K.; Vyas, V.; Majhi, P.K.; Mahanta, D.K.; Komal, J.; Singh, S.; Kumar, P.V.D.; Acharya, L.K. Resistance to Fungicides in Entomopathogenic Fungi: Underlying Mechanisms, Consequences, and Opportunities for Progress. Trop. Plant Pathol. 2023, 49, 5–17. [Google Scholar] [CrossRef]
- Gautam, U.K.; Hlávková, D.; Shaik, H.A.; Karaca, I.; Karaca, G.; Sezen, K.; Kodrík, D. Adipokinetic Hormones Enhance the Efficacy of the Entomopathogenic Fungus Isaria fumosorosea in Model and Pest Insects. Pathogens 2020, 9, 801. [Google Scholar] [CrossRef]
- Kang, B.R.; Han, J.H.; Kim, J.J.; Kim, Y.C. Dual Biocontrol Potential of the Entomopathogenic Fungus, Isaria javanica, for Both Aphids and Plant Fungal Pathogens. Mycobiology 2018, 46, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.S.; Hyon, J.H.; Kim, H.S.; Jang, S.H. Biological Control of the Small Leafhopper, Empoasca flavescens F. (Homoptera: Cicadellidae) Using the Entomopathogenic Fungus, Verticillium lecanii. Egypt. J. Biol. Pest Control 2023, 33, 36. [Google Scholar] [CrossRef]
- García-Nevárez, G.; Hidalgo-Jaminson, E. Comparación de Cepas Locales y Comerciales de Simplicillium y Lecanicillium Colonizando Pústulas de Hemileia vastatrix. Rev. Mex. Fitopatol. 2019. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model Application of Entomopathogenic Fungi as Alternatives to Chemical Pesticides: Prospects, Challenges, and Insights for Next-Generation Sustainable Agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef] [PubMed]
- Babbal; Adivitiya; Khasa, Y.P. Microbes as Biocontrol Agents. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 507–552. ISBN 978-981-10-3473-2. [Google Scholar]
- Srivastava, D.A.; Harris, R.; Breuer, G.; Levy, M. Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis. Plants 2021, 10, 210. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of Action and Biocontrol Potential of Trichoderma against Fungal Plant Diseases—A Review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Elamathi, E.; Malathi, P.; Viswanathan, R.; Ramesh Sundar, A. Expression Analysis on Mycoparasitism Related Genes during Antagonism of Trichoderma with Colletotrichum falcatum Causing Red Rot in Sugarcane. J. Plant Biochem. Biotechnol. 2018, 27, 351–361. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Lichius, A.; Turrà, D.; Di Pietro, A.; Zeilinger, S. Chemotropism Assays for Plant Symbiosis and Mycoparasitism Related Compound Screening in Trichoderma atroviride. Front. Microbiol. 2020, 11, 601251. [Google Scholar] [CrossRef]
- Baiyee, B.; Pornsuriya, C.; Ito, S.; Sunpapao, A. Trichoderma spirale T76-1 Displays Biocontrol Activity against Leaf Spot on Lettuce (Lactuca Sativa L.) Caused by Corynespora cassiicola or Curvularia aeria. Biol. Control 2019, 129, 195–200. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; del-Val, E.; Larsen, J. The Interactions of Trichoderma at Multiple Trophic Levels: Inter-Kingdom Communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Zaki, O.; Weekers, F.; Thonart, P.; Tesch, E.; Kuenemann, P.; Jacques, P. Limiting Factors of Mycopesticide Development. Biol. Control 2020, 144, 104220. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.J.; Ton, J.; et al. The Induced Resistance Lexicon: Do’s and Don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.D.M.; Varanda, C.M.R.; Félix, M.R.F. Induced Resistance during the Interaction Pathogen x Plant and the Use of Resistance Inducers. Phytochem. Lett. 2016, 15, 152–158. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Pacios-Michelena, S.; Palacios-Ponce, A.S.; Chávez-González, M.L.; Aguilar, C.N. Trichoderma as a Biological Control Agent: Mechanisms of Action, Benefits for Crops and Development of Formulations. World J. Microbiol. Biotechnol. 2023, 39, 269. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced Systemic Resistance (ISR) in Plants: Mechanism of Action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Fontana, D.C.; De Paula, S.; Torres, A.G.; De Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Srinivas, V.; Sharma, M. Deciphering the Tri-Dimensional Effect of Endophytic Streptomyces sp. on Chickpea for Plant Growth Promotion, Helper Effect with Mesorhizobium ciceri and Host-Plant Resistance Induction against Botrytis cinerea. Microb. Pathog. 2018, 122, 98–107. [Google Scholar] [CrossRef]
- Molitor, A.; Zajic, D.; Voll, L.M.; Pons-Kühnemann, J.; Samans, B.; Kogel, K.-H.; Waller, F. Barley Leaf Transcriptome and Metabolite Analysis Reveals New Aspects of Compatibility and Piriformospora Indica–Mediated Systemic Induced Resistance to Powdery Mildew. Mol. Plant-Microbe Interact. 2011, 24, 1427–1439. [Google Scholar] [CrossRef]
- Xu, X.; He, Q.; Chen, C.; Zhang, C. Differential Communications between Fungi and Host Plants Revealed by Secretome Analysis of Phylogenetically Related Endophytic and Pathogenic Fungi. PLoS ONE 2016, 11, e0163368. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.A.; Bae, H.; Strem, M.D.; Crozier, J.; Thomas, S.E.; Samuels, G.J.; Vinyard, B.T.; Holmes, K.A. Antibiosis, Mycoparasitism, and Colonization Success for Endophytic Trichoderma Isolates with Biological Control Potential in Theobroma cacao. Biol. Control 2008, 46, 24–35. [Google Scholar] [CrossRef]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial Biopesticides: Current Status and Advancement for Sustainable Agriculture and Environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. ISBN 978-0-12-820526-6. [Google Scholar]
- Georgis, R.; Koppenhöfer, A.M.; Lacey, L.A.; Bélair, G.; Duncan, L.W.; Grewal, P.S.; Samish, M.; Tan, L.; Torr, P.; Van Tol, R.W.H.M. Successes and Failures in the Use of Parasitic Nematodes for Pest Control. Biol. Control 2006, 38, 103–123. [Google Scholar] [CrossRef]
- Chitra, P.; Sujatha, K.; Jeyasankar, A. Entomopathogenic Nematode as a Biocontrol Agent—Recent Trends—A Review. Int. J. Adv. Res. Biol. Sci. 2017, 4, 9–20. [Google Scholar] [CrossRef]
- Kumar, D.; Kumari, P.; Kamboj, R.; Kumar, A.; Banakar, P.; Kumar, V. Entomopathogenic Nematodes as Potential and Effective Biocontrol Agents against Cutworms, Agrotis Spp.: Present and Future Scenario. Egypt. J. Biol. Pest Control 2022, 32, 42. [Google Scholar] [CrossRef]
- Smagghe, F.; Spooner-Hart, R.; Chen, Z.-H.; Donovan-Mak, M. Biological Control of Arthropod Pests in Protected Cropping by Employing Entomopathogens: Efficiency, Production and Safety. Biol. Control 2023, 186, 105337. [Google Scholar] [CrossRef]
- Sharma, M.P.; Sharma, A.N.; Hussaini, S.S. Entomopathogenic Nematodes, a Potential Microbial Biopesticide: Mass Production and Commercialisation Status—A Mini Review. Arch. Phytopathol. Plant Prot. 2011, 44, 855–870. [Google Scholar] [CrossRef]
- Li, X.; Cowles, E.A.; Cowles, R.S.; Gaugler, R.; Cox-Foster, D.L. Characterization of Immunosuppressive Surface Coat Proteins from Steinernema glaseri That Selectively Kill Blood Cells in Susceptible Hosts. Mol. Biochem. Parasitol. 2009, 165, 162–169. [Google Scholar] [CrossRef]
- Bojko, J.; Reinke, A.W.; Stentiford, G.D.; Williams, B.; Rogers, M.S.J.; Bass, D. Microsporidia: A New Taxonomic, Evolutionary, and Ecological Synthesis. Trends Parasitol. 2022, 38, 642–659. [Google Scholar] [CrossRef]
- Khalaf, A.; Lawniczak, M.K.N.; Blaxter, M.L.; Jaron, K.S. Polyploidy Is Widespread in Microsporidia. Microbiol. Spectr. 2024, 12, e03669-23. [Google Scholar] [CrossRef]
- Murareanu, B.M.; Sukhdeo, R.; Qu, R.; Jiang, J.; Reinke, A.W. Generation of a Microsporidia Species Attribute Database and Analysis of the Extensive Ecological and Phenotypic Diversity of Microsporidia. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.; Francis, O.; Blaxter, M.L. Genome Evolution in Intracellular Parasites: Microsporidia and Apicomplexa. J. Eukaryot. Microbiol. 2024, 71, e13033. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, L.P. Biopesticides in Organic Farming: Recent Advances 2021; Taylor & Francis Group: Abingdon, UK, 2021. [Google Scholar]
- Mehrabadi, M.; Darsouei, R.; Karimi, J. Insect Pathogenic Viruses, Microsporidians and Endosymbionts. In Biological Control of Insect and Mite Pests in Iran; Springer International Publishing: Cham, Switzerland, 2021; pp. 505–534. ISBN 978-3-030-63990-7. [Google Scholar]
- Hajek, A.E.; Gardescu, S.; Delalibera, I. Summary of Classical Biological Control Introductions of Entomopathogens and Nematodes for Insect Control. BioControl 2020, 66, 167–180. [Google Scholar] [CrossRef]
- Lewis, L.C.; Bruck, D.J.; Prasifka, J.R.; Raun, E.S. Nosema Pyrausta: Its Biology, History, and Potential Role in a Landscape of Transgenic Insecticidal Crops. Biol. Control 2009, 48, 223–231. [Google Scholar] [CrossRef]
- Malysh, J.M.; Chertkova, E.A.; Tokarev, Y.S. The Microsporidium Nosema pyrausta as a Potent Microbial Control Agent of the Beet Webworm Loxostege sticticalis. J. Invertebr. Pathol. 2021, 186, 107675. [Google Scholar] [CrossRef] [PubMed]
- Dakhel, W.H.; Jaronski, S.T.; Schell, S. Control of Pest Grasshoppers in North America. Insects 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H. Potential integrated management practices against desert locusts (Schistocerca gregaria) in nepal: A mini review. Nat. Resour. Sustain. Dev. 2020, 10, 115–124. [Google Scholar] [CrossRef]
- Pocco, M.E.; Laura De Wysiecki, M.; Lange, C.E. Infectivity of Paranosema locustae (Microsporidia) against Gregarious-Phase South American Locust (Orthoptera) When Treated En Masse. J. Invertebr. Pathol. 2020, 177, 107504. [Google Scholar] [CrossRef]
- Fuxa, J.R.; Brooks, W.M. Effects of Vairimorpha Necatrix in Sprays and Corn Meal on Heliothis Species in Tobacco, Soybeans, and Sorghum123. J. Econ. Entomol. 1979, 72, 462–467. [Google Scholar] [CrossRef]
- Jordan, C.; de Carvalho, V.R.; Mascarin, G.M.; dos Santos Oliveira, L.R.; Dunlap, C.A.; Wilcken, C.F. First Record of a New Microsporidium Pathogenic to Gonipterus platensis in Brazil. Sci. Rep. 2021, 11, 10971. [Google Scholar] [CrossRef]
- Deans, C.; Krischik, V. The Current State and Future Potential of Microbial Control of Scarab Pests. Appl. Sci. 2023, 13, 766. [Google Scholar] [CrossRef]
- Vaselek, S. The Role of Protists, Nematodes and Mites as Natural Control Agents of Sandfly Populations. Front. Trop. Dis. 2024, 5, 1369007. [Google Scholar] [CrossRef]
- Pinheiro, D.H.; Valicente, F.H. Identification of Bacillus thuringiensis Strains for the Management of Lepidopteran Pests. Neotrop. Entomol. 2021, 50, 804–811. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Musolino, V.; Lupia, C.; Palma, E.; Britti, D.; Musella, V. Entomopathogenic Fungi for Pests and Predators Control in Beekeeping. Vet. Sci. 2022, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, A.; Badosa, E.; Cabrefiga, J.; Francés, J.; Montesinos, E. Prospects and Limitations of Microbial Pesticides for Control of Bacterial and Fungal Pomefruit Tree Diseases. Trees 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Harte, S.J.; Bray, D.P.; Nash-Woolley, V.; Stevenson, P.C.; Fernández-Grandon, G.M. Antagonistic and Additive Effect When Combining Biopesticides against the Fall Armyworm, Spodoptera frugiperda. Sci. Rep. 2024, 14, 6029. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 275–293. ISBN 978-3-030-53238-3. [Google Scholar]
- Kala, S.; Sogan, N.; Agarwal, A.; Naik, S.N.; Patanjali, P.K.; Kumar, J. Biopesticides: Formulations and Delivery Techniques. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–220. ISBN 978-0-12-819304-4. [Google Scholar]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern Seed Technology: Seed Coating Delivery Systems for Enhancing Seed and Crop Performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Mishra, R.K.; Bohra, A.; Kamaal, N.; Kumar, K.; Gandhi, K.; GK, S.; Saabale, P.R.; SJ, S.N.; Sarma, B.K.; Kumar, D.; et al. Utilization of Biopesticides as Sustainable Solutions for Management of Pests in Legume Crops: Achievements and Prospects. Egypt. J. Biol. Pest Control 2018, 28, 3. [Google Scholar] [CrossRef]
- Talaviya, T.; Shah, D.; Patel, N.; Yagnik, H.; Shah, M. Implementation of Artificial Intelligence in Agriculture for Optimisation of Irrigation and Application of Pesticides and Herbicides. Artif. Intell. Agric. 2020, 4, 58–73. [Google Scholar] [CrossRef]
- Marciano, A.F.; Mascarin, G.M.; Franco, R.F.F.; Golo, P.S.; Jaronski, S.T.; Fernandes, É.K.K.; Bittencourt, V.R.E.P. Innovative Granular Formulation of Metarhizium robertsii Microsclerotia and Blastospores for Cattle Tick Control. Sci. Rep. 2021, 11, 4972. [Google Scholar] [CrossRef]
- Sullivan, C.F.; Parker, B.L.; Skinner, M. A Review of Commercial Metarhizium- and Beauveria-Based Biopesticides for the Biological Control of Ticks in the USA. Insects 2022, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, A.; Theologidis, I.; Varympopi, A.; Papafotis, D.; Mermigka, G.; Tzima, A.; Panopoulos, N.J.; Skandalis, N. Shifting Perspectives of Translational Research in Bio-Bactericides: Reviewing the Bacillus amyloliquefaciens Paradigm. Biology 2021, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, R.; Liu, Y.; Liu, Y. Evaluation of the Biocontrol Efficiency of Bacillus subtilis Wettable Powder on Pepper Root Rot Caused by Fusarium solani. Pathogens 2023, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Navi, S.S.; Yang, X.B. Efficacy of Seed Treatments with Bradyrhizobium japonicum to Reduce Occurrence of Soybean Sudden Death Syndrome in Early-Planted Soybeans. In Fungal Diversity, Ecology and Control Management; Springer Nature: Singapore, 2022; pp. 415–437. ISBN 9789811688775. [Google Scholar]
- Suárez-Estrella, F.; Jurado, M.M.; López-González, J.A.; Toribio, A.; Martínez-Gallardo, M.R.; Estrella-González, M.J.; López, M.J. Seed Priming by Application of Microbacterium Spp. Strains for Control of Botrytis cinerea and Growth Promotion of Lettuce Plants. Sci. Hortic. 2023, 313, 111901. [Google Scholar] [CrossRef]
- Dhananjayan, V.; Jayakumar, S.; Ravichandran, B. Conventional Methods of Pesticide Application in Agricultural Field and Fate of the Pesticides in the Environment and Human Health. In Controlled Release of Pesticides for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–39. ISBN 978-3-030-23396-9. [Google Scholar]
- Holka, M.; Kowalska, J. The Potential of Adjuvants Used with Microbiological Control of Insect Pests with Emphasis on Organic Farming. Agriculture 2023, 13, 1659. [Google Scholar] [CrossRef]
- Lin, F.; Mao, Y.; Zhao, F.; Idris, A.L.; Liu, Q.; Zou, S.; Guan, X.; Huang, T. Towards Sustainable Green Adjuvants for Microbial Pesticides: Recent Progress, Upcoming Challenges, and Future Perspectives. Microorganisms 2023, 11, 364. [Google Scholar] [CrossRef]
- Damak, M.; de Ruiter, J.; Panat, S.; Varanasi, K.K. Dynamics of an Impacting Emulsion Droplet. Sci. Adv. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, Y. A Review: Development of Plant Protection Methods and Advances in Pesticide Application Technology in Agro-Forestry Production. Agriculture 2023, 13, 2165. [Google Scholar] [CrossRef]
- Lysov, A.K.; Kornilov, T.V.; Krasnobaeva, I.L. Improvement of the Technology of Application of Biological Products for Plant Protection. Russ. Agric. Sci. 2023, 49, S141–S147. [Google Scholar] [CrossRef]
- Xun, L.; Campos, J.; Salas, B.; Fabregas, F.X.; Zhu, H.; Gil, E. Advanced Spraying Systems to Improve Pesticide Saving and Reduce Spray Drift for Apple Orchards. Precis. Agric. 2023, 24, 1526–1546. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The Effect of Trichoderma Biofertilizer on the Quality of Flowering Chinese Cabbage and the Soil Environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Nikolova, I. Effect of Biopesticides on the Spotted Alfalfa Aphid, Therioaphis trifolii Monell, and Its Predator, Coccinella septempunctata L. Crop Prot. 2024, 177, 106532. [Google Scholar] [CrossRef]
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L. Novel Endotherapy-Based Applications of Ozonated Water to Bobal Grapevines: Effect on Grape Quality. Agronomy 2020, 10, 1218. [Google Scholar] [CrossRef]
- Gyuris, R.; Szabó, Á.; László, A.M.; Gutermuth, Á.; Sörös, C. An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). Horticulturae 2024, 10, 278. [Google Scholar] [CrossRef]
- Archer, L.; Albrecht, U.; Crane, J. Trunk Injection to Deliver Crop Protection Materials: An Overview of Basic Principles and Practical Considerations: HS1426, 11/2021. EDIS 2021. [Google Scholar] [CrossRef]
- Crane, J.H.; Carrillo, D.; Evans, E.A.; Gazis, R.; Schaffer, B.A.; Ballen Orozco, F.H.; Wasielewski, J. Recommendations for Control and Mitigation of Laurel Wilt and Ambrosia Beetle Vectors in Commercial Avocado Groves in Florida. EDIS 2020, 2020. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk Injection as a Tool to Deliver Plant Protection Materials—An Overview of Basic Principles and Practical Considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Murolo, S.; Concas, J.; Romanazzi, G. Use of Biocontrol Agents as Potential Tools in the Management of Chestnut Blight. Biol. Control 2019, 132, 102–109. [Google Scholar] [CrossRef]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological Control of Emerging Forest Diseases: How Can We Move from Dreams to Reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Benigno, A.; Aglietti, C.; Cacciola, S.O.; Moricca, S. Trunk Injection Delivery of Biocontrol Strains of Trichoderma Spp. Effectively Suppresses Nut Rot by Gnomoniopsis Castaneae in Chestnut (Castanea sativa Mill.). Biology 2024, 13, 143. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Recent Advances in Downstream Processing and Formulations of Bacillus Thuringiensis Based Biopesticides. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Screening of Different Adjuvants for Wastewater/Wastewater Sludge-Based Bacillus thuringiensis Formulations. J. Econ. Entomol. 2006, 99, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, T.; Gong, W.; Gao, Y.; Zhao, G. Additive Screening and Formula Optimization of Microbial Inhibitor Having Disease Prevention and Growth Promotion Effects on Avena Sativa. Front. Microbiol. 2023, 14, 1208591. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, L.; Groves, R.L.; Bradford, B.; Harding, R.S.; Nault, B.A. Evaluating Combinations of Bioinsecticides and Adjuvants for Managing Thrips tabaci (Thysanoptera: Thripidae) in Onion Production Systems. Crop Prot. 2021, 142, 105527. [Google Scholar] [CrossRef]
- Bayramoglu, Z.; Gencer, D.; Demir, I. Development of Novel Betabaculovirus (HycuGV-Hc1) as a Biopesticide (HycuGV-TR61) and Its Efficacy on the Fall Webworm, Hyphantria cunea Drury (Lepidoptera: Erebidae) Larvae. Egypt. J. Biol. Pest Control 2023, 33, 21. [Google Scholar] [CrossRef]
- Kim, J.S.; Je, Y.H.; Woo, E.O. Roles of Adjuvants in Aphicidal Activity of Enzymes from Beauveria bassiana (Ascomycota: Hypocreales) SFB-205 Supernatant. J. Asia-Pac. Entomol. 2010, 13, 345–350. [Google Scholar] [CrossRef]
- Bhuiyan, M.Z.R.; Del Río Mendoza, L.E.; Lakshman, D.K.; Qi, A.; Khan, M.F.R. Evaluation of Adjuvants Added to Fungicides for Controlling Cercospora Leaf Spot on Sugar Beet. Crop Prot. 2024, 175, 106471. [Google Scholar] [CrossRef]
- Preininger, C.; Sauer, U.; Bejarano, A.; Berninger, T. Concepts and Applications of Foliar Spray for Microbial Inoculants. Appl. Microbiol. Biotechnol. 2018, 102, 7265–7282. [Google Scholar] [CrossRef]
- Idziak, R.; Sobczak, A.; Waligora, H.; Szulc, P. Impact of Multifunctional Adjuvants on Efficacy of Sulfonylurea Herbicide Applied in Maize (Zea mays L.). Plants 2023, 12, 1118. [Google Scholar] [CrossRef]
- Gava, C.A.T.; de Castro, A.P.C.; Pereira, C.A.; Gonçalves, J.S.; Araújo, L.F.C.; da Paz, C.D. Photoprotector Adjuvants to Enhance UV Tolerance of Yeast Strains for Controlling Mango Decay Using Pre-Harvest Spraying. Biocontrol Sci. Technol. 2018, 28, 811–822. [Google Scholar] [CrossRef]
- Lopes, M.M.; de Oliveira-Paiva, C.A.; Farinas, C.S. Modification of Pectin/Starch-Based Beads with Additives to Improve Bacillus subtilis Encapsulation for Agricultural Applications. Int. J. Biol. Macromol. 2023, 246, 125646. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhao, L.; Sun, W.; Hu, Y.; Han, H. Surface Characteristics and Wettability Enhancement of Respirable Sintering Dust by Nonionic Surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 323–333. [Google Scholar] [CrossRef]
- Karamchandani, B.M.; Dalvi, S.G.; Bagayatkar, M.; Banat, I.M.; Satpute, S.K. Prospective Applications of Chitosan and Chitosan-Based Nanoparticles Formulations in Sustainable Agricultural Practices. Biocatal. Agric. Biotechnol. 2024, 58, 103210. [Google Scholar] [CrossRef]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.M.R.; De France, K. Biotechnological Development of Trichoderma-Based Formulations for Biological Control. Appl. Microbiol. Biotechnol. 2023, 107, 5595–5612. [Google Scholar] [CrossRef]
- Nguyen Thi, H.; Nguyen, Q.N.; Dang Thi, N.Q.; Nguyen, N.L.; Do, A.D. Mass Production of Entomopathogenic Fungi Purpureocillium Lilacinum PL1 as a Biopesticide for the Management of Amrasca devastans (Hemiptera: Cicadellidae) in Okra Plantation. Egypt. J. Biol. Pest Control 2023, 33, 85. [Google Scholar] [CrossRef]
- Zhang, C.; Ali Khan, R.A.; Wei, H.; Wang, R.; Hou, J.; Liu, T. Rapid and Mass Production of Biopesticide Trichoderma brev T069 from Cassava Peels Using Newly Established Solid-State Fermentation Bioreactor System. J. Environ. Manag. 2022, 313, 114981. [Google Scholar] [CrossRef]
- Santos, P.d.S.; Abati, K.; Mendoza, N.V.R.; Mascarin, G.M.; Delalibera Júnior, I. Nutritional Impact of Low-Cost Substrates on Biphasic Fermentation for Conidia Production of the Fungal Biopesticide Metarhizium anisopliae. Bioresour. Technol. Rep. 2021, 13, 100619. [Google Scholar] [CrossRef]
- Lobo, C.B.; Juárez Tomás, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of Low-Cost Formulations of Plant Growth-Promoting Bacteria to Be Used as Inoculants in Beneficial Agricultural Technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- dos Santos, H.M.; de S. Varize, C.; Valença, C.A.S.; Dossi, F.C.A.; de Aragão Batista, M.V.; Fernandes, R.P.M.; Severino, P.; Souto, E.B.; Dolabella, S.S.; da C. Mendonça, M.; et al. Use of Agro-Industrial Bio-Waste for the Growth and Production of a Previously Isolated Bacillus thuringiensis Strain. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 5. [Google Scholar] [CrossRef]
- Mulatu, A.; Alemu, T.; Megersa, N.; Vetukuri, R.R. Optimization of Culture Conditions and Production of Bio-Fungicides from Trichoderma Species under Solid-State Fermentation Using Mathematical Modeling. Microorganisms 2021, 9, 1675. [Google Scholar] [CrossRef] [PubMed]
- Ndao, A.; Sellamuthu, B.; Kumar, L.R.; Tyagi, R.D.; Valéro, J.R. Biopesticide Production Using Bacillus thuringiensis Kurstaki by Valorization of Starch Industry Wastewater and Effluent from Aerobic, Anaerobic Digestion. Syst. Microbiol. Biomanuf. 2021, 1, 494–504. [Google Scholar] [CrossRef]
- Saberi, F.; Marzban, R.; Ardjmand, M.; Pajoum Shariati, F.; Tavakoli, O. Optimization of the Culture Medium, Fermentation Process, and Effectiveness of a Biopesticide from an Iranian Bacillus thuringiensis Var. Tenebrionis (BN2). J. Agric. Sci. Technol. 2023, 25, 469–484. [Google Scholar] [CrossRef]
- Fayad, N.; Abboud, J.; Driss, F.; Louka, N.; Kallassy Awad, M. Optimization of Culture Conditions and Wheat Bran Class Selection in the Production of Bacillus thuringiensis-Based Biopesticides. Fermentation 2022, 8, 666. [Google Scholar] [CrossRef]
- Morales-Borrell, D.; González-Fernández, N.; Mora-González, N.; Pérez-Heredia, C.; Campal-Espinosa, A.; Bover-Fuentes, E.; Salazar-Gómez, E.; Morales-Espinosa, Y. Design of a Culture Medium for Optimal Growth of the Bacterium Pseudoxanthomonas Indica H32 Allowing Its Production as Biopesticide and Biofertilizer. AMB Express 2020, 10, 190. [Google Scholar] [CrossRef]
- Sala, A.; Barrena, R.; Sánchez, A.; Artola, A. Fungal Biopesticide Production: Process Scale-up and Sequential Batch Mode Operation with Trichoderma Harzianum Using Agro-Industrial Solid Wastes of Different Biodegradability. Chem. Eng. J. 2021, 425, 131620. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst. Microbiol. Biomanuf. 2020, 1, 142–165. [Google Scholar] [CrossRef]
- Sala, A.; Artola, A.; Sánchez, A.; Barrena, R. Rice Husk as a Source for Fungal Biopesticide Production by Solid-State Fermentation Using B. bassiana and T. harzianum. Bioresour. Technol. 2020, 296, 122322. [Google Scholar] [CrossRef]
- Sala, A.; Vittone, S.; Barrena, R.; Sánchez, A.; Artola, A. Scanning Agro-Industrial Wastes as Substrates for Fungal Biopesticide Production: Use of Beauveria bassiana and Trichoderma harzianum in Solid-State Fermentation. J. Environ. Manag. 2021, 295, 113113. [Google Scholar] [CrossRef]
- Hamrouni, R.; Regus, F.; Claeys-Bruno, M.; Farnet Da Silva, A.-M.; Orsière, T.; Laffont-Schwob, I.; Boudenne, J.-L.; Dupuy, N. Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum. J. Fungi 2023, 9, 1123. [Google Scholar] [CrossRef]
- de Rezende, L.C.; de Andrade Carvalho, A.L.; Costa, L.B.; de Almeida Halfeld-Vieira, B.; Silva, L.G.; Pinto, Z.V.; Morandi, M.A.B.; de Medeiros, F.H.V.; Mascarin, G.M.; Bettiol, W. Optimizing Mass Production of Trichoderma asperelloides by Submerged Liquid Fermentation and Its Antagonism against Sclerotinia sclerotiorum. World J. Microbiol. Biotechnol. 2020, 36, 113. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, M.; Malusà, E.; Sas-Paszt, L.; Trzcinski, P.; Galvez, A.; Flor-Peregrin, E.; Shilev, S.; Canfora, L.; Mocali, S.; Vassilev, N. Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality. Microorganisms 2021, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.F.; Kubeneck, S.; Bonatto, C.; Bazoti, S.F.; Nerling, J.P.; Klein, G.H.; Michelon, W.; Alves, S.L.; Mossi, A.J.; Fongaro, G.; et al. Trichoderma koningiopsis Fermentation in Airlift Bioreactor for Bioherbicide Production. Bioprocess Biosyst. Eng. 2024, 47, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, G.O.; Pimentel, M.F.; Bueno, L.A.; Lobo Junior, M.; Mascarin, G.M.; Luna Finkler, C.L. Production of Microsclerotia by Trichoderma asperellum through Submerged Liquid Fermentation Using Low-Cost Nitrogen and Carbon Sources. Biocatal. Agric. Biotechnol. 2022, 44, 102455. [Google Scholar] [CrossRef]
- Ordaz-Hernández, A.; Montesinos-Matías, R.; Mellín-Rosas, M.A.; Pérez-Aguirre, T.; Loera, O.; Angel-Cuapio, A. Improvement of the Production and Quality of Cordyceps javanica Conidia for the Control of Diaphorina citri Adults. World J. Microbiol. Biotechnol. 2024, 40, 115. [Google Scholar] [CrossRef]
- Roswanjaya, Y.P.; Saryanah, N.A.; Devy, L. Conidia Production of Beauveria bassiana in Solid Substrate Fermentation Using a Biphasic System. KnE Life Sci. 2022. [Google Scholar] [CrossRef]
- Cuatlayotl-Cottier, R.; Huerta-de la Peña, A.; Peña-Chora, G.; Salazar-Magallón, J.A. Insecticidal Activity of Industrial By-Products Fermented by Bacillus thuringiensis Strain GP139 against Mites (Prostigmata: Tetranychidae) and Aphids (Hemiptera: Aphidoidea). Biocontrol Sci. Technol. 2021, 32, 103–109. [Google Scholar] [CrossRef]
- Iwanicki, N.S.; Mascarin, G.M.; Moreno, S.G.; Eilenberg, J.; Delalibera Júnior, I. Growth Kinetic and Nitrogen Source Optimization for Liquid Culture Fermentation of Metarhizium robertsii Blastospores and Bioefficacy against the Corn Leafhopper Dalbulus maidis. World J. Microbiol. Biotechnol. 2020, 36, 71. [Google Scholar] [CrossRef]
- Iannaccone, F.; Alborino, V.; Dini, I.; Balestrieri, A.; Marra, R.; Davino, R.; Di Francia, A.; Masucci, F.; Serrapica, F.; Vinale, F. In Vitro Application of Exogenous Fibrolytic Enzymes from Trichoderma Spp. to Improve Feed Utilization by Ruminants. Agriculture 2022, 12, 573. [Google Scholar] [CrossRef]
- Li, T.; Tang, J.; Karuppiah, V.; Li, Y.; Xu, N.; Chen, J. Co-Culture of Trichoderma atroviride SG3403 and Bacillus subtilis 22 Improves the Production of Antifungal Secondary Metabolites. Biol. Control 2020, 140, 104122. [Google Scholar] [CrossRef]
- Bhandari, P.; Pant, M.; Patanjali, P.K.; Raza, S.K. Advances in Bio-Botanicals Formulations with Incorporation of Nanotechnology in Intensive Crop Management. In Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 291–305. ISBN 978-3-319-42990-8. [Google Scholar]
- Waghunde, R.R.; Shinde, C.U.; Pandey, P.; Singh, C. Fungal Biopesticides for Agro-Environmental Sustainability. In Industrially Important Fungi for Sustainable Development; Springer International Publishing: Cham, Switzerland, 2021; pp. 479–508. ISBN 978-3-030-67561-5. [Google Scholar]
- Borges, D.F.; Lopes, E.A.; Fialho Moraes, A.R.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Moreira Valente, V.M. Formulation of Botanicals for the Control of Plant-Pathogens: A Review. Crop Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Awan, U.A.; Meng, L.; Xia, S.; Raza, M.F.; Zhang, Z.; Zhang, H. Isolation, Fermentation, and Formulation of Entomopathogenic Fungi Virulent against Adults of Diaphorina citri. Pest Manag. Sci. 2021, 77, 4040–4053. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Das, U.; Panda, S.K.; Saranraj, P. Microorganisms in Fermentation. In Learning Materials in Biosciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–39. ISBN 978-3-030-16230-6. [Google Scholar]
- Ptaszek, M.; Canfora, L.; Pugliese, M.; Pinzari, F.; Gilardi, G.; Trzciński, P.; Malusà, E. Microbial-Based Products to Control Soil-Borne Pathogens: Methods to Improve Efficacy and to Assess Impacts on Microbiome. Microorganisms 2023, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaushik, N.; Sharma, A.; Djébali, N. Development of Novel Liquid Formulation of Bacillus siamensis with Antifungal and Plant Growth Promoting Activity. ACS Agric. Sci. Technol. 2022, 3, 55–66. [Google Scholar] [CrossRef]
- Lei, C.J.; Ahmad, R.H.I.R.; Halim, N.A.; Asib, N.; Zakaria, A.; Azmi, W.A. Bioefficacy of an Oil-Emulsion Formulation of Entomopathogenic Fungus, Metarhizium anisopliae against Adult Red Palm Weevil, Rhynchophorus ferrugineus. Insects 2023, 14, 482. [Google Scholar] [CrossRef]
- Yaakov, N.; Kottakota, C.; Mani, K.A.; Naftali, S.M.; Zelinger, E.; Davidovitz, M.; Ment, D.; Mechrez, G. Encapsulation of Bacillus thuringiensis in an Inverse Pickering Emulsion for Pest Control Applications. Colloids Surf. B Biointerfaces 2022, 213, 112427. [Google Scholar] [CrossRef] [PubMed]
- Amar Feldbaum, R.; Yaakov, N.; Ananth Mani, K.; Yossef, E.; Metbeev, S.; Zelinger, E.; Belausov, E.; Koltai, H.; Ment, D.; Mechrez, G. Single Cell Encapsulation in a Pickering Emulsion Stabilized by TiO2 Nanoparticles Provides Protection against UV Radiation for a Biopesticide. Colloids Surf. B Biointerfaces 2021, 206, 111958. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.C.; Spannemberg, S.S.; Brun, T.; Schmaltz, S.; Escobar, O.; Sanchotene, D.M.; Dornelles, S.H.B.; Zabot, G.L.; Tres, M.V.; Kuhn, R.C.; et al. Development of a Solid Bioherbicide Formulation by Spray Drying Technology. Agriculture 2020, 10, 215. [Google Scholar] [CrossRef]
- Quiroga-Cubides, G.; Toloza-Moreno, D.; Barrera, G.; Gómez, J.; Ruiz, J.; Gómez, M.; Cortes-Rojas, D. Formulation Process of a Biopesticide Based on the Erinnyis Ello Betabaculovirus ErelGV: Unit Operations Analysis. Rev. Mex. Ing. Quím. 2021, 20, 989–1004. [Google Scholar] [CrossRef]
- Han, S.; Wu, C.; Fan, Y.; Cao, J.; Wang, A. Optimization of Water Dispersible Granules Containing Biocontrol Bacteria and Stress Alleviation on Cotton. Chil. J. Agric. Res. 2024, 84, 195–210. [Google Scholar] [CrossRef]
- Şenol Kotan, M.; Dikbaş, N.; Kotan, R. Solid Carrier Bacterial Formulations against Fusarium Root and Stem Rot Disease in Cucumber. J. Plant Pathol. 2023, 106, 153–163. [Google Scholar] [CrossRef]
- Advances in Agricultural Entomology; AkiNik Publications: New Delhi, India, 2021; ISBN 978-93-90541-37-9.
- Vimala Devi, P.S.; Duraimurugan, P.; Chandrika, K.S.V.P.; Vineela, V. Development of a Water Dispersible Granule (WDG) Formulation of Bacillus thuringiensis for the Management of Spodoptera litura (Fab.). Biocontrol Sci. Technol. 2021, 31, 850–864. [Google Scholar] [CrossRef]
- WHO Chemical Safety: Pesticides. Available online: https://www.who.int/news-room/questions-and-answers/item/chemical-safety-pesticides (accessed on 7 May 2024).
- Pesticides Use, Pesticides Trade and Pesticides Indicators; FAO: Rome, Italy, 2022; ISBN 978-92-5-136614-1.
- Baima Ferreira Freitas, I.; Duarte-Neto, P.J.; Sorigotto, L.R.; Cardoso Yoshii, M.P.; de Palma Lopes, L.F.; de Almeida Pereira, M.M.; Girotto, L.; Badolato Athayde, D.; Veloso Goulart, B.; Montagner, C.C.; et al. Effects of Pasture Intensification and Sugarcane Cultivation on Non-Target Species: A Realistic Evaluation in Pesticide-Contaminated Mesocosms. Sci. Total Environ. 2024, 922, 171425. [Google Scholar] [CrossRef] [PubMed]
- FAO CCPR/Committee Plays an Increasingly Important Role in Development of International Food Safety System. Available online: https://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1643101 (accessed on 7 May 2024).
- Lu, T.; Lei, C.; Gao, M.; Lv, L.; Zhang, C.; Qian, H.; Tang, T. A Risk Entropy Approach for Linking Pesticides and Soil Bacterial Communities. J. Hazard. Mater. 2024, 469, 133970. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.R.; Delabona, P.D.S. Strategies for Bioremediation of Pesticides: Challenges and Perspectives of the Brazilian Scenario for Global Application—A Review. Environ. Adv. 2022, 8, 100220. [Google Scholar] [CrossRef]
- Brüggemann, M.; Mayer, S.; Brown, D.; Terry, A.; Rüdiger, J.; Hoffmann, T. Measuring Pesticides in the Atmosphere: Current Status, Emerging Trends and Future Perspectives. Environ. Sci. Eur. 2024, 36, 39. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z. Assessing Pesticides in the Atmosphere: A Global Study on Pollution, Human Health Effects, Monitoring Network and Regulatory Performance. Environ. Int. 2024, 187, 108653. [Google Scholar] [CrossRef]
- Hulin, M.; Leroux, C.; Mathieu, A.; Gouzy, A.; Berthet, A.; Boivin, A.; Bonicelli, B.; Chubilleau, C.; Hulin, A.; Leoz Garziandia, E.; et al. Monitoring of Pesticides in Ambient Air: Prioritization of Substances. Sci. Total Environ. 2021, 753, 141722. [Google Scholar] [CrossRef]
- Kruse-Plaß, M.; Hofmann, F.; Wosniok, W.; Schlechtriemen, U.; Kohlschütter, N. Pesticides and Pesticide-Related Products in Ambient Air in Germany. Environ. Sci. Eur. 2021, 33, 114. [Google Scholar] [CrossRef]
- Yera, A.; Nascimento, M.; da Rocha, G.; de Andrade, J.; Vasconcellos, P. Occurrence of Pesticides Associated to Atmospheric Aerosols: Hazard and Cancer Risk Assessments. J. Braz. Chem. Soc. 2020, 31, 1317–1326. [Google Scholar] [CrossRef]
- Veludo, A.F.; Martins Figueiredo, D.; Degrendele, C.; Masinyana, L.; Curchod, L.; Kohoutek, J.; Kukučka, P.; Martiník, J.; Přibylová, P.; Klánová, J.; et al. Seasonal Variations in Air Concentrations of 27 Organochlorine Pesticides (OCPs) and 25 Current-Use Pesticides (CUPs) across Three Agricultural Areas of South Africa. Chemosphere 2022, 289, 133162. [Google Scholar] [CrossRef] [PubMed]
- Guida, Y.; de Carvalho, G.O.; Capella, R.; Pozo, K.; Lino, A.S.; Azeredo, A.; Carvalho, D.F.P.; Braga, A.L.F.; Torres, J.P.M.; Meire, R.O. Atmospheric Occurrence of Organochlorine Pesticides and Inhalation Cancer Risk in Urban Areas at Southeast Brazil. Environ. Pollut. 2021, 271, 116359. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; Colin, P.; Yahyaoui, A.; Petrique, O.; Yusà, V.; Mellouki, A.; Pastor, A. Occurrence of Currently Used Pesticides in Ambient Air of Centre Region (France). Atmos. Environ. 2010, 44, 3915–3925. [Google Scholar] [CrossRef]
- Datta, A.; Parkhey, P.; Bhatnagar, T. Bioremediation Studies on Pesticides in Soil by Novel Microorganisms Isolated from Agricultural Fields. Int. Res. J. Adv. Eng. Manag. (IRJAEM) 2024, 2, 317–325. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, G.; Bai, X.; Su, Y.; Zhang, Z.; Han, J. Research Progress on Remediation of Organochlorine Pesticide Contamination in Soil. Environ. Geochem. Health 2024, 46, 25. [Google Scholar] [CrossRef] [PubMed]
- Wahla, V.; Shukla, S. Role of Microorganisms in Bioremediation of Pesticides. In Research Anthology on Emerging Techniques in Environmental Remediation; IGI Global: Hershey, PA, USA, 2022; pp. 150–176. [Google Scholar]
- da Silva Júnior, A.H.; de Oliveira, C.R.S.; Leal, T.W.; Mapossa, A.B.; Fiates, J.; Ulson de Souza, A.A.; Ulson de Souza, S.M.d.A.G.; da Silva, A. Organochlorine Pesticides Remediation Techniques: Technological Perspective and Opportunities. J. Hazard. Mater. Lett. 2024, 5, 100098. [Google Scholar] [CrossRef]
- Raimondo, E.E.; Saez, J.M.; Aparicio, J.D.; Fuentes, M.S.; Benimeli, C.S. Bioremediation of Lindane-Contaminated Soils by Combining of Bioaugmentation and Biostimulation: Effective Scaling-up from Microcosms to Mesocosms. J. Environ. Manag. 2020, 276, 111309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Wang, Y.; Saleem, M.; Mu, Y.; Zheng, Y.; Zhang, Q. Pesticide Contamination Remediation by Biochar-Immobilized Microorganisms: A Review. Int. J. Environ. Sci. Technol. 2023, 21, 2225–2238. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, C.; Zhang, M.; Wu, Y.; Zhang, Z.; Zhang, H. Degradation Characteristics and Soil Remediation of Thifensulfuron-Methyl by Immobilized Serratia marcecens N80 Beads. Environ. Technol. Innov. 2021, 24, 102059. [Google Scholar] [CrossRef]
- Sharma, R.; Saroop, S. Role of Microbes in Pesticide Bioremediation: Recent Advances and Biotechnological Implications. In Pesticides in the Environment; Elsevier: Amsterdam, The Netherlands, 2024; pp. 223–250. ISBN 978-0-323-99427-9. [Google Scholar]
- Sun, L.-N.; Zhang, J.; Kwon, S.-W.; He, J.; Zhou, S.-G.; Li, S.-P. Paracoccus huijuniae sp. Nov., an Amide Pesticide-Degrading Bacterium Isolated from Activated Sludge of a Wastewater Biotreatment System. Int. J. Syst. Evol. Microbiol. 2013, 63, 1132–1137. [Google Scholar] [CrossRef]
- Tao, Y.; Hu, S.; Han, S.; Shi, H.; Yang, Y.; Li, H.; Jiao, Y.; Zhang, Q.; Akindolie, M.S.; Ji, M.; et al. Efficient Removal of Atrazine by Iron-Modified Biochar Loaded Acinetobacter Lwoffii DNS32. Sci. Total Environ. 2019, 682, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A Bio-Functions Integration Microcosm: Self-Immobilized Biochar-Pellets Combined with Two Strains of Bacteria to Remove Atrazine in Water and Mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.D.A.; Gebler, L.; Niemeyer, J.C.; Itako, A.T. Destination of Pesticide Residues on Biobeds: State of the Art and Future Perspectives in Latin America. Chemosphere 2020, 248, 126038. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abdullah, A.M.; Badr El Din, N.I.; Mishaqa, E.S.I. Biosorption Potential of the Microchlorophyte Chlorella vulgaris for Some Pesticides. J. Fertil. Pestic. 2017, 8, 177. [Google Scholar] [CrossRef]
- Plangklang, P.; Reungsang, A. Bioaugmentation of Carbofuran by Burkholderia cepacia PCL3 in a Bioslurry Phase Sequencing Batch Reactor. Process Biochem. 2010, 45, 230–238. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Hu, M.; Hao, W.; Geng, P.; Zhang, Y. Biodegradation of Carbofuran by Pichia anomala Strain HQ-C-01 and Its Application for Bioremediation of Contaminated Soils. Biol. Fertil. Soils 2011, 47, 917–923. [Google Scholar] [CrossRef]
- Da Luz, D.S.; Guimarães, P.S.; Castro, M.S.; Primel, E.G.; Giroldo, D.; Martins, C.D.M.G. Effects of the Pesticide Carbofuran on Two Species of Chlorophyceae (Desmodesmus communis and Pseudopediastrum boryanum) and Their Pesticide Bioremediation Ability. Environ. Toxicol. Chem. 2024, 43, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Bhasker Reddy, G.V.; Joshi, D.K.; Gold, M.H. Degradation of Chlorophenoxyacetic Acids by the Lignin-Degrading Fungus Dichomitus Squalens. Microbiology 1997, 143, 2353–2360. [Google Scholar] [CrossRef]
- Sun, X.; Li, D.; Li, B.; Sun, S.; Yabo, S.D.; Geng, J.; Ma, L.; Qi, H. Exploring the Disparity of Inhalable Bacterial Communities and Antibiotic Resistance Genes between Hazy Days and Non-Hazy Days in a Cold Megacity in Northeast China. J. Hazard. Mater. 2020, 398, 122984. [Google Scholar] [CrossRef]
- Shi, T.; Fang, L.; Qin, H.; Chen, Y.; Wu, X.; Hua, R. Rapid Biodegradation of the Organophosphorus Insecticide Chlorpyrifos by Cupriavidus nantongensis X1T. Int. J. Environ. Res. Public Health 2019, 16, 4593. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Suppahia, A.; Nadh, A.G.; Nair, A.S. Identification and Characterization of a Pesticide Degrading Flavobacterium Species EMBS0145 by 16S rRNA Gene Sequencing. Interdiscip. Sci. Comput. Life Sci. 2015, 7, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bempelou, E.D.; Vontas, J.G.; Liapis, K.S.; Ziogas, V.N. Biodegradation of Chlorpyrifos and 3,5,6-Trichloro-2-Pyridinol by the Epiphytic Yeasts Rhodotorula glutinis and Rhodotorula rubra. Ecotoxicology 2018, 27, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, Y.; Ma, L.; Gao, G.; Wang, Y. Combination of Biochar and Immobilized Bacteria in Cypermethrin-Contaminated Soil Remediation. Int. Biodeterior. Biodegrad. 2017, 120, 15–20. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Y.; Ai, G.-M.; Liu, Y.; Liu, Z.-P. Novel Chryseobacterium sp. PYR2 Degrades Various Organochlorine Pesticides (OCPs) and Achieves Enhancing Removal and Complete Degradation of DDT in Highly Contaminated Soil. J. Environ. Manag. 2015, 161, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Bhattacharya, A.; Kumar, S.; Singh, D.K.; Khare, S.K. Biodegradation of 1,1,1-Trichloro-2,2- Bis (4-Chlorophenyl) Ethane (DDT) by Using Serratia marcescens NCIM 2919. J. Environ. Sci. Health Part B 2016, 51, 809–816. [Google Scholar] [CrossRef]
- Fang, H.; Dong, B.; Yan, H.; Tang, F.; Yu, Y. Characterization of a Bacterial Strain Capable of Degrading DDT Congeners and Its Use in Bioremediation of Contaminated Soil. J. Hazard. Mater. 2010, 184, 281–289. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, L.; Wang, J. Combined Treatment of Contaminated Soil with a Bacterial Stenotrophomonas Strain DXZ9 and Ryegrass (Lolium perenne) Enhances DDT and DDE Remediation. Environ. Sci. Pollut. Res. 2018, 25, 31895–31905. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, T.; Najafpour, G.D.; Younesi, H.; Mohammadi, M. Rapid Biodegradation of Diazinon Using a Novel Strain of Candida pseudolambica. Environ. Technol. Innov. 2022, 25, 102218. [Google Scholar] [CrossRef]
- Kurade, M.B.; Kim, J.R.; Govindwar, S.P.; Jeon, B.-H. Insights into Microalgae Mediated Biodegradation of Diazinon by Chlorella vulgaris: Microalgal Tolerance to Xenobiotic Pollutants and Metabolism. Algal Res. 2016, 20, 126–134. [Google Scholar] [CrossRef]
- Feng, F.; Chen, X.; Wang, Q.; Xu, W.; Long, L.; Nabil EL-Masry, G.; Wan, Q.; Yan, H.; Cheng, J.; Yu, X. Use of Bacillus-Siamensis-Inoculated Biochar to Decrease Uptake of Dibutyl Phthalate in Leafy Vegetables. J. Environ. Manag. 2020, 253, 109636. [Google Scholar] [CrossRef]
- Lu, P.; Liu, H.; Liu, A. Biodegradation of Dicofol by Microbacterium sp. D-2 Isolated from Pesticide-Contaminated Agricultural Soil. Appl. Biol. Chem. 2019, 62, 72. [Google Scholar] [CrossRef]
- Wared Musa, H.A.; Abass, M.H.; Al-Farttoosy, A.H. Biodegradation of Difenoconazole Using Fungal-Bacterial Consortia. IOP Conf. Ser. Earth Environ. Sci. 2024, 1371, 022016. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, X.; Wu, X.; Dong, F.; Liu, X.; Xu, J.; Wu, X.; Li, M.; Zheng, Y. Removal of Dimethachlon from Soils Using Immobilized Cells and Enzymes of a Novel Potential Degrader Providencia Stuartii JD. J. Hazard. Mater. 2019, 378, 120606. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Wu, X.; Long, Y.; An, H.; Pan, X.; Li, M.; Dong, F.; Zheng, Y. Rapid Degradation of Dimethomorph in Polluted Water and Soil by Bacillus cereus WL08 Immobilized on Bamboo Charcoal–Sodium Alginate. J. Hazard. Mater. 2020, 398, 122806. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chopra, A.; Cameotra, S.S.; Suri, C.R. Efficient Biotransformation of Herbicide Diuron by Bacterial Strain Micrococcus sp. PS-1. Biodegradation 2010, 21, 979–987. [Google Scholar] [CrossRef]
- Beltrán-Flores, E.; Sarrà, M.; Blánquez, P. Pesticide Bioremediation by Trametes versicolor: Application in a Fixed-Bed Reactor, Sorption Contribution and Bioregeneration. Sci. Total Environ. 2021, 794, 148386. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Mittal, A. Bioremediation of Endosulfan Using Aspergillus terreus and Cladosporium oxysporum. Bull. Environ. Contam. Toxicol. 2005, 75, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Okeke, B.C.; Arshad, M.; Frankenberger, W.T. Biodegradation Kinetics of Endosulfan by Fusarium ventricosum and a Pandoraea Species. J. Agric. Food Chem. 2003, 51, 8015–8019. [Google Scholar] [CrossRef]
- Singh, A.; Lal, R. Sphingobium ummariense sp. Nov., a Hexachlorocyclohexane (HCH)-Degrading Bacterium, Isolated from HCH-Contaminated Soil. Int. J. Syst. Evol. Microbiol. 2009, 59, 162–166. [Google Scholar] [CrossRef]
- Manickam, N.; Reddy, M.K.; Saini, H.S.; Shanker, R. Isolation of Hexachlorocyclohexane-Degrading Sphingomonas sp. by Dehalogenase Assay and Characterization of Genes Involved in γ-HCH Degradation. J. Appl. Microbiol. 2008, 104, 952–960. [Google Scholar] [CrossRef]
- Ceci, A.; Pinzari, F.; Riccardi, C.; Maggi, O.; Pierro, L.; Petrangeli Papini, M.; Gadd, G.M.; Persiani, A.M. Metabolic Synergies in the Biotransformation of Organic and Metallic Toxic Compounds by a Saprotrophic Soil Fungus. Appl. Microbiol. Biotechnol. 2018, 102, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Kandil, M.M.; Trigo, C.; Koskinen, W.C.; Sadowsky, M.J. Isolation and Characterization of a Novel Imidacloprid-Degrading Mycobacterium sp. Strain MK6 from an Egyptian Soil. J. Agric. Food Chem. 2015, 63, 4721–4727. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Kulp, M.; Lukk, T. Bioremediation of Lindane Contaminated Soil: Exploring the Potential of Actinobacterial Strains. Chemosphere 2021, 278, 130468. [Google Scholar] [CrossRef]
- Anupama, K.S.; Paul, S. Ex Situ and in Situ Biodegradation of Lindane by Azotobacter chroococcum. J. Environ. Sci. Health Part B 2009, 45, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wahla, A.Q.; Anwar, S.; Mueller, J.A.; Arslan, M.; Iqbal, S. Immobilization of Metribuzin Degrading Bacterial Consortium MB3R on Biochar Enhances Bioremediation of Potato Vegetated Soil and Restores Bacterial Community Structure. J. Hazard. Mater. 2020, 390, 121493. [Google Scholar] [CrossRef]
- Ortiz-Hernández, M.L.; Quintero-Ramírez, R.; Nava-Ocampo, A.A.; Bello-Ramírez, A.M. Study of the Mechanism of Flavobacterium sp. for Hydrolyzing Organophosphate Pesticides. Fundam. Clin. Pharmacol. 2003, 17, 717–723. [Google Scholar] [CrossRef]
- Madureira Barroso, G.; Dos Santos, J.B.; De Oliveira, I.T.; Rocha Nunes, T.K.M.; Alves Ferreira, E.; Marinho Pereira, I.; Valadão Silva, D.; De Freitas Souza, M. Tolerance of Bradyrhizobium sp. BR 3901 to Herbicides and Their Ability to Use These Pesticides as a Nutritional Source. Ecol. Indic. 2020, 119, 106783. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced Paraquat Removal from Contaminated Water Using Cell-Immobilized Biochar. Clean. Technol. Environ. Policy 2021, 24, 1073–1085. [Google Scholar] [CrossRef]
- Camacho-Morales, R.L.; Guillén-Navarro, K.; Sánchez, J.E. Degradation of the Herbicide Paraquat by Macromycetes Isolated from Southeastern Mexico. 3 Biotech 2017, 7, 324. [Google Scholar] [CrossRef]
- Ma, L.; Hu, T.; Liu, Y.; Liu, J.; Wang, Y.; Wang, P.; Zhou, J.; Chen, M.; Yang, B.; Li, L. Combination of Biochar and Immobilized Bacteria Accelerates Polyacrylamide Biodegradation in Soil by Both Bio-Augmentation and Bio-Stimulation Strategies. J. Hazard. Mater. 2021, 405, 124086. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, X.; Huo, S.; Lin, W.; Xia, X.; Liu, K.; Duan, B. Isolation and Identification of the Raoultella ornithinolytica-ZK4 Degrading Pyrethroid Pesticides within Soil Sediment from an Abandoned Pesticide Plant. Arch. Microbiol. 2019, 201, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Yan, B.; Xu, Y.; Awasthi, M.K.; Yang, H. Characterization of Pyridine Biodegradation by Two Enterobacter sp. Strains Immobilized on Solidago canadensis L. Stem Derived Biochar. J. Hazard. Mater. 2021, 414, 125577. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Pan, J.; Tuwang, M.; Li, H.; Ma, C.; Chen, M.; Lin, X. Isolation, Screening, and Degradation Characteristics of a Quinclorac-Degrading Bacterium, Strain D, and Its Potential for Bioremediation of Rice Fields Polluted by Quinclorac. Microbiol. Spectr. 2021, 9, e00398-21. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.B.; Barrera, V.; Bianchinotti, V. Molecular Identification of Three Isolates of Trichoderma harzianum Isolated from Agricultural Soils in Argentina, and Their Abilities to Detoxify in Vitro Metsulfuron Methyl. Botany 2015, 93, 793–800. [Google Scholar] [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial Compatibility and Immobilization with Biochar Improved Tebuconazole Degradation, Soil Microbiome Composition and Functioning. J. Hazard. Mater. 2020, 398, 122941. [Google Scholar] [CrossRef]
- Li, W.; Chen, A.; Shang, C.; Zhang, X.; Chai, Y.; Luo, S.; Shao, J.; Peng, L. Remediation of Thiamethoxam Contaminated Wetland Soil by Phanerochaete Chrysosporium and the Response of Microorganisms. J. Environ. Chem. Eng. 2022, 10, 108333. [Google Scholar] [CrossRef]
- Jenjaiwit, S.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; Ratpukdi, T.; Siripattanakul-Ratpukdi, S. Removal of Triclocarban from Treated Wastewater Using Cell-Immobilized Biochar as a Sustainable Water Treatment Technology. J. Clean. Prod. 2021, 320, 128919. [Google Scholar] [CrossRef]
- Singhal, M.; Jadhav, S.; Sonone, S. Microalgae Based Sustainable Bioremediation of Water Contaminated by Pesticides. Biointerface Res. Appl. Chem. 2021, 12, 149–169. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of Pesticides from Water and Wastewater: Chemical, Physical and Biological Treatment Approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Doolotkeldieva, T.; Konurbaeva, M.; Bobusheva, S. In Situ Microbial Bioremediation of Obsolete Pesticides at Their Disposal Sites. Bioremediat. J. 2024. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Li, S. Isolation of a Chlorpyrifos-Degrading Bacterium, Sphingomonas sp. Strain Dsp-2, and Cloning of the Mpd Gene. Res. Microbiol. 2007, 158, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Hancock, W.G.; Minard, R.D.; Freyer, A.J.; Honeycutt, R.C.; LeBaron, H.M.; Paulson, D.L.; Liu, S.; Bollag, J.M. Microbial Transformation of the Herbicide Metolachlor by a Soil Actinomycete. J. Agric. Food Chem. 1985, 33, 584–589. [Google Scholar] [CrossRef]
- Aldas-Vargas, A.; van der Vooren, T.; Rijnaarts, H.H.M.; Sutton, N.B. Biostimulation Is a Valuable Tool to Assess Pesticide Biodegradation Capacity of Groundwater Microorganisms. Chemosphere 2021, 280, 130793. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of Water Containing Pesticides by Microalgae: Mechanisms, Methods, and Prospects for Future Research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, T.; Rana, S.S.; Goyal, E.; Ratha, S.K.; Renuka, N. Harnessing the Potential of Microalgae-Based Systems for Mitigating Pesticide Pollution and Its Impact on Their Metabolism. J. Environ. Manag. 2024, 357, 120723. [Google Scholar] [CrossRef]
- Lamilla, C.; Schalchli, H.; Briceño, G.; Leiva, B.; Donoso-Piñol, P.; Barrientos, L.; Rocha, V.A.L.; Freire, D.M.G.; Diez, M.C. A Pesticide Biopurification System: A Source of Biosurfactant-Producing Bacteria with Environmental Biotechnology Applications. Agronomy 2021, 11, 624. [Google Scholar] [CrossRef]
- Marketsand, Markets Biopesticide Market. Available online: https://www.marketsandmarkets.com/Market-Reports/biopesticides-267.html#:~:text=%5B319%20Pages%20Report%5D%20According%20to,period%20in%20terms%20of%20value (accessed on 8 May 2024).

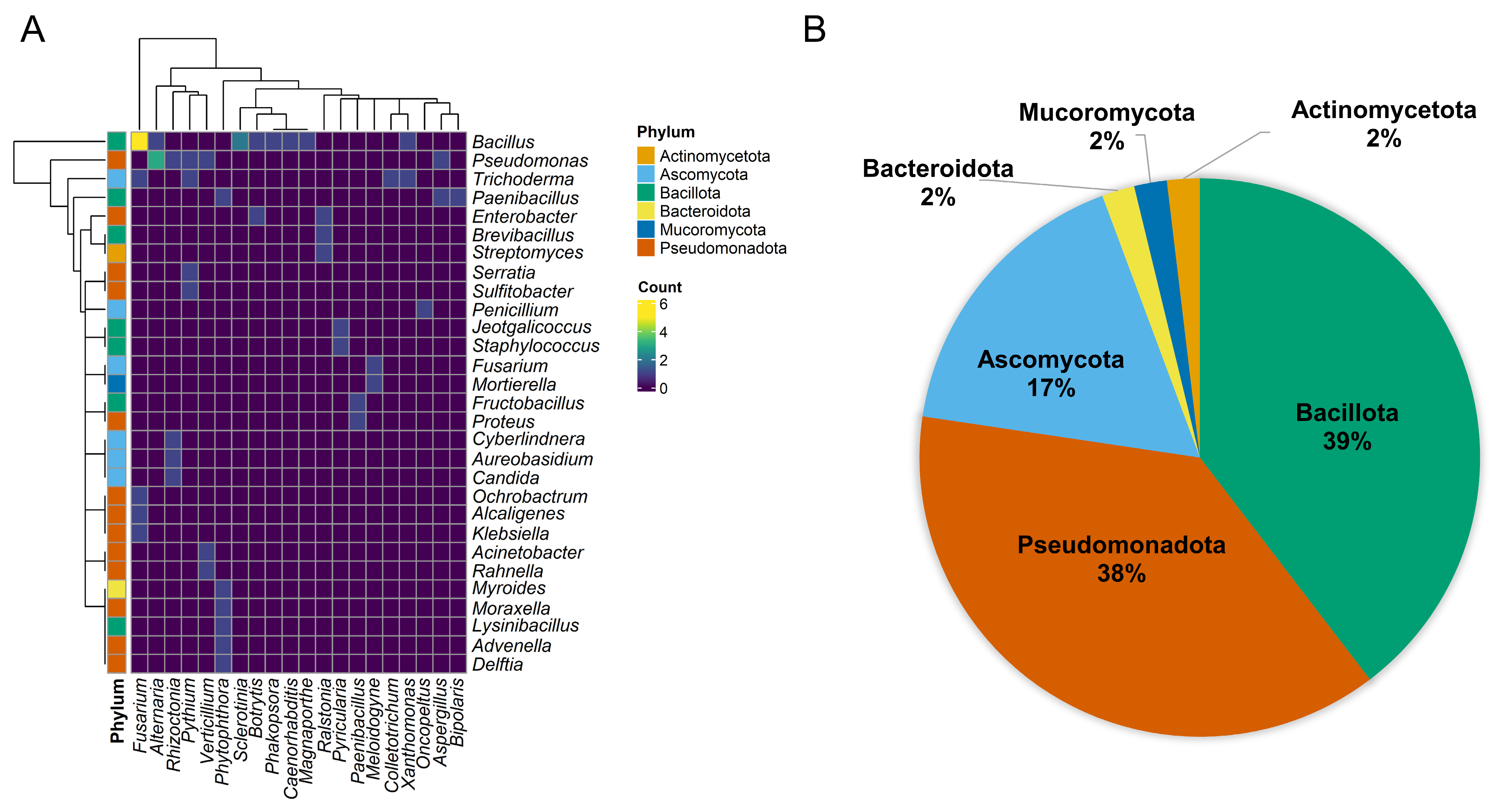


| Fungi | Disease | Target | References |
|---|---|---|---|
| Sclerotinia sclerotiorum | White mold—Sclerotinia rot | Widely distributed soybeans, common beans, cotton, etc. | [23] |
| Phytophthora infestans | Late blight—Phytophthora blight | Potatoes and tomatoes | [24] |
| Colletotrichum lindemuthianum, C. orbiculare, C. capsici | Anthracnose—Colletotrichum rot | Horticultural, ornamental, and fruit tree crops e.g., alfalfa, almond, apple, blueberry, celery | [25] |
| Stagonosporopsis cucurbitacearum (syn. Phoma ucurbitacearum; Teliomorph: Didymella bryoniae | Gummy stem blight—Didymella rot | Watermelon and other species in Cucurbitaceae. | [26] |
| Phomopsis vexans—seed-borne | Phomopsis—blight | Brinjal (Solanum melongena) | [27] |
| Alternaria solani, Sclerotiorum rolfsii | Alternaria—rot | Fruit rot in tomatoes, cauliflower | [22,28] |
| Pseudoperonospora cubensis | Mildew and rust | Broad range of monocotyledonous and dicotyledonous plants, economically important crops, and wild plants | [29] |
| Macrophomina phaseolina Rhizoctonia solani (soil-borne pathogens) | Charcoal rot or ashy stem blight | Common bean (Phaseolus vulgaris L.) | [30] |
| Moniliophthora perniciosa | Witch’s broom | Cocoa | [31] |
| Ascochyta rabiei | Ascochyta—blight | Chickpea (Cicer arietinum L.), Lentil (Lens culinaris), cowpea (Vigna unguiculata), pea (Pisum sativum), and the common bean (Phaseolus vulgaris | [32] |
| Fusarium sp. | Crown rate Stem-end rot Mango Papaya Wheat and barley | Banana Avogado, potato Leaf spot Fruit rot Fusarium head blight, FHB | [33] |
| Chemical Classification | Examples of Pesticides |
|---|---|
| Organochlorines | Diclorodifeniltricloroetano (DDT), dichlorodiphenyldichloroethylene (DDE), dichlorodiphenyldichloroethane (DDD); hexachlorocyclohexane (HCH), such as lindane; cyclodienes, including aldrin, dieldrin, endrin, heptachlor, chlordane, and endosulfan; toxaphene; mirex and chlordecone |
| Organophosphates | Malathion, parathion, diazinon, chlorpyrifos, dichlorvos, phorate |
| Carbamates | Thiobencarb, propoxur, molinate, disulfiram (Antabuse), pyridostigmine, methiocarb, carbaryl |
| Pyrethroids | Permethrin, deltamethrin, cypermethrin, fenvalerate, bifenthrin |
| Plant | Structure Used | Active Compound | Target | Refs. |
|---|---|---|---|---|
| Acorus calamus L. | Leaf, rhizome, and stem | A-asarone and β-asarone, | Fungi: Microsporum gypseum, Penicillium marneffei, Trichophyton rubrum Insect: Sitophilus zeamais | [102,103] |
| Curcuma longa L. | Root stem | Curcumin | Caterpillar: Spodoptera frugiperda, Spodoptera litura | [104,105] |
| Ocimum basilicum L. | Leaf | Major: Linalool and Cinnamic acid methyl ester | Fungi: Alternalia solani, Alternaria heveae, Phytophthora infestans | [106,107] |
| Azadirachta indica | Mainly in seeds | Azadirachtin, meliantriol, salannin, desacetyl salannin, nimbin, desacetyl nimbin, and nimbidin | Insect: Dictyoptera, Orthoptera, Heteroptera, Isoptera, Lepidoptera, Diptera, Coleoptera, Homoptera, Siphonaptera, and Hemiptera | [108,109] |
| Artemisia herba-alba Assso | Cineole, thujone, camphor, davanone | Insect: Callosobruchus maculatus Fungi: Fusarium moniliforme, F. oxysporum, F. solani | [110,111] | |
| Mentha piperita | Leaves | Menthol, menthone, iso menthone, neomenthol, acetyl menthol, pulegone, methyl acetate | Fungi: Botrytis cinerea Insect mites, mosquito larvae | [112,113] |
| Garlic (Allium sativum) | Bulbs | Allicin | Fungi: Drechslera tritici-repetis, Bipolaris sorokiniana, and Septoria tricidi | [114] |
| Commercial Product and Country | Crop | Virus | Target Insect | Reference |
|---|---|---|---|---|
| GemStar LC (Mexico, Brazil, EUA USA, Argentina) Virin-HS (Russia) DOA BIO V2 (Brazil) | Cotton, pepper soybean, tomato, and other rowed crops | NPV | Heliothis sp. Helicoverpa armigera (cotton bollworm, corn earworm, tobacco budworm, corn earworm) | [155,156,157] |
| MadmexTM (Switzerland) Cyd-X (EUA) USA Virosoft CP4 (EUA USA, Canada, European countries) CarpovirusineTM ( France) granuponTM (Switzerland) GranusalTM (Germany) Virin-CyAP (Russia) Carposin (EUA USA, China, European Union) Carpovirus SC (Argentina) | Pear, apple, and walnut | NPV | Cydia pomenella (codling moth) | [158,159,160] |
| Baculo-soja (Brazil), Multigen (Brazil), BaculovirusNitral (Brazil), Coopervirus SC (Brazil), Protege (Brazil) | Soybeans | NPV | Anticarcia gemmatalis (velvet been caterpillar) | [161] |
| Gusano Biological (Brazil) VPN-80TM (Guatemala) | Alfalfa vegetable | NPV | Autograph acalifornica (beet armyworm alfalfa looper and several other lepidopteran species) | [162,163] |
| Spodopterin (Brazil) DOA BIO V3 (Brazil) | Cotton, soybeans, tomato, peanut, cabbage, corn, and tobacco | NPV | Spodoptera litura | [164] |
| Capex 2 (Brazil) | Apple and pear | GV | Adoxophyes orana | [160,165,166,167] |
| Agrovir (Brazil) | Maize | GV | Agrotis segetum | [160,168] |
| Disparvirus (Brazil) Gypchek (EUA) Virin-ENSH (Brazil) | Forestry | NPV | Lymantria dispar | [169] |
| GemStar (Mexico) Biotrol (Brazil) Elcar (Brazil) | Cotton, vegetables | NPV | Helicoverpa zea | [170] |
| VPN-82 (Guatemala) VPN-Ultra (Guatemala) | Horticulture | NPV | Spodoptera albula | [171] |
| Spod-X (Brazil) Vir-ex (Brazil) Spod-XLC (Mexico DOA BIO V1(Brazil) Ness-A (Argentina) Ness-E (Argentina ARG) Otienem-STM (Brazil) | Vegetables | NPV | Spodoptera exigua (beet armyworm) | [148,161] |
| Spodopterin® (several countries, with emphasis on Brazil) Littovir® (Brazil) | Cotton, corn | NPV | Spodoptera littoralis | [166,172] |
| DOA BIO V3 (Brazil) | Vegetables | NPV | Spodoptera littoralis (cotton leafworm) | [166] |
| Mamestrin (Brazil) | Oilseed rapes | NPV | Mamestra brassicae | [162,166] |
| Leconti-virus (Brazil) | Forestry | NPV | Neodiprion lecontei | [173] |
| Neochek-S (South Korea) | Forestry | NPV | Neodiprion sertifer | [174] |
| Biocontrol I Virtuss (Brazil) | Forestry | NPV | Orgyia pseudotsugata | [169] |
| Virin-ABB (Brazil) | Forestry | NPV | Hyphantri acunea | [148] |
| Advantages | Disadvantages |
|---|---|
| Environmentally friendly | Slow action |
| Safe for workers | Susceptibility to abiotic stress factors: temperature and exposure to UV radiation affect the survival of fungi |
| Wide host range | Limited shelf life |
| No chemical residues in food | Difficult in standardization of rest phase of conidia |
| Sustainable production | Higher costs * |
| Metabolite (s) | Producer Fungi | Fungal Pathogen(s) | Refs. |
|---|---|---|---|
| Bicolorin D | Saccharicola bicolor | Sclerotinia sclerotiorum | [191] |
| Brefeldin A | Cladosporium sp. | Aspergillus niger | [192] |
| Pretrichodermamide A | Trichoderma harzianum Epiccocum nigrum | Ustilago maydis | [193] |
| Methyl dichloroasterrate | Aspergillus capensis | Botrytis cinerea, Monilinia fructicola, Sclerotinia sclerotiorum, Sclerotinia trifoliorum | [194] |
| Trichodermin | Trichoderma brevicompactum | Rhizoctonia solani Fusarium solani | [195] |
| Versicolorin | Aspergillus versicolor | Colletotrichum musae | [196] |
| Entomopathogenic Fungi | Plant Pathogens | Insect Pests | Ref. |
|---|---|---|---|
| Beauveria bassiana | Rhizoctonia solani Gaeumannomyces graminis var. tritici Botrytis cinerea, Alternaria alternata | Macrosiphum euphorbiae (Hemiptera) Myzus persicae (Hemiptera) Hyposidra talaca (Lepidoptera) Helopeltis theivora (Heteroptera) | [207,208,209,210] |
| Metarhizium anisopliae | Fusarium graminearum Botrytis Cinerea | Helopeltis theivora (Heteroptera) Phyllotreta striolata (Coleoptera) Monochamus alternatus (Coleoptera) | [211,212,213,214,215] |
| Paecilomyes farinosus Isaria javanica | Colletotrichum gloeosporioides Phytophthora capsici | Galleria mellonella (Lepidoptera) Planococcus ficus (Hemiptera) Pyrrhocoris apterus (Hemiptera) | [198,216,217,218] |
| Lecanicillium lecanii, (formerly known as Verticillium lecanii) | Cactodera estônica Hemileia vastatrix | Empoasca flavescens (Hemiptera) Diaphorina citri (Hemiptera) Plutella xylostella (Lepidoptera) | [219,220] |
| Compounds | Microorganisms | Environment | References |
|---|---|---|---|
| Amide | Paracoccus huijuniae sp. nov. | Culture medium | [374] |
| Atrazine | Acinetobacter lwoffii DNS32 by biochar-immobilization | Soil | [375] |
| Arthrobacter sp. ZXY-2 | Water | [376] | |
| Streptomyces sp. atz2, Aspergillus niger AN 400, Pseudomonas sp., Achromobacter sp., Basidiomycetes, Fusarium sp., Microcystis novacekii | Culture medium | [357] | |
| Stereum hirsutum | Soil | [377] | |
| Atrazine, desethylatrazine, and desisopropylatrazine | Pleurotus ostreatus INCQS 40310 | Culture medium | [357] |
| Atrazine, molinate, simazine, isoproturon, propanil, carbofuran, dimethoate, pendimethalin, metolachlor, and pyriproxyfen | Chlorella vulgaris | Soil and water | [378] |
| Carbofuran | Burkholderia cepacia PCL3 | Soil | [379] |
| Pichia anomala HQ-C-01 | [380] | ||
| Pseudopediastrum boryanum and Desmodesmus communis | Water | [381] | |
| Chlorophenoxyacetic acids | Dichomitus squalens | Culture medium | [382] |
| Chlorpyrifos | Bacillus megaterium HM01 by biochar-immobilization | Soil and water | [383] |
| Cupriavidus nantongensis X1T | Culture medium | [384] | |
| Flavobacterium EMBS0145 | Soil | [385] | |
| Rhodotorula glutinis, Rhodotorula rubra | Culture medium and tomato fruit | [386] | |
| Cypermethrin | Consortium by Bacillus zhanjiangensis TJTB48, Bacillus pseudofirmus TJTB58, and Oceanobacillus kimchii TJTB66 by biochar-immobilization | Soil | [387] |
| DDT | Chryseobacterium sp. PYR2 | [388] | |
| Serratia marcescens NCIM 2919 | [389] | ||
| DDT, DDE | Sphingobacterium sp. D6 | [390] | |
| Stenotrophomonas sp. DXZ9 | [391] | ||
| Diazinon | Candida pseudolambica | Culture medium | [392] |
| Scenedesmus obliquus, Chlamydomonas mexicana, Chlorella vulgaris, and Chlamydomonas pitschmannii | Water | [393] | |
| Dibutyl phthalate | Bacillus siamensis T7 by biochar-immobilization | Soil | [394] |
| Dicofol | Microbacterium sp. D-2 | [395] | |
| Difenoconazole | Consortium by Aspergillus flavus and Bacillus cereus S1 | Culture medium | [396] |
| Dimethachlon | Providencia stuartii JD by biochar-immobilization | Soil | [397] |
| Dimethomorph | Bacillus cereus WL08 by biochar-immobilization | Soil and water | [398] |
| Diuron | Micrococcus sp. PS-1 | Soil | [399] |
| Pluteus cubensis SXS 320 | Culture medium | [357] | |
| Diuron and bentazon | Trametes versicolor | Water | [400] |
| Endosulfan | Cladosporium oxysporum | Culture medium | [401] |
| β-endosulfan | Pandoraea sp. | [402] | |
| Hexachlorocyclohexane (HCH) | Sphingobium ummariense | [403] | |
| Sphingomonas sp. NM05 | Soil | [404] | |
| Penicillium griseofulvum | Culture medium | [405] | |
| Imidacloprid | Mycobacterium sp. MK6 | [406] | |
| Lidane | Thermobifida cellulosilytica TB100 | Soil | [407] |
| Azotobacter chroococcum JL102 | [408] | ||
| Streptomyces sp. | Culture medium | [357] | |
| Metribuzin | Consortium by Bacillus tequilensis AQ2, Bacillus aryabhattai AQ3, Bacillus safensis AQ4, and Rhodococcus rhodochrous AQ1 by biochar-immobilization | Soil | [409] |
| Organophosphate | Flavobacterium sp. ATCC 27551 | Culture medium | [410] |
| Oxyfluorfen, 2,4-D, diuron, and sulfentrazone | Bradyrhizobium sp. BR 3901 | [411] | |
| Paraquat | Pseudomonas putida | Water | [412] |
| Hypholoma dispersum ECS-705 | Culture medium | [413] | |
| Polyacrylamide | Klebsiella sp. PCX | Soil | [414] |
| Pyrethroid | Raoultella ornithinolytica ZK4 | Culture medium | [415] |
| Pyridine | Consortium by Enterobacter cloacae complex sp. BD17 and Enterobacter sp. BD19 | Water | [416] |
| Quinclorac | Cellulosimicrobium cellulans D | Soil | [417] |
| Sulfonylurea | Trichoderma harzianum | Culture medium | [418] |
| Tebuconazole | Alcaligenes faecalis WZ-2 | Soil | [419] |
| Thiamethoxam | Phanerochaete chrysosporium | [420] | |
| Thifensulfuron-methyl | Serratia marcescens N80 | [372] | |
| Triclocarban | Pseudomonas fluorescens MC46 | Water | [421] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermelho, A.B.; Moreira, J.V.; Akamine, I.T.; Cardoso, V.S.; Mansoldo, F.R.P. Agricultural Pest Management: The Role of Microorganisms in Biopesticides and Soil Bioremediation. Plants 2024, 13, 2762. https://doi.org/10.3390/plants13192762
Vermelho AB, Moreira JV, Akamine IT, Cardoso VS, Mansoldo FRP. Agricultural Pest Management: The Role of Microorganisms in Biopesticides and Soil Bioremediation. Plants. 2024; 13(19):2762. https://doi.org/10.3390/plants13192762
Chicago/Turabian StyleVermelho, Alane Beatriz, Jean Vinícius Moreira, Ingrid Teixeira Akamine, Veronica S. Cardoso, and Felipe R. P. Mansoldo. 2024. "Agricultural Pest Management: The Role of Microorganisms in Biopesticides and Soil Bioremediation" Plants 13, no. 19: 2762. https://doi.org/10.3390/plants13192762
APA StyleVermelho, A. B., Moreira, J. V., Akamine, I. T., Cardoso, V. S., & Mansoldo, F. R. P. (2024). Agricultural Pest Management: The Role of Microorganisms in Biopesticides and Soil Bioremediation. Plants, 13(19), 2762. https://doi.org/10.3390/plants13192762











