Residual Effect of Microbial-Inoculated Biochar with Nitrogen on Rice Growth and Salinity Reduction in Paddy Soil
Abstract
1. Introduction
2. Results
2.1. Chemical Properties of Post-Harvest Soil
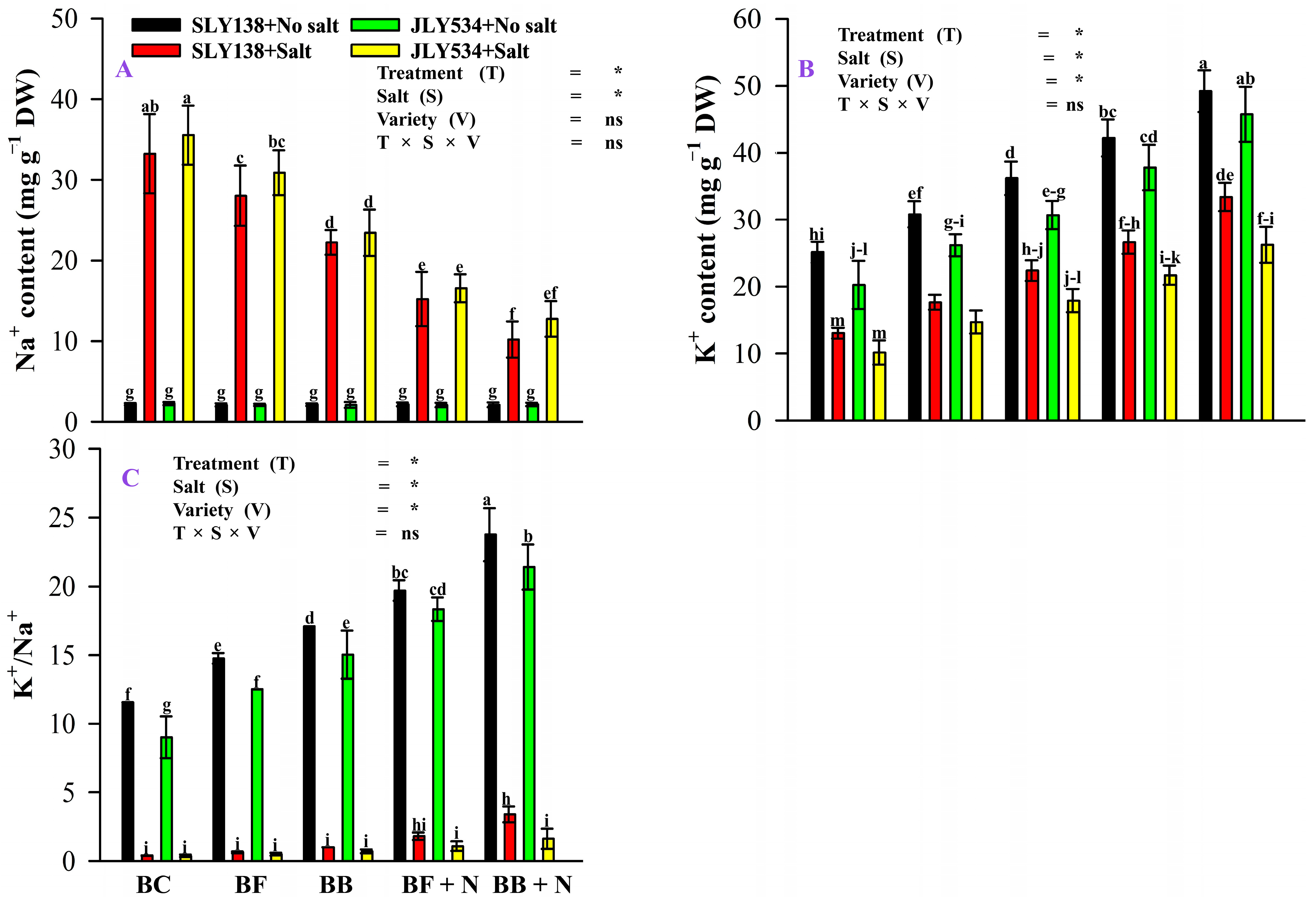
2.2. Synergistic Effect of Microbial Biochar and Nitrogen Fertilizer Modulated K+/Na+ Balance in Rice Leaves under Saline Conditions
2.3. Microbial Biochar and Nitrogen Fertilizer Reduced the ROS Damaging Effects in Rice Leaves and Increased SOD, POD, and CAT Activities in Rice Plants
2.4. Microbial Biochar and Nitrogen Fertilizer Maintain RWC and MSI in Rice Plants under Salinity Stress
2.5. Microbial Biochar and Nitrogen Fertilizer Mitigate Salinity Stress and Enhance Photosystem II Function in Rice Plants
2.6. Effect of Microbial Biochar and Nitrogen Fertilizer on Roots Anatomical Properties under Saline Stress
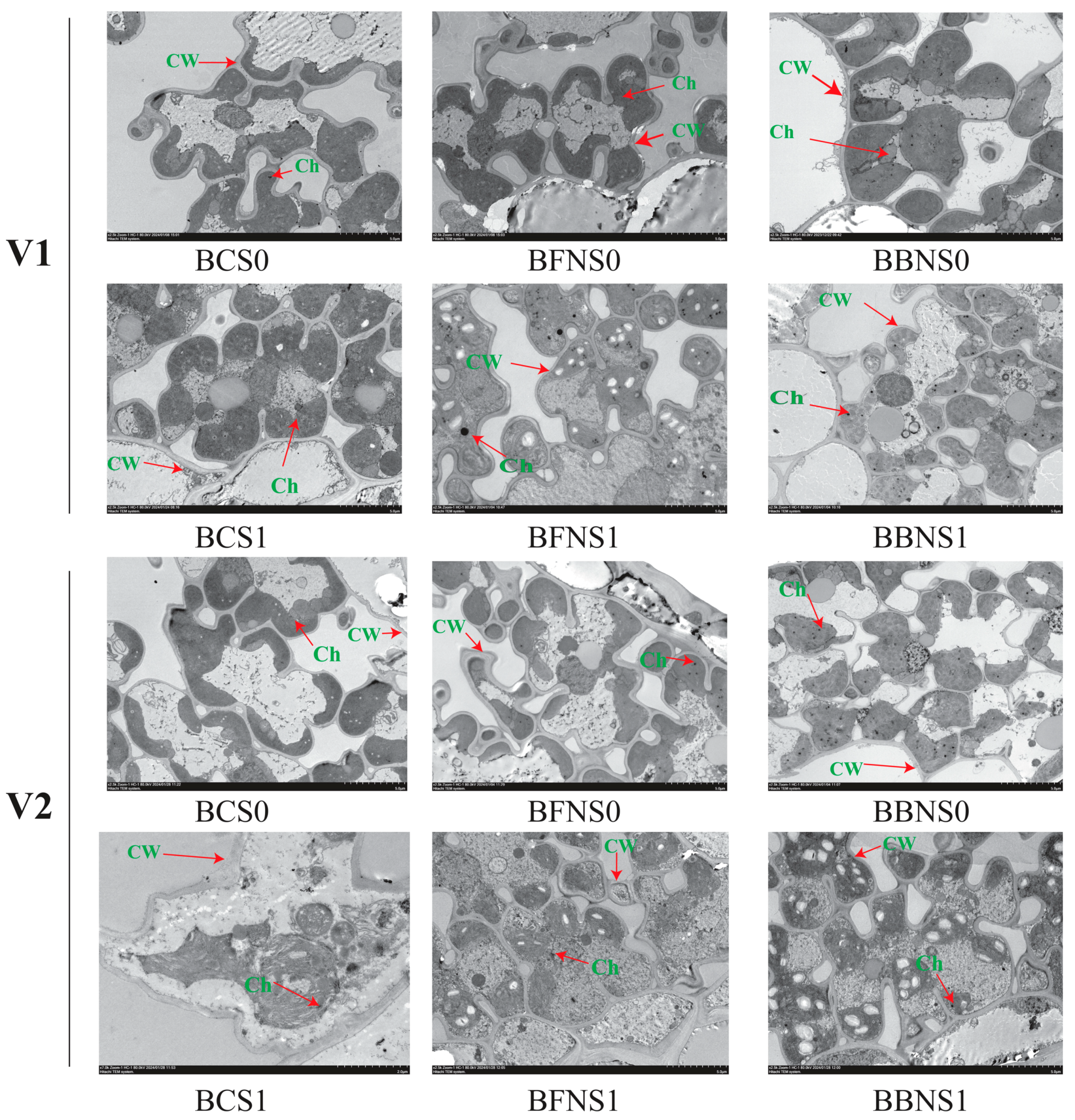
2.7. Ultrastructure Traits of Rice Leaf
2.8. Agronomic Parameters and SPAD Values of Rice
3. Discussion
4. Materials and Methods
4.1. Experimental Setup
4.2. Physiological Performance of Rice Plants
4.3. Biochemical Analysis of Rice Plant Leaves
4.4. Measurement of Na+ and K+ Content in Leaves
4.5. Scanning Electron Microscopy (SEM) of Root Cross Section and Transmission Electron Microscopy (TEM) of Leaf Cells
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alabdallah, N.M.; Saleem, K.; Al-Shammari, A.S.; AlZahrani, S.S.; Javed, H.H.; Raza, A.; Asghar, M.A.; Yong, J.W.H. Dose-Dependent Regulation of Morphological, Physio-Biochemical, Nutritional, and Metabolic Responses by Cobalt in Tagestes erecta L. Plants Exposed to Salinity Stress. Plant Stress 2024, 13, 100507. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Yao, R.; Chen, X.; Wang, X. Biochar and Fulvic Acid Amendments Mitigate Negative Effects of Coastal Saline Soil and Improve Crop Yields in a Three Year Field Trial. Sci. Rep. 2020, 10, 8946. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.A.; Alqahtani, M.M.; Alwutayd, K.M.; Aloufi, A.S.; Osama, O.; Azab, E.S.; Abdelsattar, M.; Hassanin, A.A.; Okasha, S.A. Exploring Salinity Tolerance Mechanisms in Diverse Wheat Genotypes Using Physiological, Anatomical, Agronomic and Gene Expression Analyses. Plants 2023, 12, 3330. [Google Scholar] [CrossRef] [PubMed]
- Chahal, S.S.; Choudhary, O.P.; Mavi, M.S. Microbial Activity Is Constrained by the Quality of Carbon and Nitrogen under Long-Term Saline Water Irrigation. Commun. Soil Sci. Plant Anal. 2018, 49, 1266–1280. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, T.; Lv, Z.; Yue, Y.; Liu, A.; Long, X.; Zhou, Z.; Gao, X.; Rengel, Z. The Mechanisms of Improving Coastal Saline Soils by Planting Rice. Sci. Total Environ. 2020, 703, 135529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration Effects of Coastal Saline-Alkali Soil by Ball-Milled Red Phosphorus-Loaded Biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Wang, G.; Weng, L.; Huang, Y.; Ling, Y.; Zhen, Z.; Lin, Z.; Hu, H.; Li, C.; Guo, J.; Zhou, J.L. Microbiome-Metabolome Analysis Directed Isolation of Rhizobacteria Capable of Enhancing Salt Tolerance of Sea Rice 86. Sci. Total Environ. 2022, 843, 156817. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Ghaffar, A.; Zahra, N.; Ahmad, N.; Hussain, S.; Li, J. Plant Growth Promoters Boost the Photosynthesis Related Mechanisms and Secondary Metabolism of Late-Sown Wheat under Contrasting Saline Regimes. Plant Stress 2024, 12, 100480. [Google Scholar] [CrossRef]
- Jameel, J.; Anwar, T.; Majeed, S.; Qureshi, H.; Siddiqi, E.H.; Sana, S.; Zaman, W.; Ali, H.M. Effect of Salinity on Growth and Biochemical Responses of Brinjal Varieties: Implications for Salt Tolerance and Antioxidant Mechanisms. BMC Plant Biol. 2024, 24, 128. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, K.; Chang, T.; Shaghaleh, H.; Qi, Z.; Zhang, J.; Ye, H.; Hamoud, Y.A. Interactive Effects of Microbial Fertilizer and Soil Salinity on the Hydraulic Properties of Salt-Affected Soil. Plants 2024, 13, 473. [Google Scholar] [CrossRef]
- Joseph, S.; Graber, E.R.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Pan, G.-X. Shifting Paradigms: Development of High-Efficiency Biochar Fertilizers Based on Nano-Structures and Soluble Components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Moghaddam, M.; Lakzian, A. Amelioration of Soil Properties, Growth and Leaf Mineral Elements of Summer Savory under Salt Stress and Biochar Application in Alkaline Soil. Sci. Hortic. 2020, 267, 109319. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar Stability in Soil: Meta-analysis of Decomposition and Priming Effects. Gcb Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Wu, R.; Long, M.; Tai, X.; Wang, J.; Lu, Y.; Sun, X.; Tang, D.; Sun, L. Microbiological Inoculation with and without Biochar Reduces the Bioavailability of Heavy Metals by Microbial Correlation in Pig Manure Composting. Ecotoxicol. Environ. Saf. 2022, 248, 114294. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Glaser, B.; Geraldes Teixeira, W.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen Retention and Plant Uptake on a Highly Weathered Central Amazonian Ferralsol Amended with Compost and Charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Djatouf, N.; Celletti, S.; Loppi, S. Can Lettuce Plants Grow in Saline Soils Supplemented with Biochar? Heliyon 2024, 10, e26526. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and Effective Microorganisms Promote Sesbania Cannabina Growth and Soil Quality in the Coastal Saline-Alkali Soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Jia, J.; Wang, W.; Wang, X.; Zhao, Q.; Lu, Q. Shifts of Soil Microbial Community Composition along a Short-Term Invasion Chronosequence of Spartina Alterniflora in a Chinese Estuary. Sci. Total Environ. 2019, 657, 222–233. [Google Scholar] [CrossRef]
- Abbas, H.M.M.; Rais, U.; Altaf, M.M.; Rasul, F.; Shah, A.; Tahir, A.; Nafees-Ur-Rehman, M.; Shaukat, M.; Sultan, H.; Zou, R. Microbial-Inoculated Biochar for Remediation of Salt and Heavy Metal Contaminated Soils. Sci. Total Environ. 2024, 954, 176104. [Google Scholar] [CrossRef]
- Shen, J.; Tang, H.; Liu, J.; Wang, C.; Li, Y.; Ge, T.; Jones, D.L.; Wu, J. Contrasting Effects of Straw and Straw-Derived Biochar Amendments on Greenhouse Gas Emissions within Double Rice Cropping Systems. Agric. Ecosyst. Environ. 2014, 188, 264–274. [Google Scholar] [CrossRef]
- Khan, Z.; Nauman Khan, M.; Luo, T.; Zhang, K.; Zhu, K.; Rana, M.S.; Hu, L.; Jiang, Y. Compensation of High Nitrogen Toxicity and Nitrogen Deficiency with Biochar Amendment through Enhancement of Soil Fertility and Nitrogen Use Efficiency Promoted Rice Growth and Yield. Gcb Bioenergy 2021, 13, 1765–1784. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, G.; Zhou, M.; Wu, F.; Chen, J. Effects of Aluminum and Cadmium Toxicity on Growth and Antioxidant Enzyme Activities of Two Barley Genotypes with Different Al Resistance. Plant Soil 2004, 258, 241–248. [Google Scholar] [CrossRef]
- Li, Z.; Xing, B.; Ding, Y.; Li, Y.; Wang, S. A High-Performance Biochar Produced from Bamboo Pyrolysis with in-Situ Nitrogen Doping and Activation for Adsorption of Phenol and Methylene Blue. Chin. J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Zhang, J.; Zhang, H.; Luo, J.; Bolan, N. Nitrogen Amendment Stimulated Decomposition of Maize Straw-Derived Biochar in a Sandy Loam Soil: A Short-Term Study. PLoS ONE 2015, 10, e0133131. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Zhao, Q.; Wu, K.; Ullah, S.; Iqbal, A.; Liang, H.; Zhang, J.; Muhammad, I.; Amanullah; Khan, A. Biochar in Combination with Nitrogen Fertilizer Is a Technique: To Enhance Physiological and Morphological Traits of Rice (Oryza sativa L.) by Improving Soil Physio-Biochemical Properties. J. Plant Growth Regul. 2021, 41, 2406–2420. [Google Scholar] [CrossRef]
- Huang, M.; Fan, L.; Chen, J.; Jiang, L.; Zou, Y. Continuous Applications of Biochar to Rice: Effects on Nitrogen Uptake and Utilization. Sci. Rep. 2018, 8, 11461. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, Y.-G.; Ren, C.-Z.; Guo, L.-C.; Wang, C.-L.; Jiang, Y.; Wang, X.-J.; Phendukani, H.; Zeng, Z.-H. Effects of Nitrogen Application on Chlorophyll Fluorescence Parameters and Leaf Gas Exchange in Naked Oat. J. Integr. Agric. 2013, 12, 2164–2171. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M.; Šimůnek, J. Leaching and Reclamation of a Biochar and Compost Amended Saline–Sodic Soil with Moderate SAR Reclaimed Water. Agric. Water Manag. 2015, 158, 255–265. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar Application for the Remediation of Salt-Affected Soils: Challenges and Opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Drake, J.A.; Cavagnaro, T.R.; Cunningham, S.C.; Jackson, W.R.; Patti, A.F. Does Biochar Improve Establishment of Tree Seedlings in Saline Sodic Soils? L. Degrad. Dev. 2016, 27, 52–59. [Google Scholar] [CrossRef]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of Biochar-Compost to Improve Properties and Productivity of the Degraded Coastal Soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Van Tan, L.; Thanh, T. The Effects of Salinity on Changes in Characteristics of Soils Collected in a Saline Region of the Mekong Delta, Vietnam. Open Chem. 2021, 19, 471–480. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and Bacteria Inoculated Biochar Enhanced Cd and Cu Immobilization and Enzymatic Activity in a Polluted Soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, R. The Amelioration Effects of Low Temperature Biochar Generated from Nine Crop Residues on an Acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Serkalem, W.M. Effect of Prosopis Juliflora Biochar Amendment on Some Soil Properties: The Case of Salic Fluvisols from Melkawerer Research Station, Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Xu, H.-J.; Wang, X.-H.; Li, H.; Yao, H.-Y.; Su, J.-Q.; Zhu, Y.-G. Biochar Impacts Soil Microbial Community Composition and Nitrogen Cycling in an Acidic Soil Planted with Rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.-H. Effect of Biochar on Reclaimed Tidal Land Soil Properties and Maize (Zea mays L.) Response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef]
- Rodriguez, M.B.; Godeas, A.; Lavado, R.S. Soil Acidity Changes in Bulk Soil and Maize Rhizosphere in Response to Nitrogen Fertilization. Commun. Soil Sci. Plant Anal. 2008, 39, 2597–2607. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, A.; Pandey, M. A Review: Effects Of Nitrogenous Fertilizers On Soil (Ph, Microbial Community, Greenhouse Gases Emission and Carbon Pool). Environ. Contam. Rev. 2022, 5, 44–48. [Google Scholar] [CrossRef]
- Lakhdar, A.; Rabhi, M.; Ghnaya, T.; Montemurro, F.; Jedidi, N.; Abdelly, C. Effectiveness of Compost Use in Salt-Affected Soil. J. Hazard. Mater. 2009, 171, 29–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, C.; Liu, Y.; Li, S.; Li, X.; Xi, B.; Duan, J. Xylem Anatomical and Hydraulic Traits Vary within Crown but Not Respond to Water and Nitrogen Addition in Populus Tomentosa. Agric. Water Manag. 2023, 278, 108169. [Google Scholar] [CrossRef]
- Dong, X.; Li, M.; Lin, Q.; Li, G.; Zhao, X. Soil Na+ Concentration Controls Salt-Affected Soil Organic Matter Components in Hetao Region China. J. Soils Sediments 2019, 19, 1120–1129. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black Carbon (Biochar) in Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Liu, X.; Luo, P.; Liang, W.; Liu, N.; Yang, J.; Han, X. Adsorption and Desorption Characteristics of Nitrogen, Phosphorus and Potassium by Biochars from Different Raw Materials. J. Plant Nutr. Fertil. 2019, 25, 1763–1772. [Google Scholar]
- Lashari, M.S.; Liu, Y.; Li, L.; Pan, W.; Fu, J.; Pan, G.; Zheng, J.; Zheng, J.; Zhang, X.; Yu, X. Effects of Amendment of Biochar-Manure Compost in Conjunction with Pyroligneous Solution on Soil Quality and Wheat Yield of a Salt-Stressed Cropland from Central China Great Plain. F. Crop. Res. 2013, 144, 113–118. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, K.; Tan, P.; Liu, J.; Xie, J.; Yao, X.; Chu, G.; Peng, F. Ammonia–Nitrate Mixture Dominated by NH4+–N Promoted Growth, Photosynthesis and Nutrient Accumulation in Pecan (Carya illinoinensis). Forests 2021, 12, 1808. [Google Scholar] [CrossRef]
- Li, S.-X.; Wang, Z.-H.; Stewart, B.A. Responses of Crop Plants to Ammonium and Nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of Biochar Application Rate on Sandy Desert Soil Properties and Sorghum Growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Xie, X.; Huang, Z.; Lv, W.; Zhu, H.; Hui, G.; Li, R.; Lei, X.; Li, Z. Influence of Nitrogen Application Rate on the Importance of NO3−-N and NH4+-N Transfer via Extramycelia of Arbuscular Mycorrhiza to Tomato with Expression of LeNRT2. 3 and LeAMT1. 1. Plants 2023, 12, 314. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar Applied with Appropriate Rates Can Reduce N Leaching, Keep N Retention and Not Increase NH3 Volatilization in a Coastal Saline Soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota–a Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; De Neve, S. Biochar Amendment to Soils with Contrasting Organic Matter Level: Effects on N Mineralization and Biological Soil Properties. Gcb Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, J.; Wang, H.; Cui, J.; Shi, X.; Song, J.; Li, W.; Zhong, M.; Qiu, Y.; Xu, T. Nitrogen Application Alleviates Salt Stress by Enhancing Osmotic Balance, ROS Scavenging, and Photosynthesis of Rapeseed Seedlings (Brassica napus). Plant Signal. Behav. 2022, 17, 2081419. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, Y.; Li, J.; Zeng, G.; Li, H.; Xu, F.; Feng, S.; Xu, H. Effect of Serratia Sp. K3 Combined with Organic Materials on Cadmium Migration in Soil-Vetiveria Zizanioides L. System and Bacterial Community in Contaminated Soil. Chemosphere 2020, 242, 125164. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, Y.; Feng, Q.; Zhang, X.; Gao, Y.; Ou, Y.; Yang, F.; Xie, W.; de Dios, V.R.; Ma, J. Acclimation to Nitrogen× Salt Stress in Populus Bolleana Mediated by Potassium/Sodium Balance. Ind. Crops Prod. 2021, 170, 113789. [Google Scholar] [CrossRef]
- Singh, V.; Singh, A.P.; Bhadoria, J.; Giri, J.; Singh, J.; TV, V.; Sharma, P.C. Differential Expression of Salt-Responsive Genes to Salinity Stress in Salt-Tolerant and Salt-Sensitive Rice (Oryza sativa L.) at Seedling Stage. Protoplasma 2018, 255, 1667–1681. [Google Scholar] [CrossRef]
- Azeem, M.; Pirjan, K.; Qasim, M.; Mahmood, A.; Javed, T.; Muhammad, H.; Yang, S.; Dong, R.; Ali, B.; Rahimi, M. Salinity Stress Improves Antioxidant Potential by Modulating Physio-Biochemical Responses in Moringa oleifera Lam. Sci. Rep. 2023, 13, 2895. [Google Scholar] [CrossRef]
- Naseer, M.A.; Zhang, Z.Q.; Mukhtar, A.; Asad, M.S.; Wu, H.Y.; Yang, H.; Zhou, X.B. Strigolactones: A Promising Tool for Nutrient Acquisition through Arbuscular Mycorrhizal Fungi Symbiosis and Abiotic Stress Tolerance. Plant Physiol. Biochem. 2024, 215, 109057. [Google Scholar] [CrossRef]
- Khan, M.N. Growth and Physiological Attributes of Tomato (Lycopersicon esculentum Mill.) Genotypes as Affected by NaCl Stress. Am. J. Plant Sci. 2016, 7, 453–460. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Naseer, M.A.; Hussain, S.; Mukhtar, A.; Rui, Q.; Ru, G.; Ahmad, H.; Zhang, Z.Q.; Shi, L.B.; Asad, M.S.; Chen, X. Chlorophyll Fluorescence, Physiology, and Yield of Winter Wheat under Different Irrigation and Shade Durations during the Grain-Filling Stage. Front. Plant Sci. 2024, 15, 1396929. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive Oxygen Species and Antioxidant Enzymes Involved in Plant Tolerance to Stress. Abiotic Biot. Stress Plants-Recent Adv. Futur. Perspect. 2016, 17, 463–480. [Google Scholar]
- Manan, A.; Ayyub, C.M.; Ahmad, R.; Bukhari, M.A.; Mustafa, Z. Salinity Induced Deleterious Effects on Biochemical and Physiological Processes of Tomato. Pakistan J. Life Soc. Sci. 2016, 14, 83–90. [Google Scholar]
- Awaji, S.M.; Hanjagi, P.S.; Repudi, S.R.; Suravi, U.S.; Baig, M.J.; Swain, P. Identification and Characterization of Drought Tolerant Rice Genotypes Using Physiological and Biochemical Traits. Oryza-An Int. J. Rice 2022, 59, 221–231. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and Biochemical Response of Mungbean [Vigna radiata (L.) Wilczek] Varieties at Different Developmental Stages under Drought Stress. Turkish J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon Improves Rice Salinity Resistance by Alleviating Ionic Toxicity and Osmotic Constraint in an Organ-Specific Pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Lyu, S.; Du, G.; Liu, Z.; Zhao, L.; Lyu, D. Effects of Biochar on Photosystem Function and Activities of Protective Enzymes in Pyrus ussuriensis Maxim. under Drought Stress. Acta Physiol. Plant. 2016, 38, 220. [Google Scholar] [CrossRef]
- Ahmad, S.; Sehrish, A.K.; Alomrani, S.O.; Zhang, L.; Waseem, M.; Noureen, S.; Ullah, I.; Tabassam, R.; Abbas, G.; Ali, S. Combined Application of Biochar and Metal-Tolerant Bacteria Alleviates Cadmium Toxicity by Modulating the Antioxidant Defense Mechanism and Physicochemical Attributes in Rice (Oryza sativa L.) Grown in Cadmium-Contaminated Soil. Plant Stress 2024, 11, 100348. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhawat, N.; Alshaal, T. Glycine Betaine Counters Salinity Stress by Maintaining High K+/Na+ Ratio and Antioxidant Defense via Limiting Na+ Uptake in Common Bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 2020, 200, 110732. [Google Scholar] [CrossRef]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice. Agronomy 2021, 11, 1631. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Loudari, A.; Mayane, A.; Naciri, R.; Zeroual, Y.; Colinet, G.; Oukarroum, A. Root Morphological and Anatomical Responses to Increasing Phosphorus Concentration of Wheat Plants Grown under Salinity. Plant Stress 2022, 6, 100121. [Google Scholar] [CrossRef]
- Kong, Y.; Xu, X.; Zhu, L. Cyanobactericidal Effect of Streptomyces Sp. HJC-D1 on Microcystis Auruginosa. PLoS ONE 2013, 8, e57654. [Google Scholar] [CrossRef] [PubMed]
- Ruan, R.; Wang, Y. Effects of Biochar Amendment on Root Growth and Plant Water Status Depend on Maize Genotypes. Agric. Water Manag. 2024, 293, 108688. [Google Scholar] [CrossRef]
- Neves-Piestun, B.G.; Bernstein, N. Salinity-Induced Inhibition of Leaf Elongation in Maize Is Not Mediated by Changes in Cell Wall Acidification Capacity1. Plant Physiol. 2001, 125, 1419–1428. [Google Scholar] [CrossRef]
- Rodríguez Coca, L.I.; García González, M.T.; Gil Unday, Z.; Jiménez Hernández, J.; Rodríguez Jáuregui, M.M.; Fernández Cancio, Y. Effects of Sodium Salinity on Rice (Oryza sativa L.) Cultivation: A Review. Sustainability 2023, 15, 1804. [Google Scholar] [CrossRef]
- Temme, A.A.; Kerr, K.L.; Masalia, R.R.; Burke, J.M.; Donovan, L.A. Key Traits and Genes Associate with Salinity Tolerance Independent from Vigor in Cultivated Sunflower. Plant Physiol. 2020, 184, 865–880. [Google Scholar] [CrossRef]
- Gu, W.; Wang, Y.; Feng, Z.; Wu, D.; Zhang, H.; Yuan, H.; Sun, Y.; Xiu, L.; Chen, W.; Zhang, W. Long-Term Effects of Biochar Application with Reduced Chemical Fertilizer on Paddy Soil Properties and Japonica Rice Production System. Front. Environ. Sci. 2022, 10, 902752. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Wang, Y.; Du, Z.; Rengel, Z.; Zhang, A. Biochar and Nitrogen Fertilizer Promote Rice Yield by Altering Soil Enzyme Activity and Microbial Community Structure. GCB Bioenergy 2022, 14, 1266–1280. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Liu, Z.; Liu, K.; Wang, X.; Shang, J. Salt-Affected Marginal Lands: A Solution for Biochar Production. Biochar 2023, 5, 21. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Zhang, Z.; You, L.; Xu, L.; Huang, H.; Wang, X.; Gao, Y.; Cui, X. Treatment of the Saline-Alkali Soil with Acidic Corn Stalk Biochar and Its Effect on the Sorghum Yield in Western Songnen Plain. Sci. Total Environ. 2021, 797, 149190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, Y.; He, Z.; Ma, M. Cadmium Accumulation and Oxidative Burst in Garlic (Allium sativum). J. Plant Physiol. 2005, 162, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Fu, C.; Liu, X.; Li, Y.; Yan, J.; Yue, L.; Li, J.; Khan, Z.; Nie, L.; Wu, H. Nanopriming with Selenium Doped Carbon Dots Improved Rapeseed Germination and Seedling Salt Tolerance. Crop J. 2024. [Google Scholar]
- FAO. The Euphrates Pilot Irrigation Project. Methods of Soil Analysis. In Gadeb Soil Laboratory (a Laboratory Manual); FAO: Rome, Italy, 1974. [Google Scholar]
- Walkley, A. A Critical Examination of a Rapid Method for Determining Organic Carbon in Soils—Effect of Variations in Digestion Conditions and of Inorganic Soil Constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Maynard, D.G.; Kalra, Y.P.; Crumbaugh, J.A. Nitrate and Exchangeable Ammonium Nitrogen. Soil Sampl. Methods Anal. 1993, 1, 25–38. [Google Scholar]
- Weatherley, P. Studies in the Water Relations of the Cotton Plant. I. The Field Measurement of Water Deficits in Leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of Drought and Temperature Stress in Relation to Increased Antioxidant Enzyme Activity in Wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]





| Plant Height (cm) | Plant Fresh Weight (g/Pot) | Plant Dry Weight (g/Pot) | No. of Tillers/Pot | SPAD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | EC (ds/m) | SLY138 | JLY534 | SLY138 | JLY534 | SLY138 | JLY534 | SLY138 | JLY534 | SLY138 | JLY534 |
| N60 | 0.4 | 32.58 t | 28.85 tu | 37.75 vw | 33.33 wx | 16.35 s–u | 15.35 t–v | 13.00 u–x | 8.25 yz | 25.63 n–q | 23.63 p–s |
| 6.84 | 23.50 v | 17.53 w | 22.45 yz | 17.03 z | 10.23 wx | 4.10 y | 7.75 y–z | 5.00 z | 19.20 r–t | 14.08 t | |
| N120 | 0.4 | 49.48 m–o | 46.08 n–q | 53.38 rs | 49.18 st | 22.95 n–q | 19.00 q–t | 20.00 o–s | 16.50 r–v | 26.80 m–q | 24.65 q–r |
| 6.84 | 38.18 rs | 33.85 st | 37.15 vw | 28.08 xy | 14.33 u–w | 6.70 xy | 12.25 v–y | 10.75 w–y | 21.43 q–s | 18.43 st | |
| BC | 0.4 | 41.23 qr | 38.83 rs | 45.40 tu | 41.25 uv | 20.38 q–s | 18.58 r–t | 17.00 r–u | 14.00 t–w | 33.83 e–l | 33.23 f–l |
| 6.84 | 33.90 st | 27.03 uv | 28.63 xy | 24.43 y | 12.80 u–w | 6.38 x–y | 9.75 w–y | 9.25 x–z | 30.05 k–o | 28.13 l–p | |
| BF | 0.4 | 61.08 ij | 59.03 jk | 114.90 j | 111.55 jk | 29.93 k | 29.00 i–l | 25.50 i–m | 22.75 l–q | 37.30 b–i | 34.68 d–k |
| 6.84 | 49.78 mn | 47.28 n–p | 95.45 no | 89.23 op | 21.65 o–r | 13.15 u–w | 18.25 q–t | 16.00 s–v | 34.73 d–k | 32.90 g–l | |
| BB | 0.4 | 73.55 ef | 71.93 e–g | 129.75 hi | 125.30 i | 35.05 e–g | 33.63 f–h | 29.25 f–i | 28.00 g–j | 38.80 b–g | 36.13 c–j |
| 6.84 | 60.90 i–k | 56.23 j–l | 107.75 kl | 104.05 lm | 25.45 l–o | 16.95 s–u | 23.00 k–p | 19.00 p–s | 37.20 b–i | 31.80 i–m | |
| BC + N60 | 0.4 | 55.78 kl | 53.70 lm | 78.40 q | 74.63 q | 29.20 i–l | 26.55 k–n | 24.25 j–o | 21.75 m–q | 36.25 c–j | 34.28 d–k |
| 6.84 | 44.35 o–q | 42.25 p–r | 55.68 r | 42.33 tu | 20.58 p–s | 11.50 vw | 17.00 r–u | 16.25 r–v | 35.78 c–k | 30.73 j–n | |
| BC + N120 | 0.4 | 68.48 f–h | 67.45 f–h | 105.05 lm | 100.63 mn | 34.58 e–g | 32.08 g–i | 28.00 g–j | 25.25 i–n | 38.88 b–g | 35.98 c–k |
| 6.84 | 55.85 j–l | 52.95 lm | 87.25 p | 77.08 q | 24.75 m–p | 15.65 t–v | 20.75 n–r | 19.25 p–s | 34.90 c–k | 32.48 h–m | |
| BF + N60 | 0.4 | 82.30 c | 80.35 c | 146.40 de | 139.85 f | 42.58 cd | 38.70 de | 33.50 c–f | 32.00 d–g | 39.25 b–f | 38.48 b–h |
| 6.84 | 71.83 e–g | 65.45 hi | 125.55 i | 116.83 j | 31.40 g–j | 21.60 o–r | 27.00 h–l | 23.50 j–p | 35.35 c–k | 35.33 c–k | |
| BF + N120 | 0.4 | 91.65 b | 89.65 b | 157.13 bc | 153.65 c | 46.45 bc | 43.95 c | 36.50 b–d | 35.50 b–e | 40.85 bc | 39.75 b–e |
| 6.84 | 78.85 cd | 71.25 e–g | 136.50 fg | 128.73 hi | 35.18 e–g | 27.28 j–m | 30.25 f–h | 27.00 h–l | 37.70 b–i | 34.90 c–k | |
| BB + N60 | 0.4 | 93.15 b | 92.10 b | 161.65 b | 156.78 bc | 48.60 b | 45.50 bc | 38.25 b | 37.00 bc | 40.18 b–d | 36.83 c–i |
| 6.84 | 83.38 c | 74.23 de | 140.55 ef | 131.98 gh | 37.15 ef | 30.10 h–k | 31.50 e–h | 27.50 g–k | 36.98 b–i | 34.18 d–k | |
| BB + N120 | 0.4 | 106.25 a | 102.50 a | 176.58 a | 175.35 a | 55.30 a | 53.68 a | 43.25 a | 44.25 a | 46.98 a | 42.88 ab |
| 6.84 | 92.68 b | 81.65 c | 152.45 cd | 146.53 de | 43.93 c | 36.43 ef | 36.00 b–e | 30.50 f–h | 37.95 b–h | 34.63 d–k | |
| S | * | * | * | * | * | ||||||
| T | * | * | * | * | * | ||||||
| V | * | * | * | * | * | ||||||
| T × S × V | ns | ns | ns | ns | ns | ||||||
| Plant Height (cm) | Plant Fresh Weight (g/Pot) | Plant Dry Weight (g/Pot) | No. of Tillers/Pot | SPAD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | EC (ds/m) | SLY138 | JLY534 | SLY138 | JLY534 | SLY138 | JLY534 | SLY138 | JLY534 | SLY138 | JLY534 |
| BC | 0.4 | 41.23 h | 38.83 hi | 45.40 j | 41.25 j | 20.38 g | 18.58 g | 17.00 h | 14.00 hi | 33.83 d–h | 33.23 e–h |
| 6.84 | 33.90 i | 27.03 j | 28.63 k | 24.43 k | 12.80 h | 6.38 i | 9.75 i | 9.25 i | 30.05 gh | 28.13 h | |
| BF | 0.4 | 61.08 f | 59.03 f | 114.90 f | 111.55 fg | 29.93 de | 29.00 de | 25.50 d–f | 22.75 fg | 37.30 b–f | 34.68 c–g |
| 6.84 | 49.78 g | 47.28 g | 95.45 i | 89.23 i | 21.65 fg | 13.15 h | 18.25 gh | 16.00 h | 34.73 c–g | 32.90 e–h | |
| BB | 0.4 | 73.55 de | 71.93 e | 129.75 de | 125.30 e | 35.05 c | 33.63 cd | 29.25 d | 28.00 de | 38.80 b–e | 36.13 c–g |
| 6.84 | 60.90 f | 56.23 f | 107.75 gh | 104.05 h | 25.45 ef | 16.95 gh | 23.00 e–g | 19.00 gh | 37.20 b–f | 31.80 f–h | |
| BF + N120 | 0.4 | 91.65 b | 89.65 b | 157.13 b | 153.65 b | 46.45 b | 43.95 b | 36.50 b | 35.50 bc | 40.85 a–c | 39.75 b–d |
| 6.84 | 78.85 cd | 71.25 e | 136.50 d | 128.73 e | 35.18 c | 27.28 e | 30.25 d | 27.00 d–f | 37.70 b–f | 34.90 c–g | |
| BB + N120 | 0.4 | 106.25 a | 102.50 a | 176.58 a | 175.35 a | 55.30 a | 53.68 a | 43.25 a | 44.25 a | 46.98 a | 42.88 ab |
| 6.84 | 92.68 b | 81.65 c | 152.45 bc | 146.53 c | 43.93 b | 36.43 c | 36.00 b | 30.50 cd | 37.95 b–f | 34.63 c–h | |
| S | * | * | * | * | * | ||||||
| T | * | * | * | * | * | ||||||
| V | * | * | * | * | * | ||||||
| T × S × V | ns | ns | ns | ns | ns | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, H.M.M.; Rais, U.; Sultan, H.; Tahir, A.; Bahadur, S.; Shah, A.; Iqbal, A.; Li, Y.; Khan, M.N.; Nie, L. Residual Effect of Microbial-Inoculated Biochar with Nitrogen on Rice Growth and Salinity Reduction in Paddy Soil. Plants 2024, 13, 2804. https://doi.org/10.3390/plants13192804
Abbas HMM, Rais U, Sultan H, Tahir A, Bahadur S, Shah A, Iqbal A, Li Y, Khan MN, Nie L. Residual Effect of Microbial-Inoculated Biochar with Nitrogen on Rice Growth and Salinity Reduction in Paddy Soil. Plants. 2024; 13(19):2804. https://doi.org/10.3390/plants13192804
Chicago/Turabian StyleAbbas, Hafiz Muhammad Mazhar, Ummah Rais, Haider Sultan, Ashar Tahir, Saraj Bahadur, Asad Shah, Asim Iqbal, Yusheng Li, Mohammad Nauman Khan, and Lixiao Nie. 2024. "Residual Effect of Microbial-Inoculated Biochar with Nitrogen on Rice Growth and Salinity Reduction in Paddy Soil" Plants 13, no. 19: 2804. https://doi.org/10.3390/plants13192804
APA StyleAbbas, H. M. M., Rais, U., Sultan, H., Tahir, A., Bahadur, S., Shah, A., Iqbal, A., Li, Y., Khan, M. N., & Nie, L. (2024). Residual Effect of Microbial-Inoculated Biochar with Nitrogen on Rice Growth and Salinity Reduction in Paddy Soil. Plants, 13(19), 2804. https://doi.org/10.3390/plants13192804








