Identification of Black Cumin (Nigella sativa) MicroRNAs by Next-Generation Sequencing and Their Implications in Secondary Metabolite Biosynthesis
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis of N. sativa Small RNAs
2.2. Identification of Conserved and Novel miRNAs in N. sativa
2.3. Target Prediction of Conserved and Novel N. sativa miRNAs and Their Functional Analysis
2.4. Identification of N. sativa miRNA Targets Involved in Plant Secondary Metabolite Biosynthesis
2.5. Experimental Validation of N. sativa miRNAs by qPCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials, RNA Extraction, and Quality Control
4.2. Small-RNA Library Construction and Sequencing
4.3. Small-RNA Sequencing Data Analysis
4.4. Prediction of N. sativa miRNA Targets, Their Functional Annotation, and Pathway Analysis
4.5. Extraction of Small RNA and Experimental Validation of N. sativa miRNAs by qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belgaumi, U.; Patil, S.R. The Many Therapeutic Applications of Nigella sativa—A Review of Literature. Artic. J. Evol. Med. Dent. Sci. 2020, 9, 2151–2157. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Begum, S.; Mannan, A. A Review on Nigella sativa: A Marvel Herb. J. Drug Deliv. Ther. 2020, 10, 213–219. [Google Scholar] [CrossRef]
- Lebeta Wako, F. Black Cumin (Nigella sativa L.) Production: A Mini Review. Available online: https://www.researchgate.net/profile/Fikadu-Wako/publication/357323781_Black_cumin_Nigella_sativa_L_Production_A_Mini_Review/links/61c6f919d4500608166739f0/Black-cumin-Nigella-sativa-L-Production-A-Mini-Review.pdf (accessed on 13 August 2024).
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid.-Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Bekkouch, O.; Azizi, S.E.; Azghar, A.; Gseyra, N.; Kim, B. Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021). Biomolecules 2021, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.e.A.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential Influence of Nigella sativa (Black Cumin) in Reinforcing Immune System: A Hope to Decelerate the COVID-19 Pandemic. Phytomedicine 2021, 85, 153277. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ahmad, H.; Rehmanullah; Hayat, S.S.S.; Inayat, N.; Siyyar, S. Nigella sativa L.: Uses in Traditional and Contemporary Medicines—An Overview. Acta Ecol. Sin. 2021, 41, 253–258. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent Advances in MiRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Sharma, A.; Ruiz-Manriquez, L.M.; Serrano-Cano, F.I.; Reyes-Pérez, P.R.; Alfaro, C.K.T.; Andrade, Y.E.B.; Aros, A.K.H.; Srivastava, A.; Paul, S. Identification of MicroRNAs and Their Expression in Leaf Tissues of Guava (Psidium guajava L.) under Salinity Stress. Agronomy 2020, 10, 1920. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; García-Ortega, M.; Medina-Feria, S.; Srivastava, A.; Paul, S. Identification and Expression Profiling of MicroRNAs in Leaf Tissues of Foeniculum vulgare Mill. under Salinity Stress. Plant Signal. Behav. 2024, 19, 2361174. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, B.; Zhang, C. MicroRNAs and Their Roles in Plant Development. Front. Plant Sci. 2022, 13, 824240. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, B. Recent Advances in the Regulation of Plant MiRNA Biogenesis. RNA Biol. 2021, 18, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, L.; Yang, Y.; Schmid, M.; Wang, Y. MiRNA Mediated Regulation and Interaction between Plants and Pathogens. Int. J. Mol. Sci. 2021, 22, 2913. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Reyes-Pérez, P.; Angulo-Bejarano, P.I.; Srivastava, A.; Ramalingam, S.; Sharma, A. Characterization of MicroRNAs from Neem (Azadirachta indica) and Their Tissue-Specific Expression Study in Leaves and Stem. 3 Biotech 2021, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Calderón, A.; Gutiérrez-García, C.; Urióstegui-Pena, A.G.; Srivastava, A.; Aguilar-Marcelino, L.; Iqbal, H.M.N.; Ahmed, S.S.S.J.; Paul, S.; Sharma, A. Identification of Cumin (Cuminum cyminum) MicroRNAs through Deep Sequencing and Their Impact on Plant Secondary Metabolism. Plants 2023, 12, 1756. [Google Scholar] [CrossRef]
- Narjala, A.; Nair, A.; Tirumalai, V.; Vivek Hari Sundar, G.; Shivaprasad, P.V. A Conserved Sequence Signature Is Essential for Robust Plant MiRNA Biogenesis. Nucleic Acids Res. 2020, 48, 3103–3118. [Google Scholar] [CrossRef]
- Sharma, A.; Bejerano, P.I.A.; Maldonado, I.C.; Capote, M.D.D.; Madariaga-Navarrete, A.; Paul, S. Genome-Wide Computational Prediction and Experimental Validation of Quinoa (Chenopodium quinoa) Micrornas. Can. J. Plant Sci. 2019, 99, 666–675. [Google Scholar] [CrossRef]
- Millar, A.A. The Function of MiRNAs in Plants. Plants 2020, 9, 198. [Google Scholar] [CrossRef]
- Owusu Adjei, M.; Zhou, X.; Mao, M.; Rafique, F.; Ma, J. MicroRNAs Roles in Plants Secondary Metabolism. Plant Signal. Behav. 2021, 16, 1915590. [Google Scholar] [CrossRef]
- Paul, S.; de la Fuente-Jiménez, J.L.; Manriquez, C.G.; Sharma, A. Identification, Characterization and Expression Analysis of Passion Fruit (Passiflora edulis) MicroRNAs. 3 Biotech 2020, 10, 25. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Méndez-García, A.; Chamu-García, V.; Rodríguez, A.L.; Bandyopadhyay, A.; Paul, S. The Applications of CRISPR/Cas-Mediated MicroRNA and LncRNA Editing in Plant Biology: Shaping the Future of Plant Non-Coding RNA Research. Planta 2023, 259, 32. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury Paul, S.; Sharma, A.; Mehta, R.; Paul, S. In Silico Characterization of MicroRNAs and Their Target Transcripts from Cranberry (Vaccinium macrocarpon). Cytol. Genet. 2020, 54, 82–90. [Google Scholar] [CrossRef]
- Cisneros, A.E.; Martín-García, T.; Primc, A.; Kuziuta, W.; Sánchez-Vicente, J.; Aragonés, V.; Daròs, J.A.; Carbonell, A. Transgene-Free, Virus-Based Gene Silencing in Plants by Artificial MicroRNAs Derived from Minimal Precursors. Nucleic Acids Res. 2023, 51, 10719–10736. [Google Scholar] [CrossRef]
- Jeena, G.S.; Singh, N.; Shikha; Shukla, R.K. An Insight into MicroRNA Biogenesis and Its Regulatory Role in Plant Secondary Metabolism. Plant Cell Rep. 2022, 41, 1651–1671. [Google Scholar] [CrossRef]
- Misra, P.; Pandey, A.; Tiwari, M.; Chandrashekar, K.; Sidhu, O.P.; Asif, M.H.; Chakrabarty, D.; Singh, P.K.; Trivedi, P.K.; Nath, P.; et al. Modulation of Transcriptome and Metabolome of Tobacco by Arabidopsis Transcription Factor, AtMYB12, Leads to Insect Resistance. Plant Physiol. 2010, 152, 2258. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Tiwari, M.; Pandey, A.; Bhatia, C.; Sharma, A.; Trivedi, P.K. MicroRNA858 Is a Potential Regulator of Phenylpropanoid Pathway and Plant Development. Plant Physiol. 2016, 171, 944–959. [Google Scholar] [CrossRef]
- Choi, Y.A.; Jeong, A.; Woo, C.H.; Cha, S.C.; Park, D.Y.; Sagong, M. Aqueous MicroRNA Profiling in Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy by next-Generation Sequencing. Sci. Rep. 2023, 13, 1274. [Google Scholar] [CrossRef]
- Khamina, K.; Diendorfer, A.B.; Skalicky, S.; Weigl, M.; Pultar, M.; Krammer, T.L.; Fournier, C.A.; Schofield, A.L.; Otto, C.; Smith, A.T.; et al. A MicroRNA Next-Generation-Sequencing Discovery Assay (MiND) for Genome-Scale Analysis and Absolute Quantitation of Circulating MicroRNA Biomarkers. Int. J. Mol. Sci. 2022, 23, 1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Pan, X.P.; Cox, S.B.; Cobb, G.P.; Anderson, T.A. Evidence That MiRNAs Are Different from Other RNAs. Cell. Mol. Life Sci. 2006, 63, 246–254. [Google Scholar] [CrossRef]
- Delfin, J.C.; Watanabe, M.; Tohge, T. Understanding the Function and Regulation of Plant Secondary Metabolism through Metabolomics Approaches. Theor. Exp. Plant Physiol. 2018, 31, 127–138. [Google Scholar] [CrossRef]
- Isah, T.; Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kumar, P.; Sanyal, R.; Mane, A.B.; Arvind Prasanth, D.; Patil, M.; Dey, A. Unravelling the Regulatory Role of MiRNAs in Secondary Metabolite Production in Medicinal Crops. Plant Gene 2021, 27, 100303. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Saikat, A.S.M.; Jain, D.; Habib, A.; Janmeda, P.; Islam, M.T.; Radha; Daştan, S.D.; Kumar, M.; et al. Biosynthesis of Secondary Metabolites Based on the Regulation of MicroRNAs. Biomed Res. Int. 2022, 2022, 9349897. [Google Scholar] [CrossRef]
- Jeyakumar, J.M.J.; Ali, A.; Wang, W.M.; Thiruvengadam, M. Characterizing the Role of the MiR156-SPL Network in Plant Development and Stress Response. Plants 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Srivastava, S.; Shasany, A.K.; Sharma, A. Identification of MiRNAs and Their Targets Involved in the Secondary Metabolic Pathways of Mentha Spp. Comput. Biol. Chem. 2016, 64, 154–162. [Google Scholar] [CrossRef]
- Yu, Z.X.; Wang, L.J.; Zhao, B.; Shan, C.M.; Zhang, Y.H.; Chen, D.F.; Chen, X.Y. Progressive Regulation of Sesquiterpene Biosynthesis in Arabidopsis and Patchouli (Pogostemon cablin) by the MIR156-Targeted SPL Transcription Factors. Mol. Plant 2015, 8, 98–110. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, M.; Li, J.; Xu, Y.; Li, C.; Lu, S. Genome-Wide Identification and Functional Analysis of Salvia Miltiorrhiza MicroRNAs Reveal the Negative Regulatory Role of Smi-MiR159a in Phenolic Acid Biosynthesis. Int. J. Mol. Sci. 2024, 25, 5148. [Google Scholar] [CrossRef]
- Chellappan, P.; Xia, J.; Zhou, X.; Gao, S.; Zhang, X.; Coutino, G.; Vazquez, F.; Zhang, W.; Jin, H. SiRNAs from MiRNA Sites Mediate DNA Methylation of Target Genes. Nucleic Acids Res. 2010, 38, 6883. [Google Scholar] [CrossRef]
- Wang, F.; Polydore, S.; Axtell, M.J. More than Meets the Eye? Factors That Affect Target Selection by Plant MiRNAs and Heterochromatic SiRNAs. Curr. Opin. Plant Biol. 2015, 27, 118–124. [Google Scholar] [CrossRef]
- Wang, F.; Johnson, N.R.; Coruh, C.; Axtell, M.J. Genome-Wide Analysis of Single Non-Templated Nucleotides in Plant Endogenous SiRNAs and MiRNAs. Nucleic Acids Res. 2016, 44, 7395–7405. [Google Scholar] [CrossRef] [PubMed]
- Ashiq, K.; Bajwa, M.; Qayyum, M.; Tanveer, S.; Asif Bajwa, M.; Shah Rukh, A.; Jahangir, N.; Ishaq, M.; Rabbani, A.; Aslam, M.; et al. Review on Phytochemical Evaluation and Extraction of Nigella sativa (Kalongi) with Pharmacological and Traditional Applications. Int. J. Biosci. 2020, 16, 231–241. [Google Scholar] [CrossRef]
- Mehmood, A.; Naveed, K.; Jadoon, N.; Ayub, Q.; Hussain, M.; Hassaan, M. Phytochemical Screening and Antibacterial Efficacy of Black Cumin (Nigella sativa L.) Seeds. FUUAST J. Biol. 2021, 11, 23–28. [Google Scholar]
- Srinivasan, K. Cumin (Cuminum cyminum) and Black Cumin (Nigella sativa) Seeds: Traditional Uses, Chemical Constituents, and Nutraceutical Effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Manoharan, N.; Jayamurali, D.; Parasuraman, R.; Narayanan Govindarajulu, S. Traditional Indian Medicine Phytochemical Composition, Therapeutical and Pharmacological Potential of Nigella sativa: A Review. Tradit. Med. Res. 2021, 6, 32. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An Updated Knowledge of Black Seed (Nigella sativa Linn.): Review of Phytochemical Constituents and Pharmacological Properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef]
- Shaukat, A.; Zaidi, A.; Anwar, H.; Kizilbash, N. Mechanism of the Antidiabetic Action of Nigella sativa and Thymoquinone: A Review. Front. Nutr. 2023, 10, 1126272. [Google Scholar] [CrossRef]
- Stra, A.; Almarwaey, L.O.; Alagoz, Y.; Moreno, J.C.; Al-Babili, S. Carotenoid Metabolism: New Insights and Synthetic Approaches. Front. Plant Sci. 2022, 13, 1072061. [Google Scholar] [CrossRef]
- Acevedo, O.; Contreras, R.A.; Stange, C. The Carrot Phytoene Synthase 2 (DcPSY2) Promotes Salt Stress Tolerance through a Positive Regulation of Abscisic Acid and Abiotic-Related Genes in Nicotiana Tabacum. Plants 2023, 12, 1925. [Google Scholar] [CrossRef]
- Qin, Y.; Woo, H.J.; Shin, K.S.; Lim, M.H.; Lee, S.K. Comparative Transcriptome Profiling of Different Tissues from Beta-Carotene-Enhanced Transgenic Soybean and Its Non-Transgenic Counterpart. Plant Cell. Tissue Organ Cult. 2020, 140, 341–356. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.J.; Lee, H.S.; Kwak, S.S. Down-Regulation of β-Carotene Hydroxylase Increases β-Carotene and Total Carotenoids Enhancing Salt Stress Tolerance in Transgenic Cultured Cells of Sweetpotato. Phytochemistry 2012, 74, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, Health Benefits, and Biological Properties of Zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef] [PubMed]
- Couillaud, J.; Leydet, L.; Duquesne, K.; Iacazio, G. The Terpene Mini-Path, a New Promising Alternative for Terpenoids Bio-Production. Genes 2021, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Niu, M.; Yan, H.; Xiong, Y.; Zhang, Y.; Zhang, X.; Li, Y.; da Silva, J.A.T.; Ma, G. Cloning, Characterization, and Functional Analysis of Acetyl-CoA C-Acetyltransferase and 3-Hydroxy-3-Methylglutaryl-CoA Synthase Genes in Santalum Album. Sci. Rep. 2021, 11, 1082. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Z.; Tian, Z.; Zhang, H.; Zhu, C.; Yao, X.; Yang, Y.; Cai, X. Molecular Cloning and Analysis of an Acetyl-CoA C-Acetyltransferase Gene (EkAACT) from Euphorbia Kansui Liou. Plants 2022, 11, 1539. [Google Scholar] [CrossRef]
- Padhan, J.K.; Sood, H.; Chauhan, R. Prospecting NGS-Transcriptomes to Assess Regulation of MiRNA-Mediated Secondary Metabolites Biosynthesis in Swertia Chirayita, a Medicinal Herb of the North-Western Himalayas. Med. Plants 2016, 8, 219–228. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, G.; He, W.; Liu, K.; Luo, Y.; Tang, J.; He, N. Functional Characterization of the 1-Deoxy-D-Xylulose 5-Phosphate Synthase Genes in Morus Notabilis. Front. Plant Sci. 2020, 11, 521621. [Google Scholar] [CrossRef]
- Henry, L.K.; Thomas, S.T.; Widhalm, J.R.; Lynch, J.H.; Davis, T.C.; Kessler, S.A.; Bohlmann, J.; Noel, J.P.; Dudareva, N. Contribution of Isopentenyl Phosphate to Plant Terpenoid Metabolism. Nat. Plants 2018, 4, 721–729. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Li, Y.; Liu, W.; Tao, Y.; Wang, G. A Structural and Functional Study on the 2-C-Methyl-d-Erythritol-4-Phosphate Cytidyltransferase (IspD) from Bacillus subtilis. Sci. Rep. 2016, 6, 36379. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hase, T. Cyanobacterial Non-Mevalonate Pathway: (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase interacts with ferredoxin in thermosynechococcus elongatus bp-1. J. Biol. Chem. 2005, 280, 20672–20679. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene Acts as a Signaling Molecule in Gene Networks Important for Stress Responses and Plant Growth. Plant Physiol. 2019, 180, 124. [Google Scholar] [CrossRef] [PubMed]
- Sabir, I.A.; Manzoor, M.A.; Shah, I.H.; Abbas, F.; Liu, X.; Fiaz, S.; Shah, A.N.; Jiu, S.; Wang, J.; Abdullah, M.; et al. Evolutionary and Integrative Analysis of Gibberellin-Dioxygenase Gene Family and Their Expression Profile in Three Rosaceae Genomes (F. vesca, P. mume, and P. avium) Under Phytohormone Stress. Front. Plant Sci. 2022, 13, 942969. [Google Scholar] [CrossRef] [PubMed]
- Ci, J.; Wang, X.; Wang, Q.; Zhao, F.; Yang, W.; Cui, X.; Jiang, L.; Ren, X.; Yang, W. Genome-Wide Analysis of Gibberellin-Dioxygenases Gene Family and Their Responses to GA Applications in Maize. PLoS ONE 2021, 16, e0250349. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Badieyan, S.; Bevan, D.R.; Herde, M.; Gatz, C.; Tholl, D. Herbivore-Induced and Floral Homoterpene Volatiles Are Biosynthesized by a Single P450 Enzyme (CYP82G1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 21205–21210. [Google Scholar] [CrossRef]
- Gómez-Vásquez, R.; Day, R.; Buschmann, H.; Randles, S.; Beeching, J.R.; Cooper, R.M. Phenylpropanoids, Phenylalanine Ammonia Lyase and Peroxidases in Elicitor-challenged Cassava (Manihot esculenta) Suspension Cells and Leaves. Ann. Bot. 2004, 94, 87. [Google Scholar] [CrossRef]
- Herrig, V.; Ferrarese, M.D.L.L.; Suzuki, L.S.; Rodrigues, J.D.; Ferrarese-Filho, O. Peroxidase and Phenylalanine Ammonia-Lyase Activities, Phenolic Acid Contents, and Allelochemicals-Inhibited Root Growth of Soybean. Biol. Res. 2002, 35, 59–66. [Google Scholar] [CrossRef]
- Maslennikova, D.; Ivanov, S.; Petrova, S.; Burkhanova, G.; Maksimov, I.; Lastochkina, O. Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity. Plants 2023, 12, 2123. [Google Scholar] [CrossRef]
- Chen, Y.; Black, D.S.; Reilly, P.J. Carboxylic Ester Hydrolases: Classification and Database Derived from Their Primary, Secondary, and Tertiary Structures. Protein Sci. 2016, 25, 1942. [Google Scholar] [CrossRef]
- Ferro, A.P.; Flores Júnior, R.; Finger-Teixeira, A.; Parizotto, A.V.; Bevilaqua, J.M.; de Oliveira, D.M.; Molinari, H.B.C.; Marchiosi, R.; dos Santos, W.D.; Seixas, F.A.V.; et al. Inhibition of Zea Mays Coniferyl Aldehyde Dehydrogenase by Daidzin: A Potential Approach for the Investigation of Lignocellulose Recalcitrance. Process Biochem. 2020, 90, 131–138. [Google Scholar] [CrossRef]
- Lashley, A.; Miller, R.; Provenzano, S.; Jarecki, S.A.; Erba, P.; Salim, V. Functional Diversification and Structural Origins of Plant Natural Product Methyltransferases. Molecules 2023, 28, 43. [Google Scholar] [CrossRef] [PubMed]
- Bureau, T.; Lam, K.C.; Ibrahim, R.K.; Behdad, B.; Dayanandan, S. Structure, Function, and Evolution of Plant O-Methyltransferases. Genome 2007, 50, 1001–1013. [Google Scholar] [CrossRef]
- Helliwell, S.B.; Karkare, S.; Bergdoll, M.; Rahier, A.; Leighton-Davis, J.R.; Fioretto, C.; Aust, T.; Filipuzzi, I.; Frederiksen, M.; Gounarides, J.; et al. FR171456 Is a Specific Inhibitor of Mammalian NSDHL and Yeast Erg26p. Nat. Commun. 2015, 6, 8613. [Google Scholar] [CrossRef]
- Zeng, Z.; Jia, Y.; Huang, X.; Chen, Z.; Xiang, T.; Han, N.; Bian, H.; Li, C. Transcriptional and Protein Structural Characterization of Homogentisate Phytyltransferase Genes in Barley, Wheat, and Oat. BMC Plant Biol. 2023, 23, 528. [Google Scholar] [CrossRef]
- Döll, S.; Kuhlmann, M.; Rutten, T.; Mette, M.F.; Scharfenberg, S.; Petridis, A.; Berreth, D.C.; Mock, H.P. Accumulation of the Coumarin Scopolin under Abiotic Stress Conditions Is Mediated by the Arabidopsis Thaliana THO/TREX Complex. Plant J. 2018, 93, 431–444. [Google Scholar] [CrossRef]
- Gutiérrez-García, C.; Ahmed, S.S.S.J.; Ramalingam, S.; Selvaraj, D.; Srivastava, A.; Paul, S.; Sharma, A. Identification of MicroRNAs from Medicinal Plant Murraya Koenigii by High-Throughput Sequencing and Their Functional Implications in Secondary Metabolite Biosynthesis. Plants 2021, 11, 46. [Google Scholar] [CrossRef]
- Kaur, R.; Pathania, S.; Kajal, M.; Thakur, V.; Kaur, J.; Singh, K. Integrated Analysis of SmRNAome, Transcriptome, and Degradome Data to Decipher MicroRNAs Regulating Costunolide Biosynthesis in Saussurea Lappa. Plant Sci. 2023, 331, 111689. [Google Scholar] [CrossRef]
- Zeeshan, M.; Qiu, C.W.; Naz, S.; Cao, F.; Wu, F. Genome-Wide Discovery of Mirnas with Differential Expression Patterns in Responses to Salinity in the Two Contrasting Wheat Cultivars. Int. J. Mol. Sci. 2021, 22, 12556. [Google Scholar] [CrossRef]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/IAA14 Regulates MicroRNA-Mediated Cold Stress Response in Arabidopsis Roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef]
- Li, H.; Meng, H.; Sun, X.; Deng, J.; Shi, T.; Zhu, L.; Lv, Q.; Chen, Q. Integrated MicroRNA and Transcriptome Profiling Reveal Key MiRNA-MRNA Interaction Pairs Associated with Seed Development in Tartary Buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2021, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Riffo-Campos, Á.L.; Riquelme, I.; Brebi-Mieville, P. Tools for sequence-based miRNA target prediction: What to choose? Int. J. Mol. Sci. 2016, 17, 1987. [Google Scholar] [CrossRef] [PubMed]

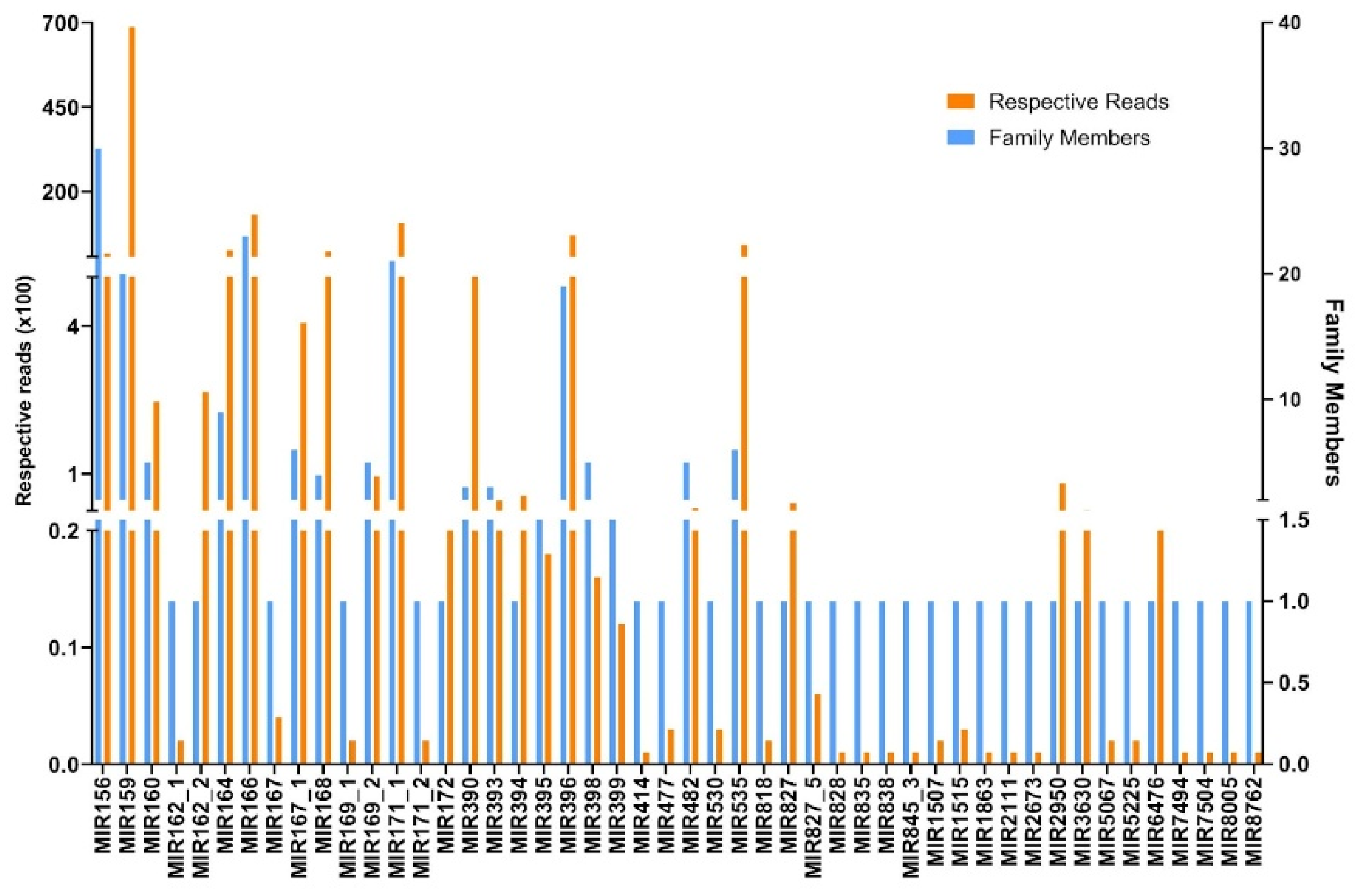

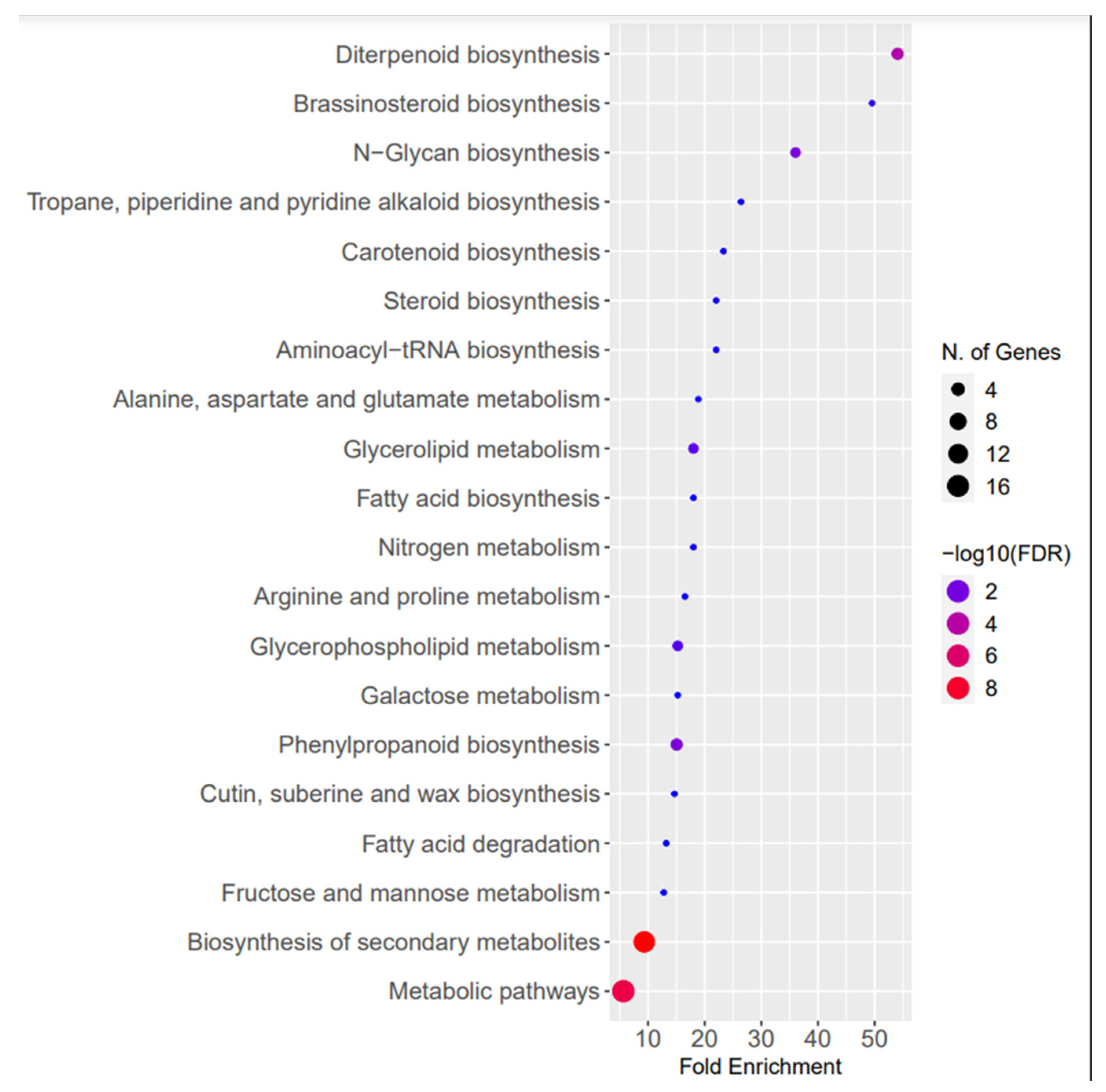
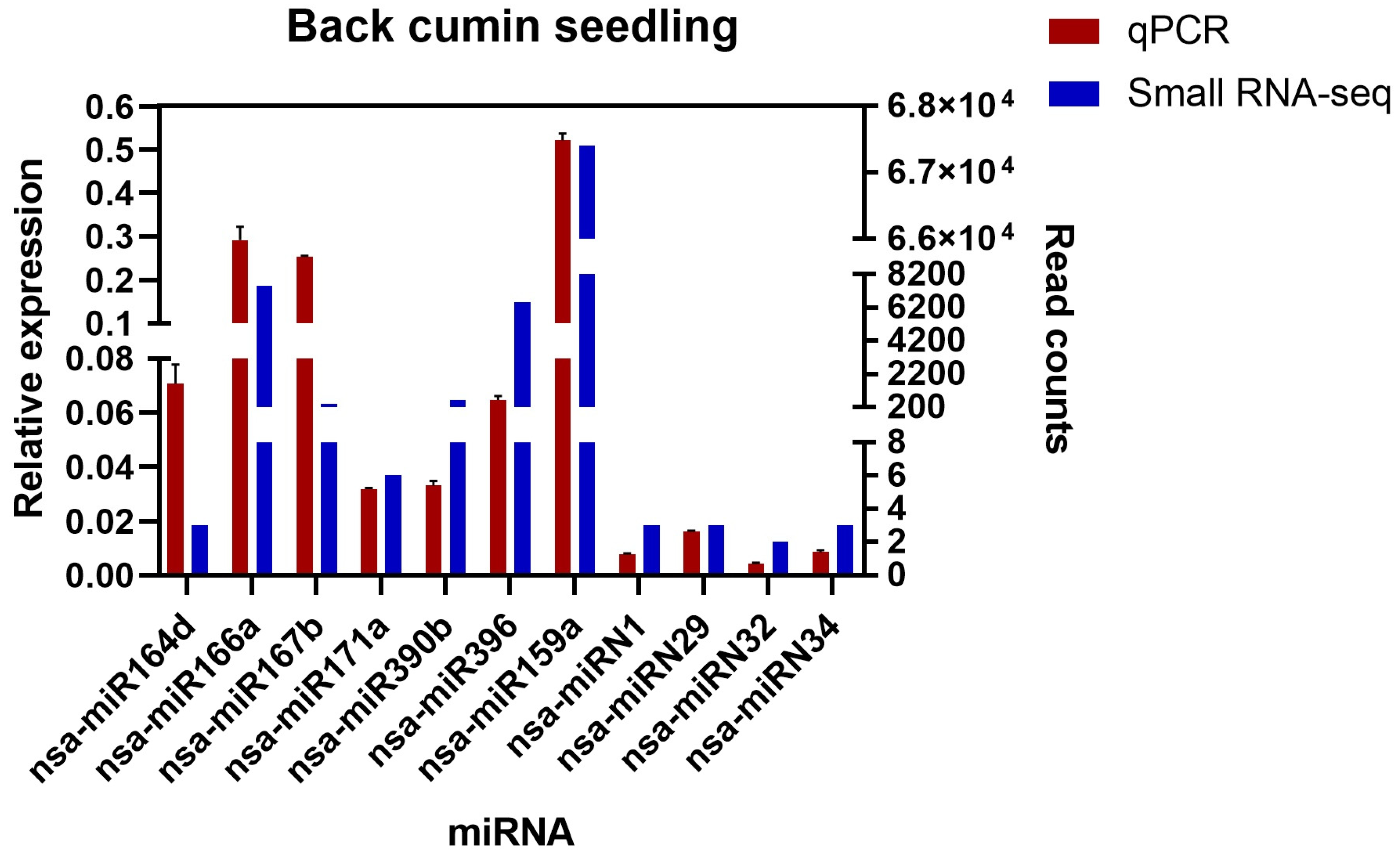
| Category | Total Reads | Unique Reads |
|---|---|---|
| Raw data generated after sequencing | 33,508,811 | 25,139,003 |
| Trimmed reads | 8,605,403 | 1,368,483 |
| Reads aligned to miRBase | 291,381 | 196,114 |
| Known miRNA Uniq | 240 | |
| Reads utilized for novel miRNA | 2,286,411 | 393,335 |
| Novel miRNA predicted | 34 | |
| Putative miRNAs | 2,168,726 | 355,560 |
| % reads aligned to ncRNA | 54.26% | |
| Reads aligned to ncRNA (rRNA, snoRNA, snRNA, tRNA) | 6,027,611 | 742,592 |
| Name | Sequence (5′-3′) | Length | Read Count | Strand | MFEI of Precursor | Potential Targets Related to Secondary Metabolite Biosynthesis |
|---|---|---|---|---|---|---|
| nsa-miRN1-3p | UCUCUCUUCCUGAUCCUCCU | 20 | 3 | + | 0.7317 | 7-deoxyloganetic acid glucosyltransferase-like acetyl-CoA acetyltransferase, cytosolic 1-like isochorismate synthase, chloroplastic-like |
| nsa-miRN2-5p | UAACCUGGUUGUGGUUGUGGU | 21 | 2 | − | 0.7723 | beta-D-glucosyl crocetin beta-1,6-glucosyltransferase-like UDP-glucosyltransferase 29-like |
| nsa-miRN3-5p | UGAUGAUGAAGAGUAGUGAUG | 21 | 1 | − | 0.8129 | probable methionine–tRNA ligase |
| nsa-miRN4-5p | AGAUAAUUUGUUACCUUUAC | 20 | 2 | − | 1.2435 | |
| nsa-miRN5-5p | AGUUGGGUAAAUUGUUGGAU | 20 | 1 | − | 0.9194 | NA |
| nsa-miRN6-3p | AAUGGUCGAAAGAUGUGGCGG | 21 | 2 | − | 0.7568 | NA |
| nsa-miRN7-5p | AGGAGGAUAAGGAUGGGAAA | 20 | 2 | − | 0.7930 | GPI transamidase component PIG-S-like |
| nsa-miRN8-3p | AGGGUUCAGCUGUUCUUCUC | 20 | 2 | + | 0.7974 | triacylglycerol lipase SDP1 |
| nsa-miRN9-3p | UUAUCAACUUCUCUCAGGUG | 20 | 1 | + | 0.7372 | adenylate isopentenyltransferase 3, chloroplastic |
| nsa-miRN10-5p | UUUAGCACCCCAUUGGACUUGU | 22 | 1 | + | 1.2967 | peroxidase N1-like phosphatidylserine decarboxylase proenzyme 2 caffeic acid 3-O-methyltransferase |
| nsa-miRN11-5p | GGUUUGGUUGAGUACAUUUCU | 21 | 1 | + | 0.7452 | NA |
| nsa-miRN12-5p | GGUUUGUUAAUGGAACCUUG | 20 | 1 | − | 0.7200 | NA |
| nsa-miRN13-3p | GAGAAGAGACAUGGCUAGAG | 20 | 2 | − | 0.7730 | glutamine synthetase cytosolic isozyme long-chain acyl-CoA synthetase 6, peroxisomal palmitoyl-acyl carrier protein thioesterase, chloroplastic-like |
| nsa-miRN14-3p | AUUUGGAUUUCAUCUGAAAU | 20 | 2 | − | 1.0207 | NA |
| nsa-miRN15-5p | CGGUUCAUAGGAAUGAGAAUGAG | 23 | 1 | + | 1.1087 | beta-glucosidase BoGH3B-like 3-ketoacyl-CoA synthase 4 |
| nsa-miRN16-5p | UGUUGAGGAAGGUAUUGUUGU | 21 | 1 | + | 0.7914 | NADPH/quinone oxidoreductase homogentisate phytyltransferase 1, chloroplastic |
| nsa-miRN17-3p | UUUAUGUGUGUGUAUUGGUUU | 21 | 1 | − | 1.5333 | NA |
| nsa-miRN18-5p | UGGAACAAGCUGGUGUGUUGCG | 22 | 1 | − | 0.7264 | NA |
| nsa-miRN19-3p | AUGUAAUAAGAAAUGGUUUAGAUU | 24 | 2 | + | 0.8467 | NA |
| nsa-miRN20-3p | CUUUUGGUGUAGUGGUUAGCG | 21 | 1 | − | 0.7979 | NA |
| nsa-miRN21-3p | CAAGAGCACCGGGAAAUGAA | 20 | 1 | + | 0.7156 | UDP-glycosyltransferase 85A8 |
| nsa-miRN22-5p | UGGAGCAGGCUCUGAAGCGG | 20 | 1 | − | 0.7328 | gibberellin 20 oxidase 1-like biotin carboxyl carrier protein of acetyl-CoA carboxylase 1, chloroplastic-like CTP synthase-like |
| nsa-miRN23-5p | AUGGUGUGGAUGUGAUUUCGUU | 22 | 1 | + | 0.7200 | geranylgeranyl pyrophosphate synthase 7, chloroplastic-like |
| nsa-miRN24-3p | AAGUUGGAGGUGGUGGUGGU | 20 | 3 | − | 0.8147 | polyphenol oxidase I, chloroplastic bifunctional riboflavin biosynthesis protein RIBA 1, chloroplastic bifunctional riboflavin biosynthesis protein RIBA 1, chloroplastic |
| nsa-miRN25-3p | UGUGUUAUGGUGCGUGGUGU | 20 | 1 | − | 0.7462 | 3-ketoacyl-CoA synthase 6-like heterodimeric geranylgeranyl pyrophosphate synthase small subunit, chloroplastic-like |
| nsa-miRN26-5p | GAAAGUGUUGACAAUCGUCCUG | 22 | 1 | − | 0.7143 | NA |
| nsa-miRN27-3p | GGUUUGUGCGGUUUGCAUGC | 20 | 1 | − | 0.9659 | S-adenosylmethionine decarboxylase proenzyme-like |
| nsa-miRN28-3p | AUUGGUAUGUUGUCUUCAUA | 20 | 1 | − | 0.7543 | glutamyl-tRNA(Gln) amidotransferase subunit A, chloroplastic/mitocondrial |
| nsa-miRN29-5p | GAAGUUCUCGGGGGUGUUUGA | 21 | 3 | + | 0.8628 | NA |
| nsa-miRN30-5p | GAGAAUAGGAUGAAACUAAACA | 22 | 2 | + | 1.3719 | NA |
| nsa-miRN31-5p | GUCUCUGGAUUGUAAGGGUU | 20 | 1 | − | 0.7812 | NA |
| nsa-miRN32-3p | GUAAAUGUUUUGGGACUUCAAGC | 23 | 2 | + | 0.8763 | NA |
| nsa-miRN33-3p | CCGUUGUUGAUGCAGCGUGG | 20 | 2 | + | 0.7292 | NA |
| nsa-miRN34-5p | GCGGCGAAGAUGGAAGUAGAG | 21 | 3 | + | 0.8914 | beta-1,3-galactosyltransferase GALT1 |
| Biosynthetic Pathway | miRNAs | E.C. | Target Enzyme |
|---|---|---|---|
| Carotenoid biosynthesis | nsa-miR167c nsa-miR167b | 2.5.1.32 | 15-cis-phytoene synthase |
| nsa-miR157d-3p | 1.14.15.24 (LUT5) | beta-carotene 3-hydroxylase | |
| Diterpenoid biosynthesis | nsa-miR394b-5p | 1.14.11.12 | gibberellin-44 dioxygenase |
| nsa-miR159 | 1.14.11.13 | gibberellin 2beta-dioxygenase | |
| nsa-miR535d | 1.14.11.15 | gibberellin 3beta-dioxygenase | |
| nsa-miR169d | 1.14.14.58 | trimethyltridecatetraene synthase | |
| Phenylpropanoid biosynthesis | nsa-miR8175 nsa-miR156r nsa-miR156f nsa-miR156w nsa-miR156b nsa-miR156p nsa-miR156l | 3.1.1 | carboxylic ester hydrolases |
| nsa-miR164b-5p | 1.2.1.68 | coniferyl aldehyde dehydrogenase | |
| nsa-miR156j nsa-miR156e nsa-miR156q nsa-miR164c nsa-miR164c-5p nsa-miR164b-5p | 1.11.1.7 | peroxidase | |
| Biosynthesis of various plant secondary metabolites | nsa-miR399i | 1.1.1.285 | 3″-deamino-3″-oxonicotianamine |
| nsa-miR167c nsa-miR167b | 2.4.1.128 | scopoletin glucosyltransferase | |
| Terpenoid backbone biosynthesis | nsa-miR156w | 2.3.1.9 | acetyl-CoA C-acetyltransferase |
| nsa-miR535d | 2.2.1.7 | 1-deoxy-D-xylulose-5-phosphate synthase | |
| nsa-miR157d-3p | 2.7.7.60 | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase | |
| nsa-miR156e | 1.17.7.1 | (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase (ferredoxin) | |
| 1.17.7.3 | (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase (flavodoxin) | ||
| nsa-miR171a-3p | 4.2.3.27 | isoprene synthase | |
| nsa-miR156j | 3.4. (FACE2) | CAAX prenyl protease 2 |
| Biosynthetic Pathway | miRNA | E.C. | Target Enzyme |
|---|---|---|---|
| Flavonoid biosynthesis | nsa-miRN1-3p nsa-miRN24-3p | 2.3.1.133 | shikimate O-hydroxycinnamoyltransferase |
| Phenylpropanoid biosynthesis | |||
| nsa-mRN10-5p nsa-mRN24-3p | 1.11.1.7 | peroxidase | |
| Terpenoid backbone biosynthesis | nsa-mRN1-3p | 2.3.1.9 | acetyl-CoA C-acetyltransferase |
| 2.7.7.60 | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase | ||
| nsa-mRN10-5p | 2.7.4.26 | isopentenyl phosphate kinase | |
| Steroid biosynthesis | nsa-miRN24-3p | 2.1.1.41 (SMT1/ERG6) | sterol 24-C-methyltransferase |
| 1.1.1.418 | plant 3beta-hydroxysteroid-4alpha-carboxylate 3-dehydrogenase (decarboxylating) | ||
| Ubiquinone and other terpenoid–quinone biosynthesis | nsa-miRN16-5p | 2.5.1.115 | homogentisate phytyltransferase |
| 2.5.1.116 | homogentisate geranylgeranyltransferase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uriostegui-Pena, A.G.; Reyes-Calderón, A.; Gutiérrez-García, C.; Srivastava, A.; Sharma, A.; Paul, S. Identification of Black Cumin (Nigella sativa) MicroRNAs by Next-Generation Sequencing and Their Implications in Secondary Metabolite Biosynthesis. Plants 2024, 13, 2806. https://doi.org/10.3390/plants13192806
Uriostegui-Pena AG, Reyes-Calderón A, Gutiérrez-García C, Srivastava A, Sharma A, Paul S. Identification of Black Cumin (Nigella sativa) MicroRNAs by Next-Generation Sequencing and Their Implications in Secondary Metabolite Biosynthesis. Plants. 2024; 13(19):2806. https://doi.org/10.3390/plants13192806
Chicago/Turabian StyleUriostegui-Pena, Andrea G., Almendra Reyes-Calderón, Claudia Gutiérrez-García, Aashish Srivastava, Ashutosh Sharma, and Sujay Paul. 2024. "Identification of Black Cumin (Nigella sativa) MicroRNAs by Next-Generation Sequencing and Their Implications in Secondary Metabolite Biosynthesis" Plants 13, no. 19: 2806. https://doi.org/10.3390/plants13192806








