Anatomical Responses of Two Species under Controlled Water Restriction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Previous Experiment: Water Restriction
2.2. Collection and Fixation of Leaf Material
2.3. Observations and Anatomical Measurements
2.4. Statistical Analysis
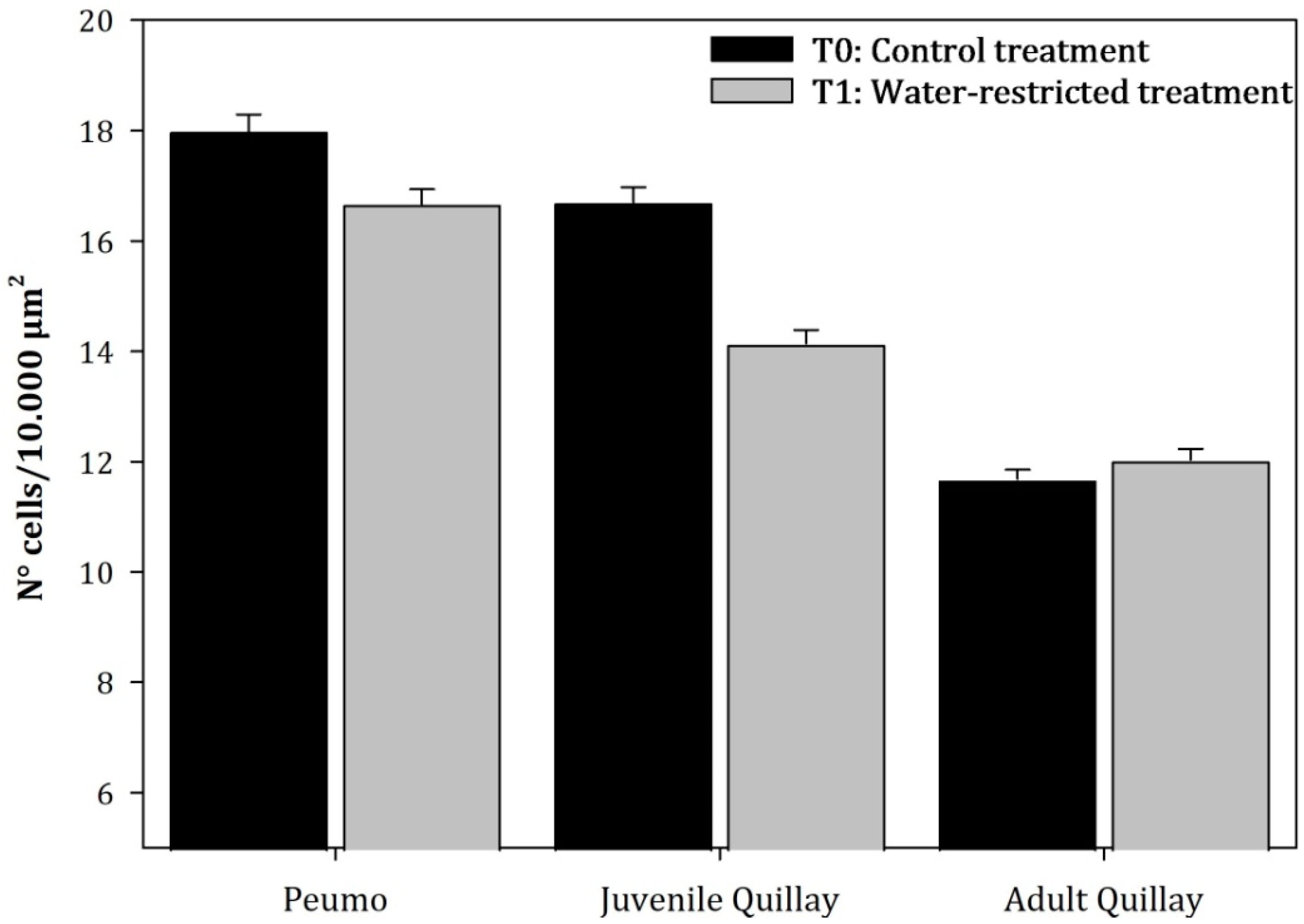
3. Results
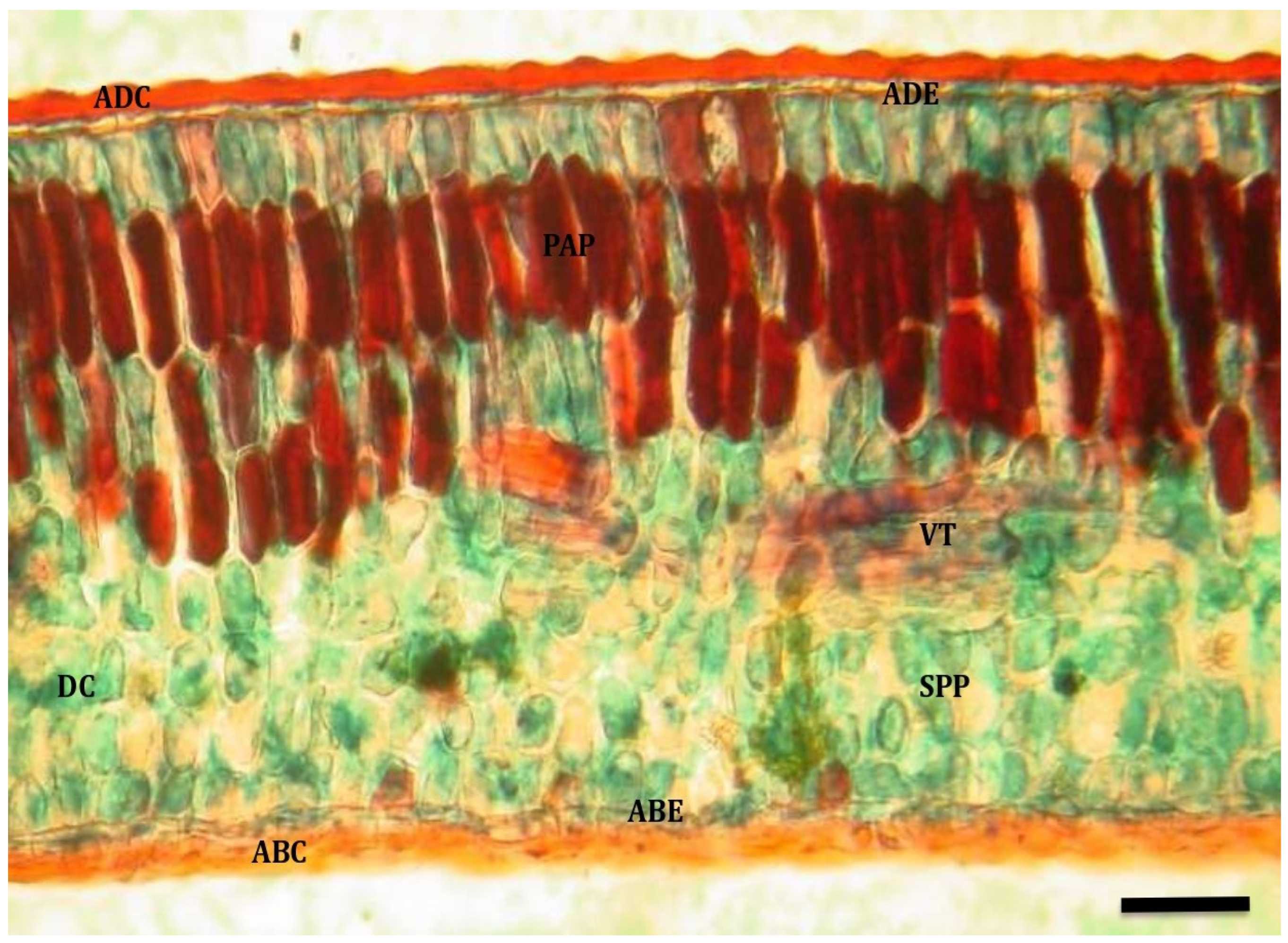
3.1. Anatomical Description
3.1.1. Quillaja saponaria
3.1.2. Cryptocarya alba
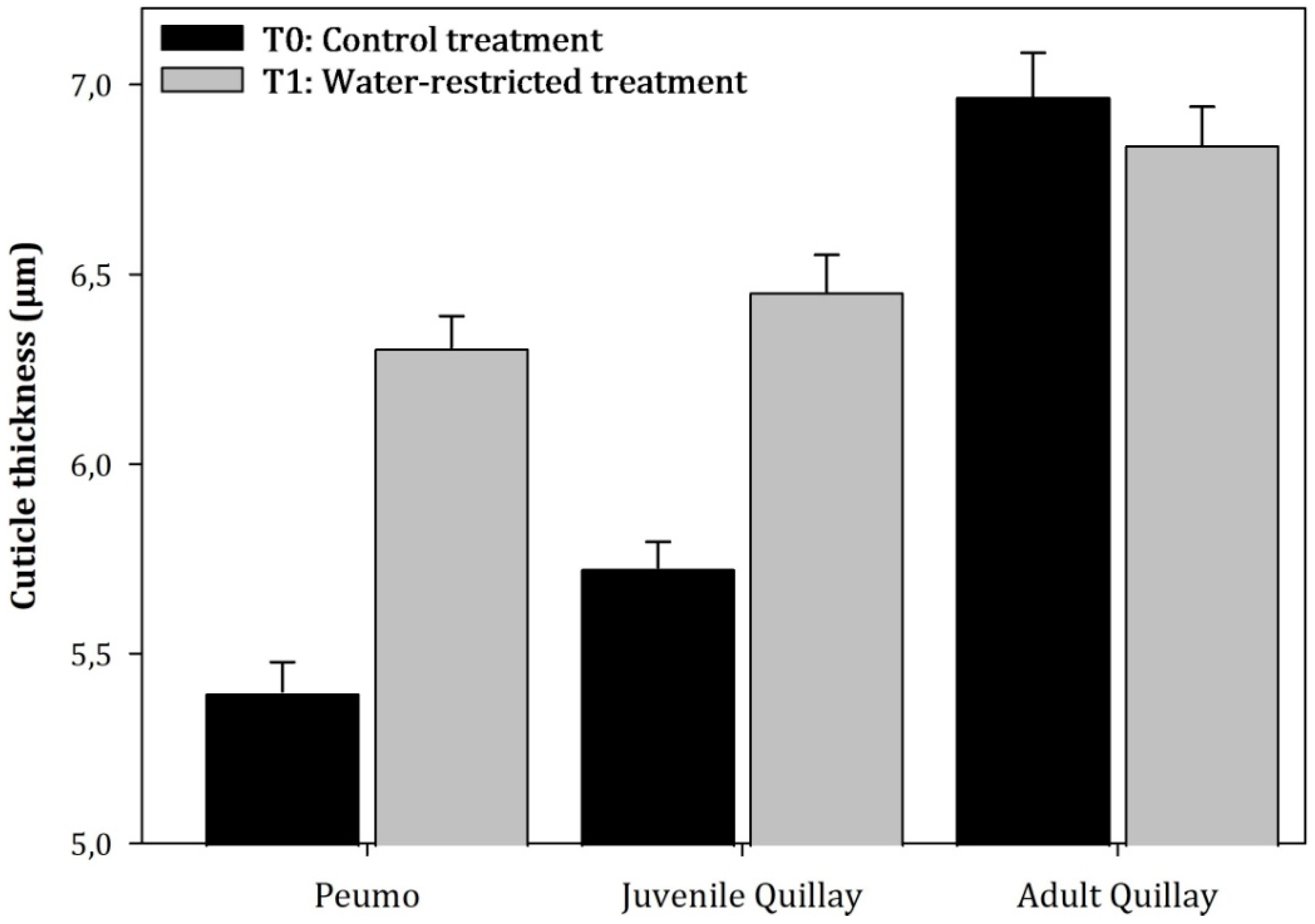
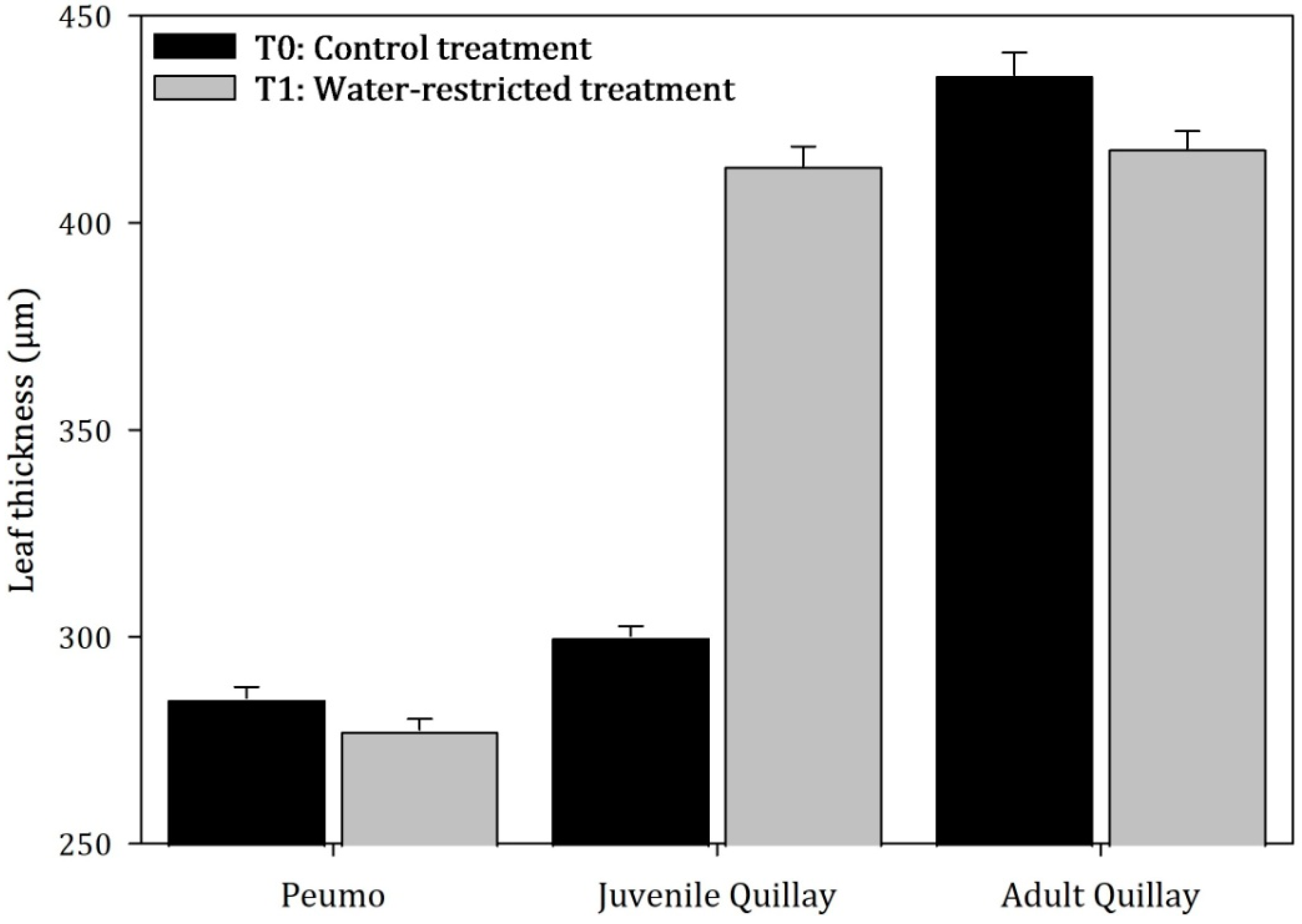
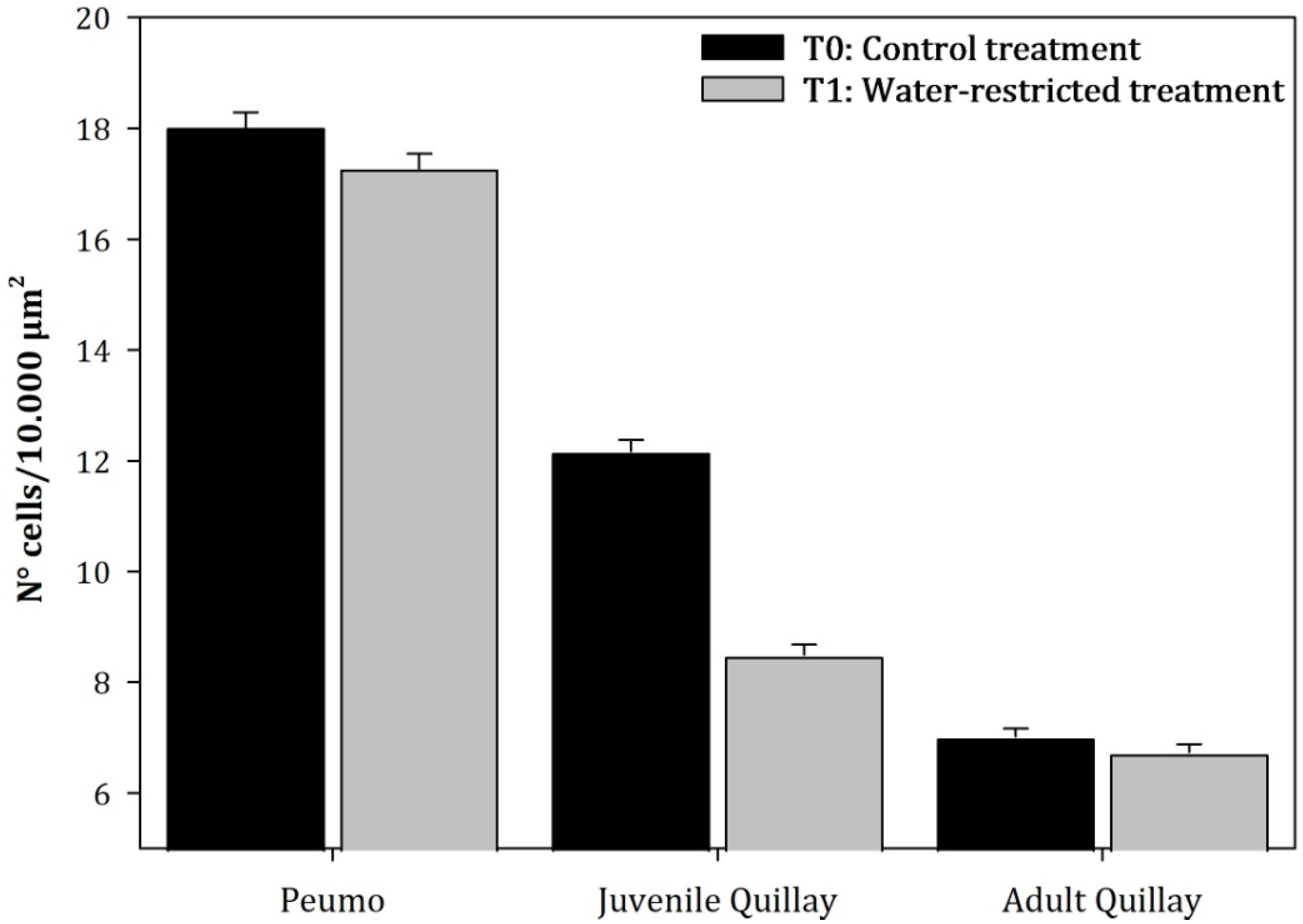
3.2. Anatomical Measurements
3.3. Parenchymal Tissue Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Wang, R.; Harrison, S.; Prentice, I.A. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, U.; Hameed, M.; Ahmad, F. Structural and functional traits underlying the capacity of Calotropis procera to face different stress conditions. J. Physiol. Biochem. 2023, 203, 107992. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Xu, L.; Chen, Z.; He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A. Hard and tough: The coordination between leaf mechanical resistance and drought tolerance. Flora 2022, 288, 152023. [Google Scholar] [CrossRef]
- Sommer, R. Phenotypic plasticity: From theory and genetics to current and future challenges. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef]
- Jansen, T.; Fleischer, K.; Luyssaert, S.; Naudts, K.; Dolman, H. Drought resistance increases from the individual to the ecosystem level in highly diverse Neotropical rainforest: A meta-analysis of leaf, tree and ecosystem responses to drought. Biogeosciences 2020, 17, 2621–2645. [Google Scholar] [CrossRef]
- Benedetti-Ruiz, S.; Delard, R.; González-Ortega, M.; Roach-Barrios, F.A. Monografía de Quillay Quillaja Saponaria; INFOR: Santiago, Chile, 2000. [Google Scholar] [CrossRef]
- Gajardo, R. La vegetación natural de Chile. In Clasificación y Distribución Geográfica; Editorial Universitaria: Santiago, Chile, 1994. [Google Scholar]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile, 2nd ed.; Editorial Universitaria: Santiago, Chile, 2018. [Google Scholar]
- Botti, C. Anatomía foliar y xerofitismo en algunas especies chilenas. Investig. Agrícola 1976, 3, 95–104. [Google Scholar]
- Montenegro, G. Atlas de Anatomía de Especies Vegetales Autóctonas de la Zona Central; Ediciones Universidad Católica de Chile: Santiago, Chile, 1984; Volume 5, p. 43. [Google Scholar]
- Chaves, M.; Maroco, J.; Pereira, J. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Donoso, S.; Peña-Rojas, K.; Pacheco, C.; Luna, G.; Aguirre, A. Respuesta fisiológica y de crecimiento en plantas de Quillaja saponaria y Cryptocarya alba sometidas a restricción hídrica. Bosque 2011, 32, 187–195. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Benedetti, S. Monografía de Peumo Cryptocarya alba (Mol.) Looser; INFOR: Santiago, Chile, 2012. [Google Scholar] [CrossRef]
- Del Fierro, P.; Pancel, L.; Rivera, H.; Castillo, J. Experiencia Silvicultural del Bosque Nativo de Chile; CONAF-GTZ: Santiago, Chile, 1998; 420p. [Google Scholar]
- Cabello, A.; Donoso, C. Cryptocarya alba (Mol.) Looser. Peumo Familia: Lauraceae. In Las Especies Arbóreas de Los Bosques Templados de Chile y Argentina; Autoecología, Marisa Cuneo Ediciones: Valdivia, Chile, 2013; pp. 206–212. [Google Scholar]
- Alfaro, R.; Sierra, V. Absorción de Humedad Atmosférica y Relaciones Hídricas en Cryptocarya alba (Mol.) Looser, Quillaja saponaria Mol., Prosopis chilensis (Mol.) Stuntz y Acacia caven. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 1973. [Google Scholar]
- Hurtado, P. Observaciones Sobre la Anatomía foliar y la Transpiración en Peumo (Cryptocarya alba (Mol.) Looser. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 1969. [Google Scholar]
- Barkla, B.; Vera-Estrella, R.; Baldera, E.; Pantoja, O. Mecanismos de tolerancia a la salinidad en plantas. In Una Ventana al Quehacer Científico; Rebolledo, F., López, A., Eds.; Instituto de Biotecnología, UNAM: Cuernava, Mexico, 2008; pp. 263–272. [Google Scholar]
- Lázaro-Nogal, A.; Matesanz, S.; Godoy, A.; Pérez-Trautman, F.; Gianoli, E.; Valladares, F. Environmental heterogeneity leads to higher plasticity in dry-edge populations of a semi-arid Chilean shrub: Insights into climate change responses. J. Ecol. 2015, 103, 338–350. [Google Scholar] [CrossRef]
- Arroyo, M.; Marquet, P.; Marticorena, C.; Simonetti, J.; Cavieres, L.; Squeo, F.; Rozzi, R.; Massardo, F. El hotspot chileno, prioridad mundial para la conservación. In Comisión Nacional del Medio Ambiente. Biodiversidad de Chile. Patrimonio y Desafíos; Saball, P., Arroyo, M., Castilla, J.C., Estades, C., De Guevara, J.M., Larraín, S., Moreno, C., Rivas, F., Rovira, J., Sánchez, A., et al., Eds.; Ocho Libros Editores: Santiago, Chile, 2006; pp. 94–97. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company Inc.: London, UK, 1940. [Google Scholar]
- Colmenares-Arteaga, M.; Rada, F.; Luque, R. Anatomía foliar de Polylepis sericea Wedd. (Rosaceae) a dos altitudes en los Altos andes venezolanos. Plántula 2005, 3, 141–148. [Google Scholar]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Noton, C. Déficit Hídrico en Plántulas de Quillay (Quillaja saponaria). Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 1976. [Google Scholar]
- Pfündel, E.; Agati, G.; Cerovic, Z. Optical properties of plant surfaces. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, MS, USA, 2006; pp. 216–249. [Google Scholar]
- Schreiber, L. Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci. 2010, 15, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Tafolla-Arellano, J.C.; González-León, A.; Tiznado-Hernández, M.; Zacarías-García, L.; Báez-Sañudo, R. Composition, physiology and biosynthesis of plant cuticle. Rev. Fitotec. Mex. 2013, 36, 3–12. [Google Scholar]
- Silva, H.; Acevedo, E.; Silva, P. Anatomía del tejido fotosintético de diez taxa de Opuntia establecidos en el secano árido mediterráneo de Chile. Rev. Chil. Hist. Nat. 2001, 74, 341–352. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stresses; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Hernández, R.; Soto, A.; Valverde, R. Influencia del régimen de humedad en el suelo sobre la morfología foliar de Rottboellia exaltata. Agron. Costarric. 1990, 2, 197–200. [Google Scholar]
- Utrillas, M.; Alegre, L. Impact of water stress on leaf anatomy and ultrastructure in Cynodon dactylon (L.) Pers. under natural conditions. Int. J. Plant Sci. 1997, 158, 313–324. [Google Scholar] [CrossRef]
- Cutler, J.; Rains, W.; Loomis, R. The importance of cell size in the water relations of plants. Physiol. Plant 1977, 40, 225–260. [Google Scholar] [CrossRef]
- Silva, H.; Martínez, J.; Baginsky, C.; Pinto, M. Efecto del déficit hídrico en la anatomía foliar de seis cultivares de poroto Phaseolus vulgaris. Rev. Chil. Hist. Nat. 1999, 72, 219–235. [Google Scholar]
- Ennajeh, M.; Vadel, A.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Silva, H.; Acevedo, E. Adaptaciones anatomomorfológicas foliares al déficit hídrico en Atriplex repanda Phil. Rev. Chil. Hist. Nat. 1984, 57, 69–78. [Google Scholar]
- Mediavilla, S.; Escudero, A.; Heilmeier, H. Internal leaf anatomy and photosynthetic resource-use efficiency: Interspecific and intraspecific comparisons. Tree Physiol. 2001, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kofidis, G.; Bosabalidis, A.; Chartzoulakis, K. Leaf anatomical alterations induced by drought stress in two avocado cultivars. J. Biol. Res. 2004, 1, 115–120. [Google Scholar]
- Maia, G.; Natal, A.; De Almeida, M. Water deficit in relation to leaf and stem anatomy of Eucalyptus camaldulensis Dehnh. shoots cultivated in vitro. Sci. Agric. 1999, 3, 723–731. [Google Scholar]
- Chartzoulakis, K.; Bosabalidis, A.; Patakas, A.; Vemmos, S. Effects of water stress on water relations, gas exchange and leaf structure of olive tree. Acta Hort. (ISHS) 2000, 537, 241–247. [Google Scholar] [CrossRef]
- La Rosa, R.; Contreras, J.; Mendoza, A.; Macabilca, Y.; Gutiérrez, A. Cambios morfofisiológicos de Ipomoea batata (L.) Lam. durante el estrés por sequía. Biologist 2008, 6, 8–12. [Google Scholar]
- Repetto-Giavelli, F.; Lohengrin, C.; Simonetti, J. Respuestas foliares de Aristotelia chilensis (Molina) Stuntz (Elaeocarpaceae) a la fragmentación del bosque maulino. Rev. Chil. Hist. Nat. 2007, 80, 469–477. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Casper, B.B.; Farseth, I.N.; Kempenich, H.; Seltzer, S.; Xavier, K. Drought prolongs leaf life span in the herbaceous desert perennial Cryptantha flava. Funct. Ecol. 2001, 15, 740–747. [Google Scholar] [CrossRef]
- Gratani, L.; Varone, L. Long-time variations in leaf mass and area of Mediterranean evergreen broad-leaf and narrow-leaf maquis species. Photosynthetica 2006, 44, 161–168. [Google Scholar] [CrossRef]
- Slama, I.; Messedi, D.; Ghnaya, T.; Savoure, A.; Abdelly, C. Effects of water deficit on growth and proline metabolism in Sesuvium portulacastrum. Environ. Exp. Bot. 2006, 56, 231–238. [Google Scholar] [CrossRef]
- Ogaya, R.; Penuelas, J. Contrasting foliar responses to drought in Quercus ilex and Phillyrea latifolia. Biol. Plant. 2006, 50, 373–382. [Google Scholar] [CrossRef]
- Otieno, D.O.; Schmidt, M.W.; Adiku, S.; Tenhunen, J. Physiological and morphological responses to water stress in two Acacia species from contrasting habitats. Tree Physiol. 2005, 25, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Setter, T. Response of cassava leaf area expansion to water deficit. Cell proliferation cell expansion and delayed development. Ann. Bot. 2004, 94, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, H. Respuestas ecofisiológicas de plantas en ecosistemas de zonas con clima mediterráneo y ambientes de alta-montaña. Rev. Chil. Hist. Nat. 2002, 75, 625–637. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Sections. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assesment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Gómez, D. El cambio climático y la respuesta de las grandes potencias. El caso de Estados Unidos y China. Análisis Político 2020, 33, 121–142. [Google Scholar]
- Gracia, C.A.; Sabaté, S.; Sánchez, A. El cambio climático y la reducción de la reserva de agua en el bosque mediterráneo. Ecosistemas 2002, 11. [Google Scholar]
- Valladares, F.; Vilagrosa, A.; Peñuelas, J.; Ogaya, R.; Camarero, J.; Corcuera, L.; Sisó, S.; Gil-Pelegrín, E. Estrés hídrico: Ecofisiología y escalas de sequía. In Ecología del Bosque Mediterráneo en un Mundo Cambiante; Valladares, F., Ed.; Ministerio de Medio Ambiente, EGRAF, S.A.: Madrid, Spain, 2004; pp. 163–190. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Rojas, K.; Donoso, S.; Badaracco, C.; Naulin, P.I.; Gotor, B.; Riquelme, A. Anatomical Responses of Two Species under Controlled Water Restriction. Plants 2024, 13, 2812. https://doi.org/10.3390/plants13192812
Peña-Rojas K, Donoso S, Badaracco C, Naulin PI, Gotor B, Riquelme A. Anatomical Responses of Two Species under Controlled Water Restriction. Plants. 2024; 13(19):2812. https://doi.org/10.3390/plants13192812
Chicago/Turabian StylePeña-Rojas, Karen, Sergio Donoso, Carolain Badaracco, Paulette I. Naulin, Bárbara Gotor, and Alejandro Riquelme. 2024. "Anatomical Responses of Two Species under Controlled Water Restriction" Plants 13, no. 19: 2812. https://doi.org/10.3390/plants13192812





