Investigating the Effects of Full-Spectrum LED Lighting on Strawberry Traits Using Correlation Analysis and Time-Series Prediction
Abstract
1. Introduction
2. Experiments and Results
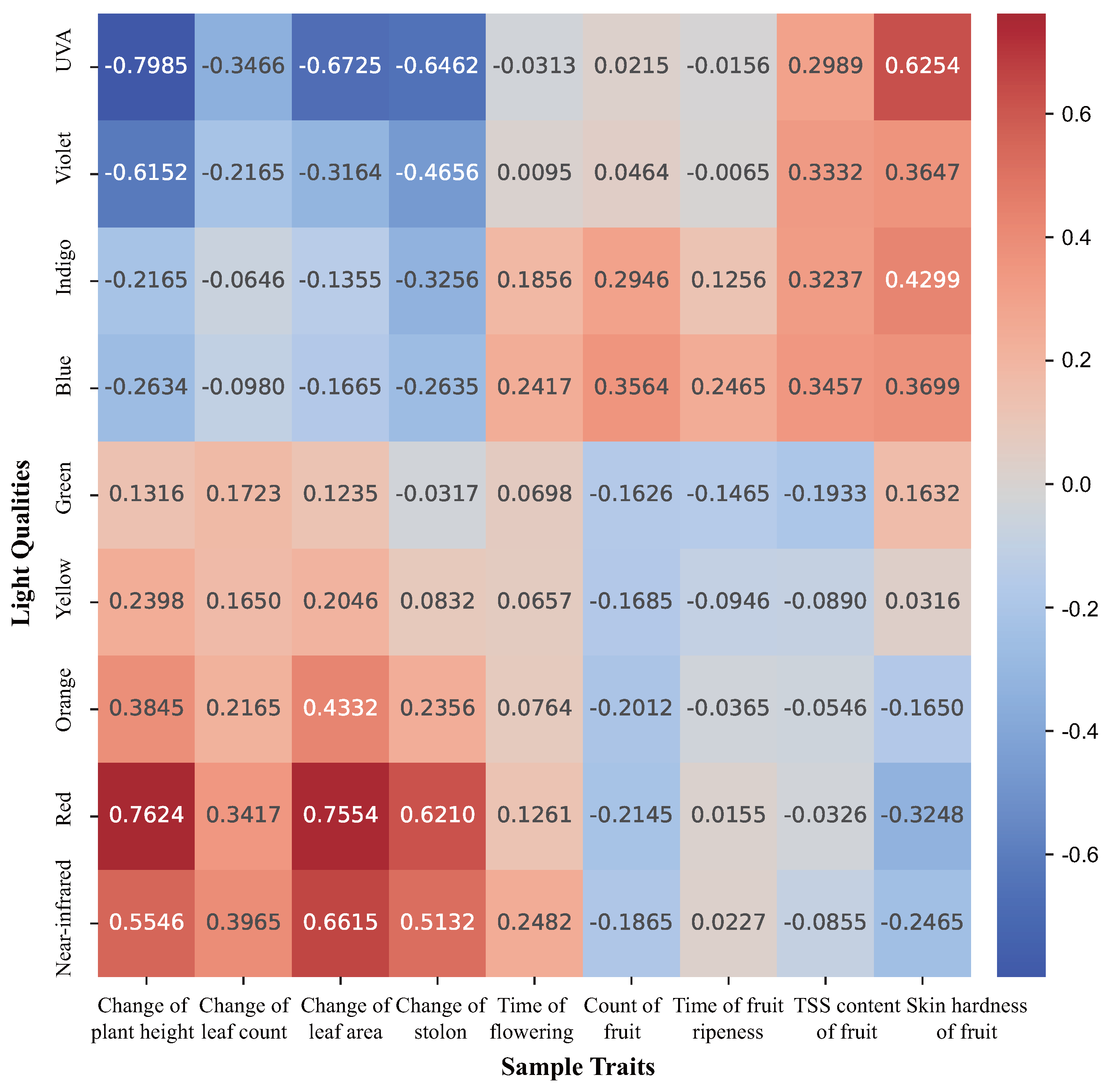
2.1. Correlation between Light Qualities and Traits
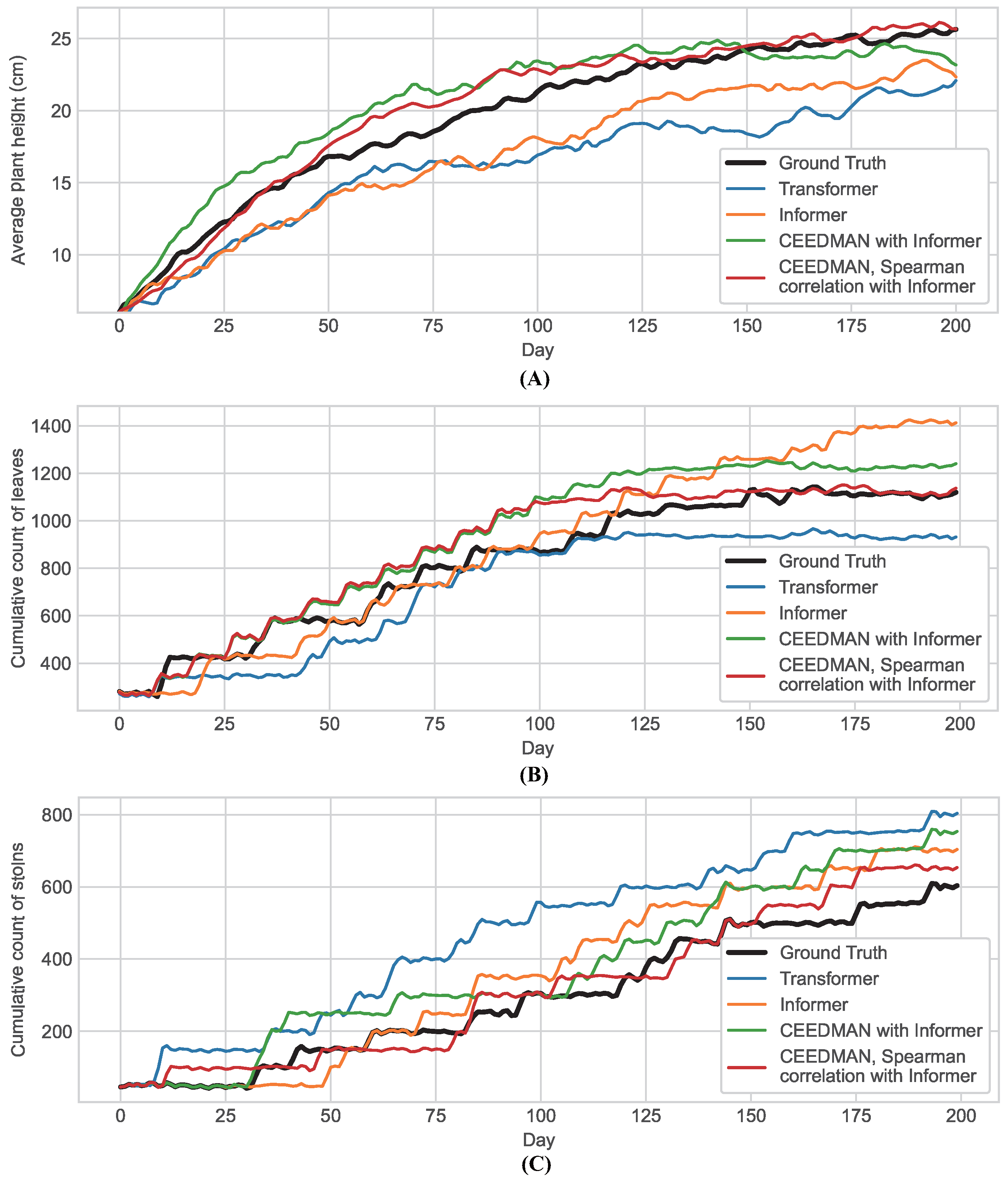
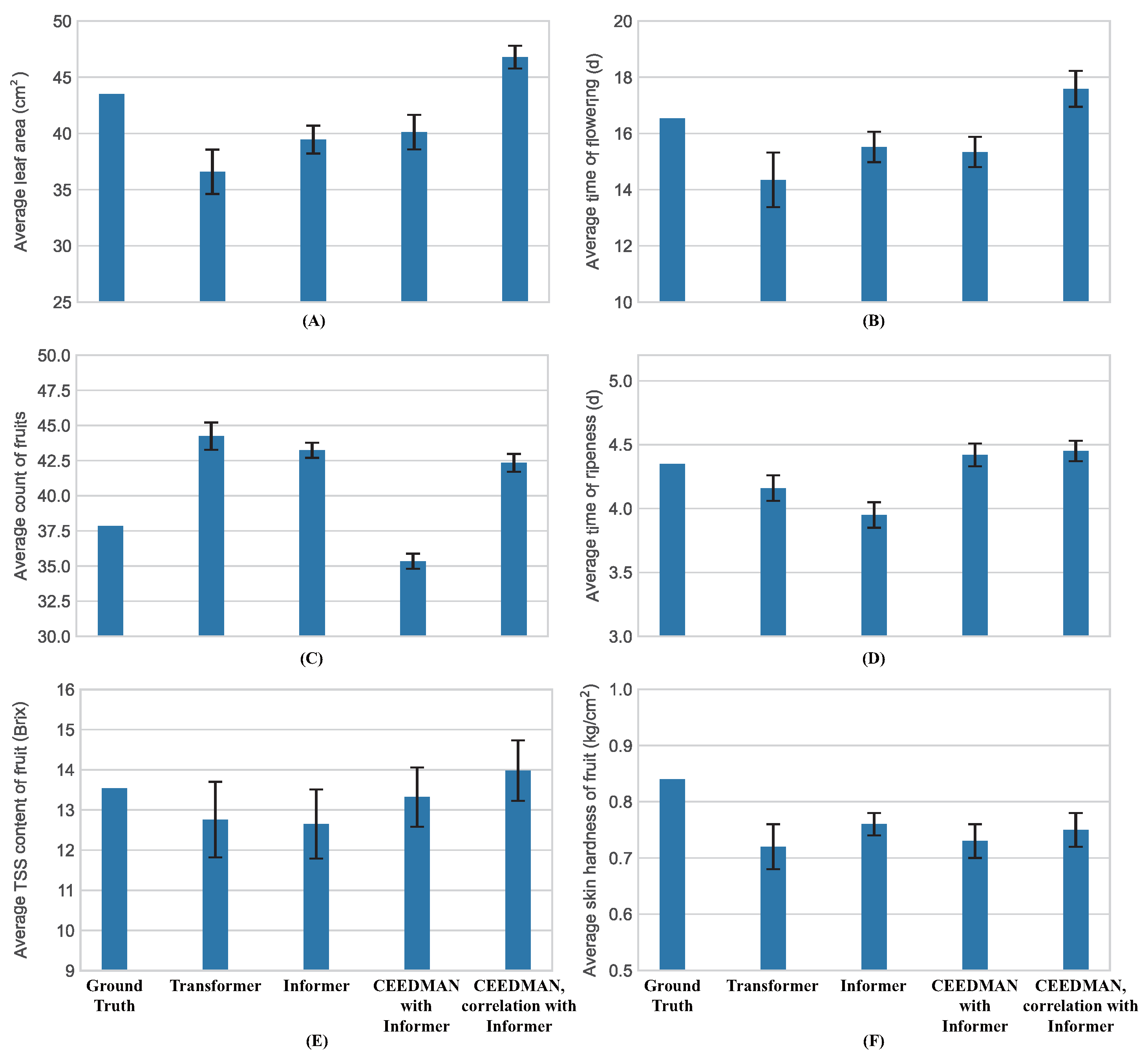
2.2. Prediction of Informer Network
3. Discussion
4. Materials and Methods
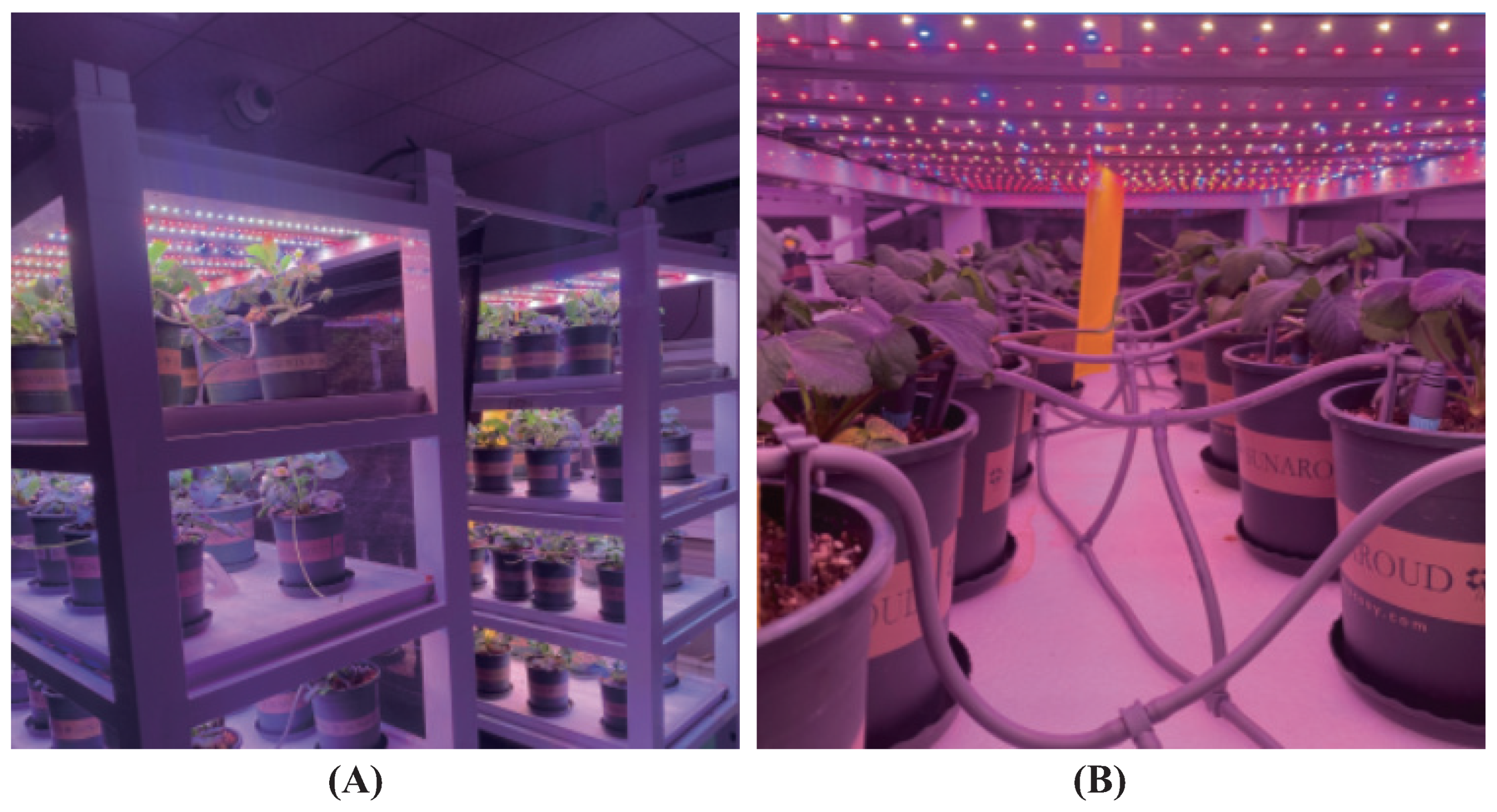
4.1. Processing of Strawberry Samples
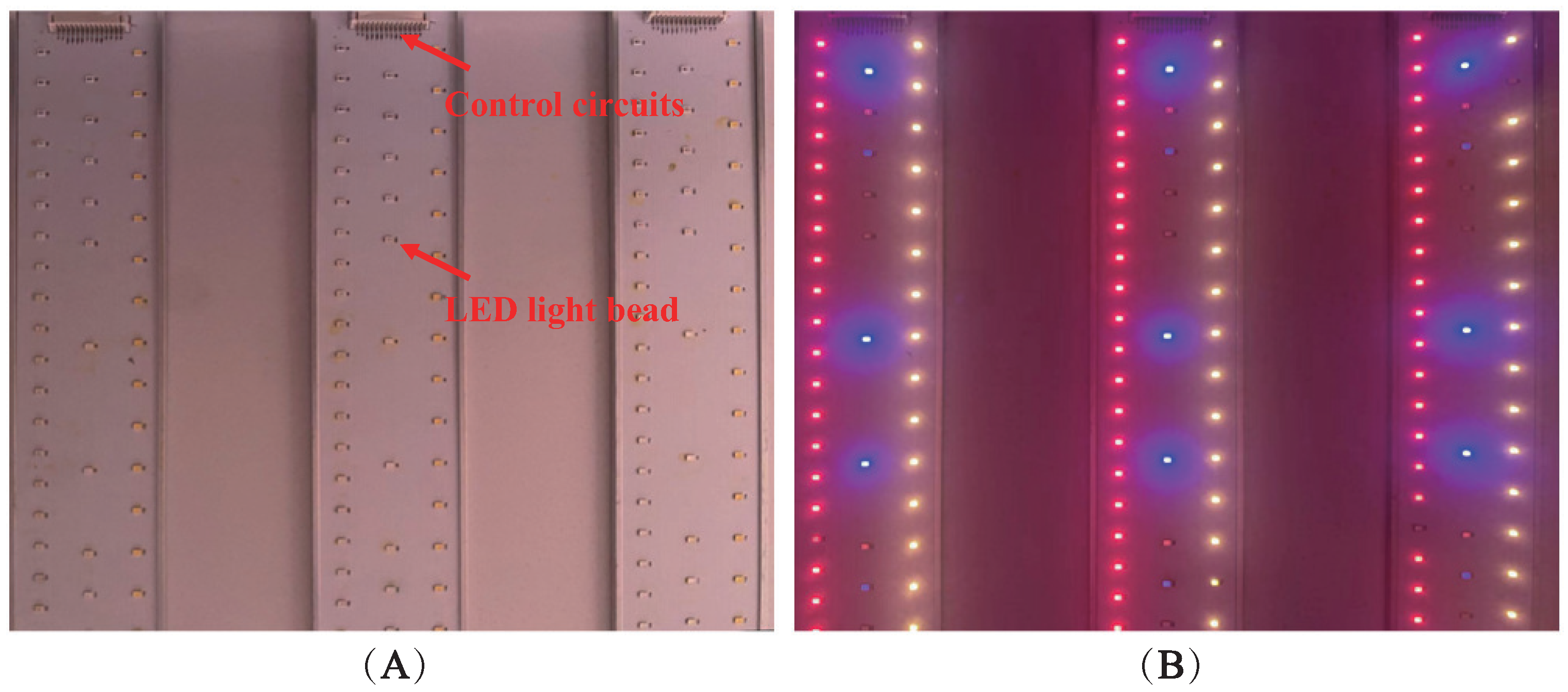
4.1.1. Light Quality Processing of Subgroups
4.1.2. Acquisition of Strawberry Traits
4.2. Pre-Processing of Data
4.2.1. CEEDMAN Decomposition Method
4.2.2. Decomposition of Original Input Signals
4.3. Informer Model
4.4. Evaluation of Correlation
4.5. Evaluation and Indicators
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Iersel, M.W. Optimizing LED lighting in controlled environment agriculture. In Light Emitting Diodes for Agriculture: Smart Lighting; Springer: Berlin/Heidelberg, Germany, 2017; pp. 59–80. [Google Scholar]
- Kozai, T. Why LED Lighting for Urban Agriculture? Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Davis, P.A.; Burns, C. Photobiology in protected horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Urbonavičiūtė, A.; Šabajevienė, G.; Duchovskis, P. The effect of red and blue light component on the growth and development of frigo strawberries. Zemdirb. Agric. 2010, 97, 99–104. [Google Scholar]
- Yoshida, H.; Hikosaka, S.; Goto, E.; Takasuna, H.; Kudou, T. Effects of light quality and light period on flowering of everbearing strawberry in a closed plant production system. In Proceedings of the VII International Symposium on Light in Horticultural Systems 956, Wageningen, The Netherlands, 14–18 October 2012; pp. 107–112. [Google Scholar]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red: Blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Folta, K.M.; Childers, K.S. Light as a growth regulator: Controlling plant biology with narrow-bandwidth solid-state lighting systems. HortScience 2008, 43, 1957–1964. [Google Scholar] [CrossRef]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Yoneda, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K.; Shimizu, H. Impact of blue, red, and far-red light treatments on gene expression and steviol glycoside accumulation in Stevia rebaudiana. Phytochemistry 2017, 137, 57–65. [Google Scholar] [CrossRef]
- Kim, H.H.; Wheeler, R.M.; Sager, J.C.; Yorio, N.C.; Goins, G.D. Light-emitting diodes as an illumination source for plants: A review of research at Kennedy Space Center. Habitation 2005, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Turcsányi, E.; Vass, I. Inhibition of Photosynthetic Electron Transport by UV-A Radiation Targets the Photosystem II Complex. Photochem. Photobiol. 2000, 72, 513–520. [Google Scholar] [CrossRef]
- Mantha, S.V.; Johnson, G.A.; Day, T.A. Day Evidence from Action and Fluorescence Spectra that UV-Induced Violet–Blue–Green Fluorescence Enhances Leaf Photosynthesis. Photochem. Photobiol. 2001, 73, 249–256. [Google Scholar]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Dubois, P.G.; Brutnell, T.P. Topology of a maize field: Distinguishing the influence of end-of-day far-red light and shade avoidance syndrome on plant height. Plant Signal. Behav. 2011, 6, 173–186. [Google Scholar] [CrossRef]
- Kasperbauer, M.; Peaslee, D. Morphology and photosynthetic efficiency of tobacco leaves that received end-of-day red and far red light during development. Plant Physiol. 1973, 52, 440–442. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, Y.Q.; Yang, Q.C.; Tao, L. Overhead supplemental far-red light stimulates tomato growth under intra-canopy lighting with LEDs. J. Integr. Agric. 2019, 18, 62–69. [Google Scholar] [CrossRef]
- Shibuya, T.; Endo, R.; Kitaya, Y.; Hayashi, S. Growth analysis and photosynthesis measurements of cucumber seedlings grown under light with different red to far-red ratios. HortScience 2016, 51, 843–846. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Cheng, Y.; Feng, L.; Wu, X.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; et al. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed]
- Zhou, H.; Zhang, S.; Peng, J.; Zhang, S.; Li, J.; Xiong, H.; Zhang, W. Informer: Beyond efficient transformer for long sequence time-series forecasting. In Proceedings of the AAAI conference on artificial intelligence, Virtual, 2–9 February 2021; Volume 35, pp. 11106–11115. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. Adv. Neural Inf. Process. Syst. 2017, 30. [Google Scholar]
- Lu, Y.; Gong, M.; Li, J.; Ma, J. Optimizing Controlled Environmental Agriculture for Strawberry Cultivation Using RL-Informer Model. Agronomy 2023, 13, 2057. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, F.; Li, D.; He, H.; Zhang, T.; Yang, S. Prediction of photovoltaic power by the Informer model based on convolutional neural network. Sustainability 2022, 14, 13022. [Google Scholar] [CrossRef]
- Newsham, K.; Greenslade, P.; McLeod, A. Effects of elevated ultraviolet radiation on Quercus robur and its insect and ectomycorrhizal associates. Glob. Chang. Biol. 1999, 5, 881–890. [Google Scholar] [CrossRef]
- Paul, N.D.; Jacobson, R.J.; Taylor, A.; Wargent, J.J.; Moore, J.P. The use of wavelength-selective plastic cladding materials in horticulture: Understanding of crop and fungal responses through the assessment of biological spectral weighting functions. Photochem. Photobiol. 2005, 81, 1052–1060. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H., Jr.; Vaughan, M.M.; Hauser, B.A.; Boote, K.J. Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agric. For. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Charles, M.T.; Arul, J. UV treatment of fresh fruits and vegetables for improved quality: A status report. Stewart Postharvest Rev. 2007, 3, 1–8. [Google Scholar]
- Aharoni, A.; Keizer, L.C.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Blaas, J.; Van Houwelingen, A.M.; De Vos, R.C.; Van Der Voet, H.; et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 2000, 12, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tabuchi, T. Tomato cultivation in a plant factory with artificial light: Effect of UV-A irradiation during the growing period on yield and quality of ripening fruit. Hortic. J. 2022, 91, 16–23. [Google Scholar] [CrossRef]
- Zhu, X.; Trouth, F.; Yang, T. Preharvest UV-B Treatment Improves Strawberry Quality and Extends Shelf Life. Horticulturae 2023, 9, 211. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB radiation as innovative tools to biofortify horticultural crops with nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.H. Ultraviolet A-specific induction of anthocyanin biosynthesis and PAL expression in tomato (Solanum lycopersicum L.). Plant Growth Regul. 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Helsper, J.P.; Ric de Vos, C.; Maas, F.M.; Jonker, H.H.; Van Den Broeck, H.C.; Jordi, W.; Pot, C.S.; Keizer, L.P.; Schapendonk, A.H. Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol. Plant. 2003, 117, 171–178. [Google Scholar] [CrossRef]
- Maffei, M.; Canova, D.; Bertea, C.; Scannerini, S. UV-A effects on photomorphogenesis and essential-oil composition in Mentha piperita. J. Photochem. Photobiol. Biol. 1999, 52, 105–110. [Google Scholar] [CrossRef]
- Gyula, P.; Schäfer, E.; Nagy, F. Light perception and signalling in higher plants. Curr. Opin. Plant Biol. 2003, 6, 446–452. [Google Scholar] [CrossRef]
- Magar, Y.; Ohyama, K.; Noguchi, A.; Amaki, W.; Furufuji, S. Effects of light quality during supplemental lighting on the flowering in an everbearing strawberry. In Proceedings of the XIII International Symposium on Plant Bioregulators in Fruit Production 1206, Chiba, Japan, 27–30 August 2017; pp. 279–284. [Google Scholar]
- Choi, H.; Kwon, J.; Moon, B.; Kang, N.; Park, K.; Cho, M.; Kim, Y. Effect of different light emitting diode (LED) lights on the growth characteristics and the phytochemical production of strawberry fruits during cultivation. Korean J. Hortic. Sci. Technol. 2013, 31, 56–64. [Google Scholar]
- Xu, D.; Ren, L.; Zhang, X. Predicting Multidimensional Environmental Factor Trends in Greenhouse Microclimates Using a Hybrid Ensemble Approach. J. Sensors 2023, 2023, 6486940. [Google Scholar] [CrossRef]
- Kai, X.; Yahping, G.; Shanglong, Z. Effect of light quality on plant growth and fruiting of Toyonoka strawberry (Fragaria × ananassa) cultivar. J. Fruit Sci. 2006, 23, 818–824. [Google Scholar]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, P.; Taylor, C.; Calders, K.; Hogervorst, M.; van Ieperen, W.; Harbinson, J.; de Visser, P.; Nicole, C.C.; Marcelis, L.F. Unraveling the effects of blue light in an artificial solar background light on growth of tomato plants. Environ. Exp. Bot. 2021, 184, 104377. [Google Scholar] [CrossRef]
- Ahmad, M.; Grancher, N.; Heil, M.; Black, R.C.; Giovani, B.; Galland, P.; Lardemer, D. Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol. 2002, 129, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, Y.; Li, X.; Chen, Q.; Zhang, Y.; Luo, Y.; Liu, Z.; Wang, Y.; Lin, Y.; Zhang, Y.; et al. Transcriptome profile analysis of strawberry leaves reveals flowering regulation under blue light treatment. Int. J. Genom. 2021, 2021, 5572076. [Google Scholar] [CrossRef]
- Stuemky, A.; Uchanski, M.E. Supplemental light-emitting diode effects on the growth, fruit quality, and yield of two greenhouse-grown strawberry (Fragaria × ananassa) cultivars. HortScience 2020, 55, 23–29. [Google Scholar] [CrossRef]
- Mohamed, F.; Omar, G.; Ismail, M. In vitro regeneration, proliferation and growth of strawberry under different light treatments. In Proceedings of the VI International Symposium on Production and Establishment of Micropropagated Plants 1155, Sanremo, Italy, 19–24 April 2015; pp. 361–368. [Google Scholar]
- Posada, F.C.; Peña-Olmos, J.E.; Ulrichs, C. Growth and photochemical efficiency of photosystem II in strawberry plants (Fragaria sp.) Affected by the light quality: Agronomic implications. Rev. Udca Actual. Divulg. Cient. 2011, 14, 43–53. [Google Scholar]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H. Phytochrome signaling: Time to tighten up the loose ends. Mol. Plant 2015, 8, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Battle, M.W.; Jones, M.A. Cryptochromes integrate green light signals into the circadian system. Plant Cell Environ. 2020, 43, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Li, Y.; Xin, G.F.; Wei, M.; Mi, Q.H.; Yang, Q.C. Effects of different proportions of red and blue light on the growth and photosynthesis of tomato seedlings. J. Appl. Ecol. 2017, 28, 1595–1602. [Google Scholar]
- Zahedi, S.; Sarikhani, H. The effect of end of day far-red light on regulating flowering of short-day strawberry (Fragaria × ananassa Duch. cv. Paros) in a long-day situation. Russ. J. Plant Physiol. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Whitelam, G.; Halliday, K. Annual Plant Reviews Volume 30: Light and Plant Development; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Rockwell, N.C.; Su, Y.S.; Lagarias, J.C. Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 2006, 57, 837–858. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Smith, M.W.; Brown, R.G.; Kamiya, Y.; Sun, T.P. Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 1998, 10, 2115–2126. [Google Scholar] [CrossRef]
- Quail, P.H. Phytochrome genes and their expression. In Photomorphogenesis in Plants; Springer: Berlin/Heidelberg, Germany, 1994; pp. 71–104. [Google Scholar]
- Quail, P.H. Phytochrome overview. In Light Sensing in Plants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 21–35. [Google Scholar]
- Devlin, P.F.; Robson, P.R.; Patel, S.R.; Goosey, L.; Sharrock, R.A.; Whitelam, G.C. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999, 119, 909–916. [Google Scholar] [CrossRef]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef]
- Robson, P.R.; Smith, H. Genetic and transgenic evidence that phytochromes A and B act to modulate the gravitropic orientation of Arabidopsis thaliana hypocotyls. Plant Physiol. 1996, 110, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Byun, M.; Liu, S.; Jang, M. Effect of nutrient solution strength on pH of drainage solution and root activity of strawberry ‘Sulhyang’ in hydroponics. Korean J. Hortic. Sci. Technol. 2011, 29, 23–28. [Google Scholar]
- Zheng, J.; He, D.; Ji, F. Effects of light intensity and photoperiod on runner plant propagation of hydroponic strawberry transplants under LED lighting. Int. J. Agric. Biol. Eng. 2019, 12, 26–31. [Google Scholar] [CrossRef]
- Guiamba, H.D.S.S.; Zhang, X.; Sierka, E.; Lin, K.; Ali, M.M.; Ali, W.M.; Lamlom, S.F.; Kalaji, H.M.; Telesiński, A.; Yousef, A.F.; et al. Enhancement of photosynthesis efficiency and yield of strawberry (Fragaria ananassa Duch.) plants via LED systems. Front. Plant Sci. 2022, 13, 918038. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, B.; Ropelewska, E.; Wrzodak, A.; Kowalski, A.; Kaniszewski, S. Yield and quality of romaine lettuce at different daily light integral in an indoor controlled environment. Agronomy 2022, 12, 1026. [Google Scholar] [CrossRef]
- Torres, M.E.; Colominas, M.A.; Schlotthauer, G.; Flandrin, P. A complete ensemble empirical mode decomposition with adaptive noise. In Proceedings of the 2011 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Prague, Czech Republic, 22–27 May 2011; pp. 4144–4147. [Google Scholar]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. London Ser. Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Katharopoulos, A.; Vyas, A.; Pappas, N.; Fleuret, F. Transformers are rnns: Fast autoregressive transformers with linear attention. In Proceedings of the International Conference on Machine Learning, PMLR, Virtual, 13–18 July 2020; pp. 5156–5165. [Google Scholar]
- Hershey, J.R.; Olsen, P.A. Approximating the Kullback Leibler divergence between Gaussian mixture models. In Proceedings of the 2007 IEEE International Conference on Acoustics, Speech and Signal Processing-ICASSP’07, Honolulu, HI, USA, 16–20 April 2007; Volume 4, p. IV-317. [Google Scholar]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Zou, J.; Bian, Z.; Yang, Q.; Li, T. UVA radiation promotes tomato growth through morphological adaptation leading to increased light interception. Environ. Exp. Bot. 2020, 176, 104073. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]



| Definition | Parameters | Acquisition Time |
|---|---|---|
| Change of plant height | Change of the maximum height from the soil part to the top of the leaf crown. | Every half day |
| Change of leaf count | Change in the count of leaves in plant samples. | Every half day |
| Change of leaf area | Change in the total leaf area in plant samples. | Every half day |
| Change of stolon | Change in the count of stolons in plant samples. | Every half day |
| Time of flowering | The number of days since the last harvest (or planting). | When the flowers bloom |
| Count of fruit | The total count of fruits produced on the sample from the beginning to the end of the experiment. | At the end of experiments of all subgroups |
| Time of fruit ripeness | The number of days from fruiting to harvest. | After each harvest |
| TSS content of fruit | Post-harvest measurements with a Brix meter. | After each harvest |
| Skin hardness of fruit | Post-harvest measurements with a hardness tester. | After each harvest |
| Method | Transformer | Informer | CEEDMAN with Informer | CEEDMAN, Correlation with Informer | ||||
|---|---|---|---|---|---|---|---|---|
| Plant height | 0.5864 | 52.1653 | 0.6016 | 42.1654 | 0.7213 | 33.2345 | 0.7651 * | 28.2684 * |
| Leaf count | 0.4675 | 60.3543 | 0.5075 | 54.3246 | 0.6854 | 46.7570 | 0.8433 * | 24.7163 * |
| Leaf area | 0.4035 | 64.1643 | 0.6324 | 46.1634 | 0.6574 | 40.1356 | 0.6831 * | 31.1538 * |
| Stolon count | 0.3785 | 72.5463 | 0.4237 * | 70.3154 | 0.4169 | 66.1395 * | 0.4094 | 68.1543 |
| Time of flowering | 0.4267 | 67.3642 | 0.5079 | 56.6237 | 0.4865 | 45.3785 | 0.5234 * | 37.3623 * |
| Count of fruit | 0.5735 | 44.7652 | 0.6095 | 46.6340 | 0.6842 * | 40.2334 | 0.6796 | 34.3266 * |
| Fruit ripeness | 0.5304 | 54.1748 | 0.4571 | 63.5465 | 0.5903 * | 42.6349 * | 0.5876 | 44.3160 |
| TSS content | 0.6264 | 31.1025 | 0.6234 | 32.3156 | 0.6762 | 28.7831 * | 0.6832 * | 29.1359 |
| Skin hardness | 0.3242 | 85.3152 | 0.4529 * | 69.3215 * | 0.4216 | 76.3165 | 0.4463 | 73.3152 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Batch size | 128 | Dropout | 0.05 |
| Epochs | 50 | Loss function | MSE |
| Leaning rate | Activation | GeLU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Gong, M.; Li, J.; Ma, J. Investigating the Effects of Full-Spectrum LED Lighting on Strawberry Traits Using Correlation Analysis and Time-Series Prediction. Plants 2024, 13, 149. https://doi.org/10.3390/plants13020149
Lu Y, Gong M, Li J, Ma J. Investigating the Effects of Full-Spectrum LED Lighting on Strawberry Traits Using Correlation Analysis and Time-Series Prediction. Plants. 2024; 13(2):149. https://doi.org/10.3390/plants13020149
Chicago/Turabian StyleLu, Yuze, Mali Gong, Jing Li, and Jianshe Ma. 2024. "Investigating the Effects of Full-Spectrum LED Lighting on Strawberry Traits Using Correlation Analysis and Time-Series Prediction" Plants 13, no. 2: 149. https://doi.org/10.3390/plants13020149
APA StyleLu, Y., Gong, M., Li, J., & Ma, J. (2024). Investigating the Effects of Full-Spectrum LED Lighting on Strawberry Traits Using Correlation Analysis and Time-Series Prediction. Plants, 13(2), 149. https://doi.org/10.3390/plants13020149





