Growth and Physiological Responses of Magnoliaceae to NaCl Stress
Abstract
:1. Introduction
2. Results
2.1. Effects of NaCl on Plant Growth
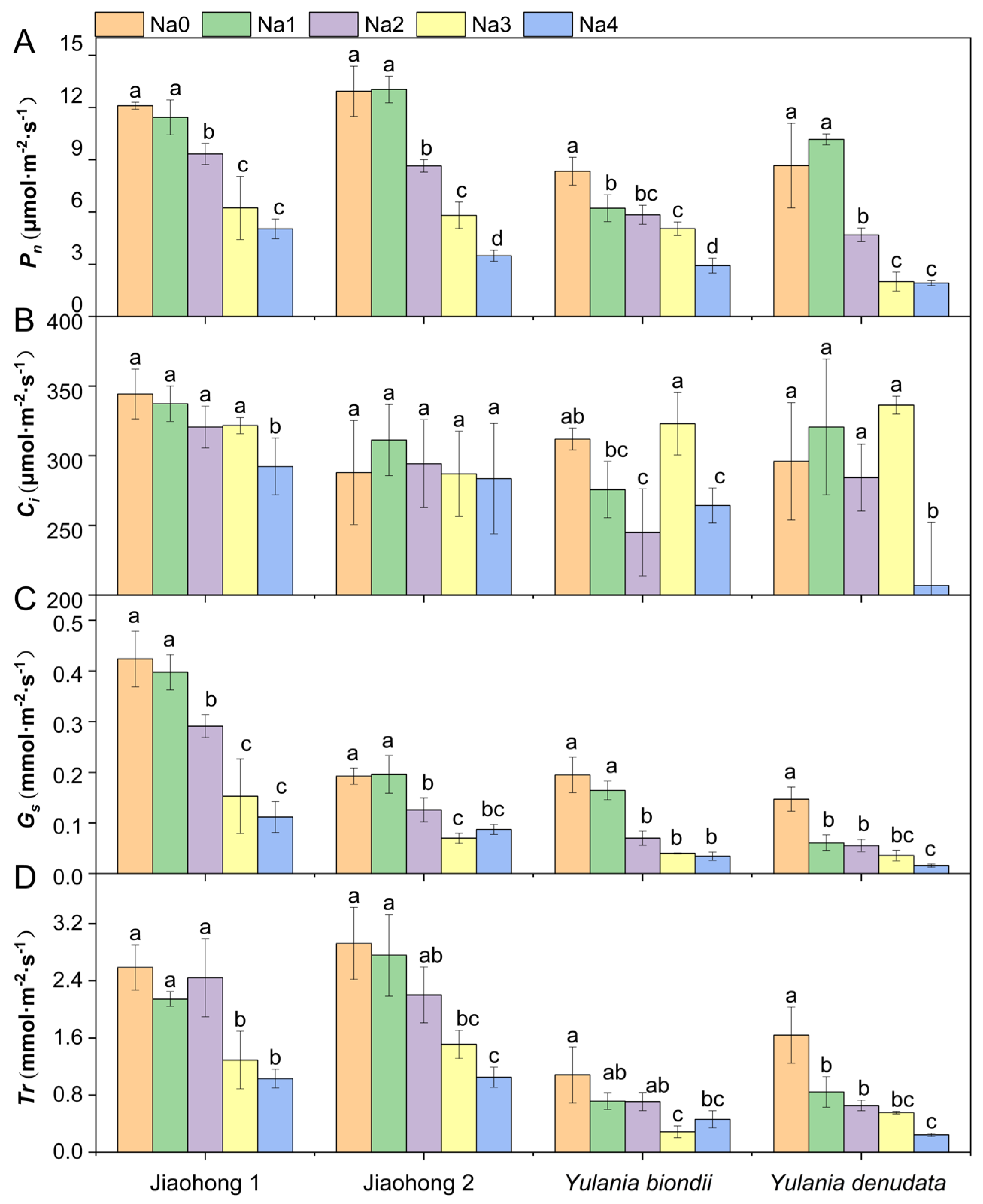
2.2. Effects of NaCl on Photosynthetic Pigment Content and Photosynthesis
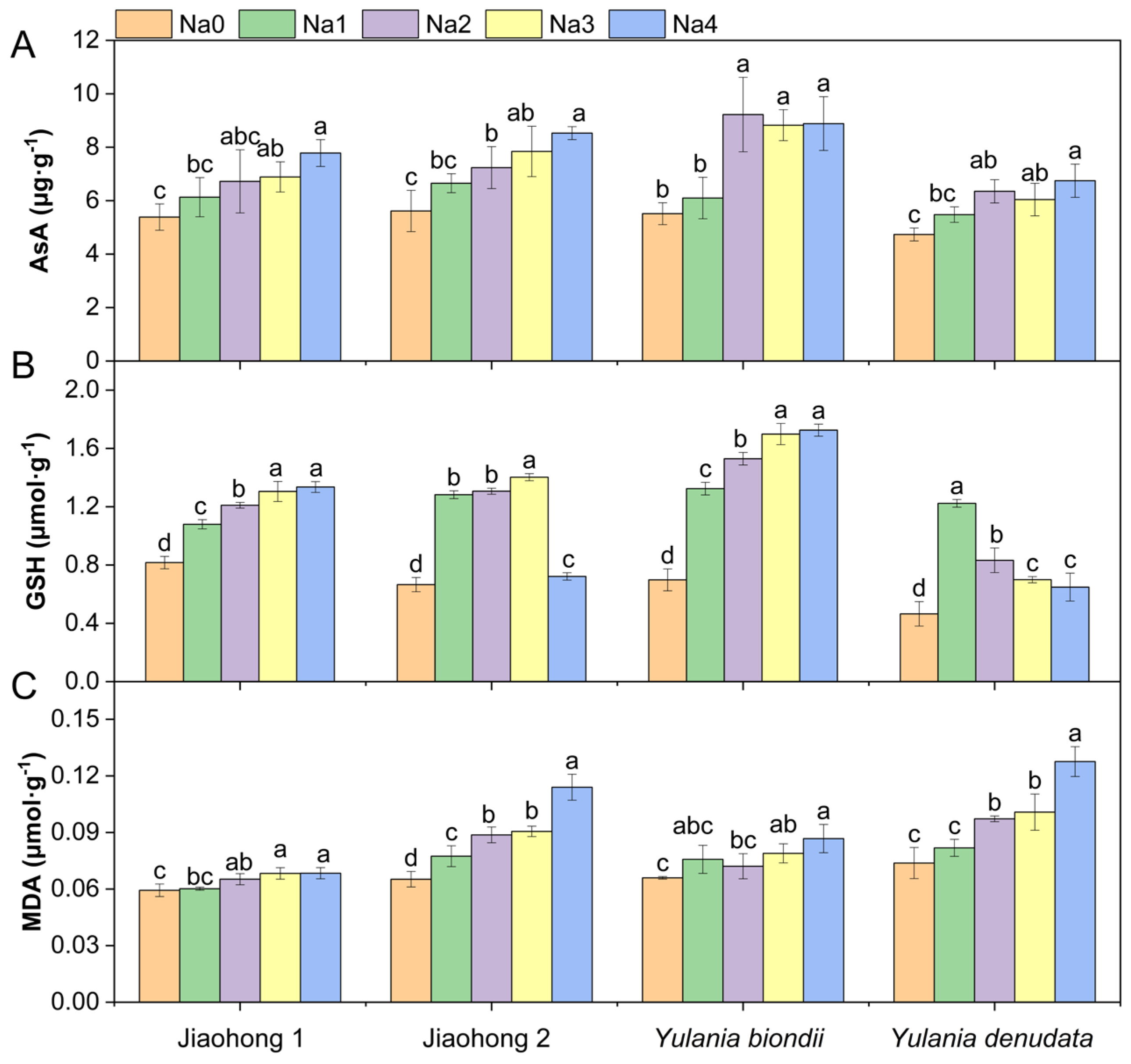
2.3. Effects of NaCl on Antioxidant Enzymes, Nonenzymatic Antioxidants, and Membrane Lipid Peroxidation
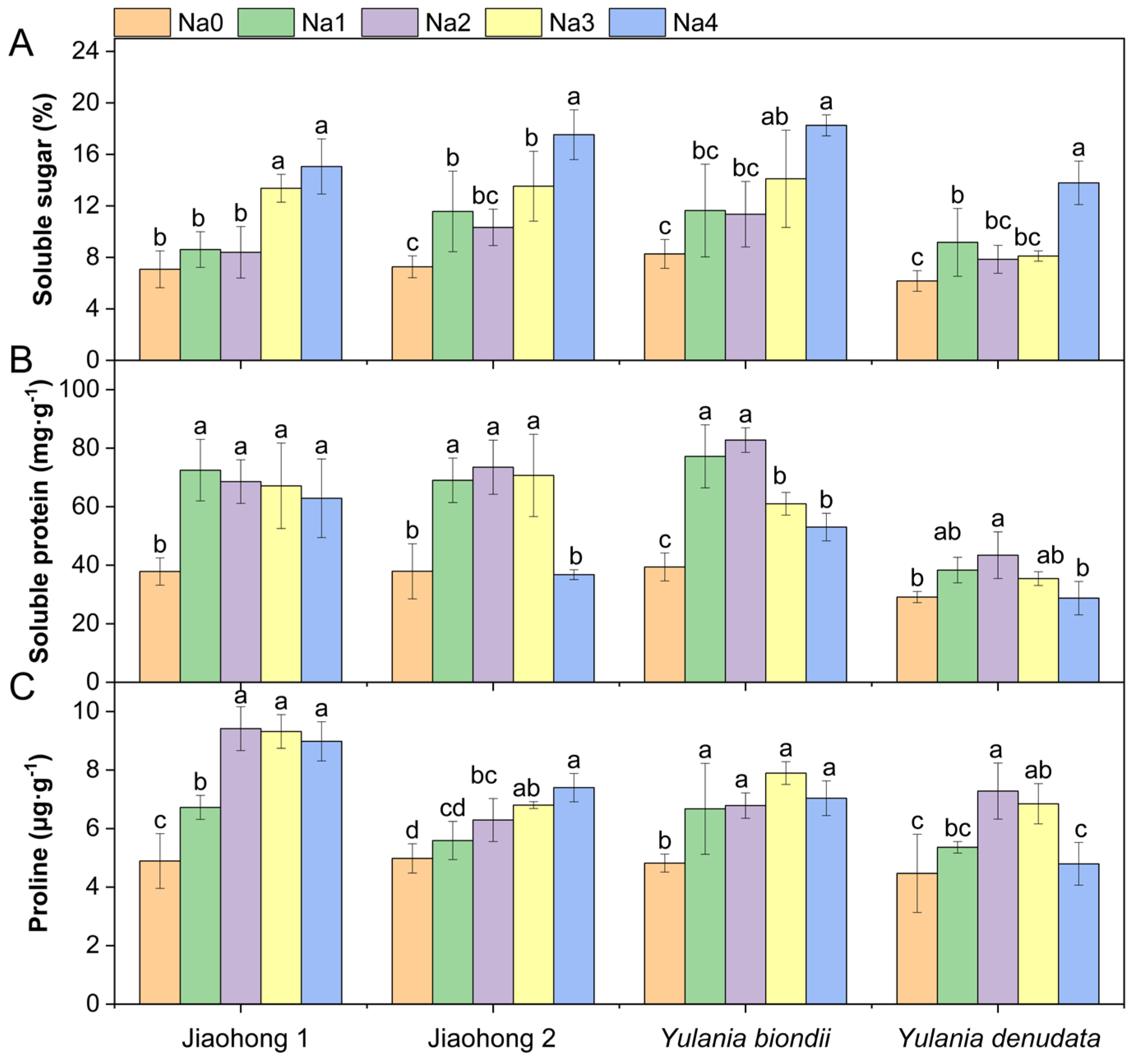
2.4. Effects of NaCl on Osmotic Adjustment
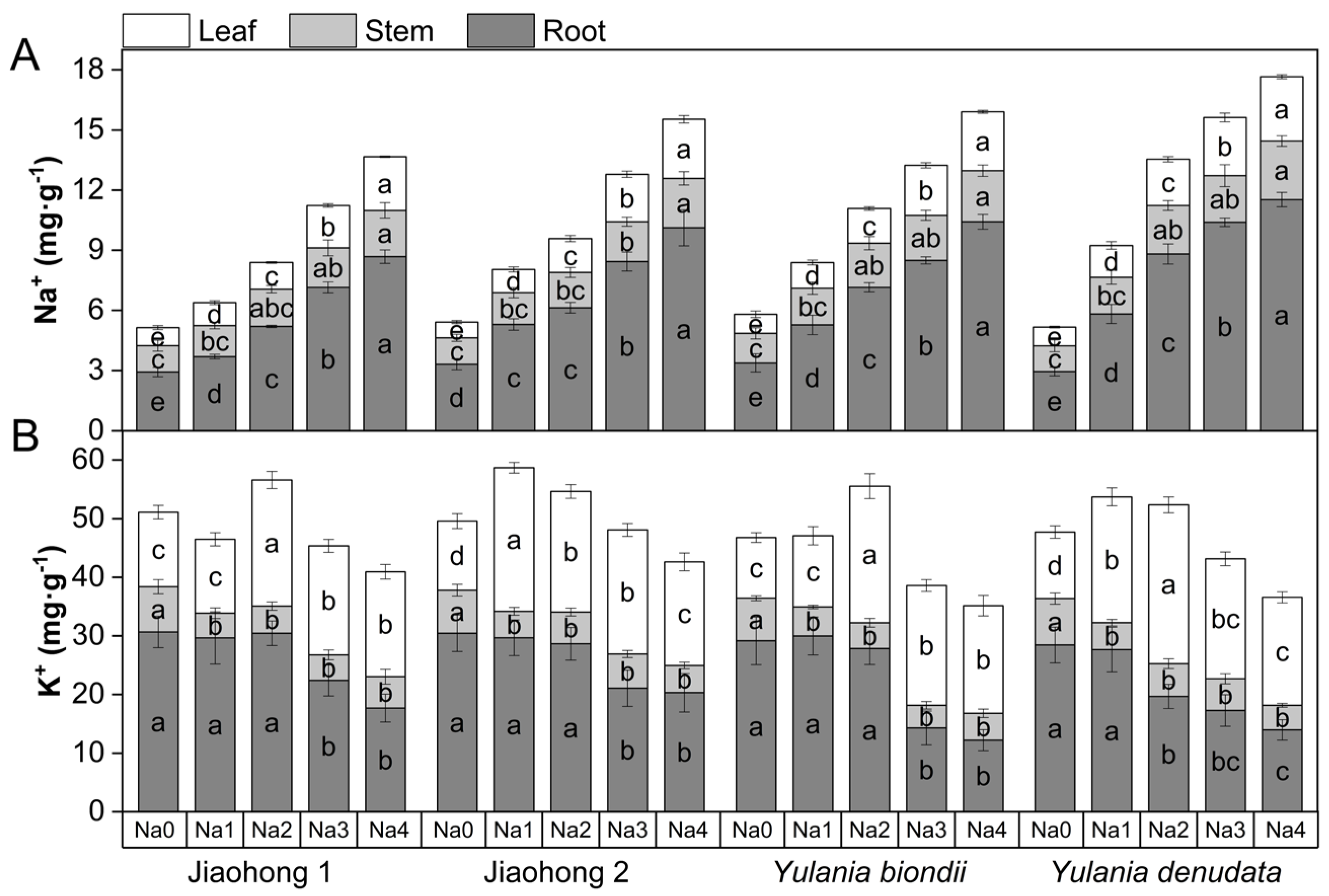
2.5. Effects of NaCl on Ion Contents
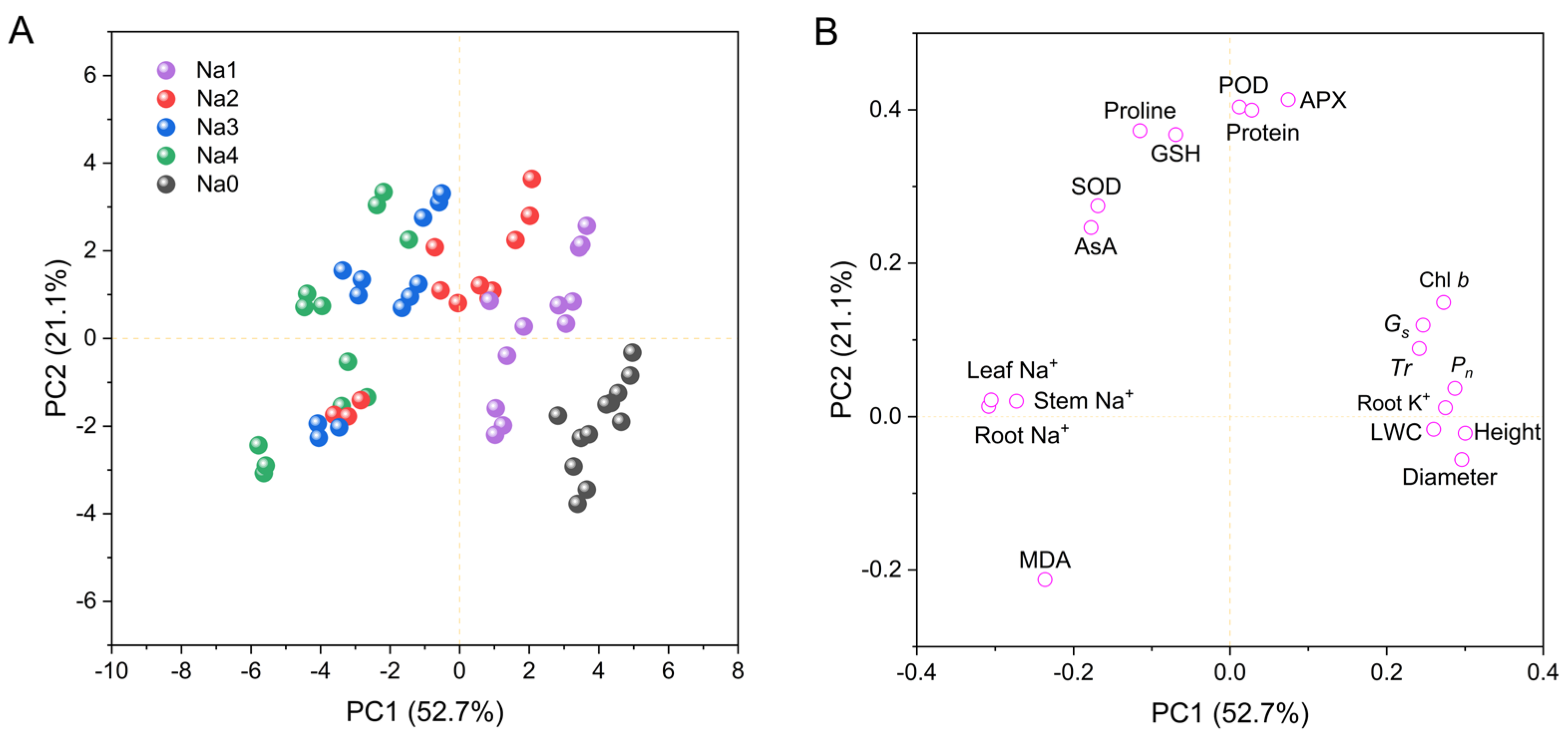
2.6. Principal Component, Heatmap, and Correlation Analyses for Growth and Physiology Indicators
3. Discussion
3.1. NaCl Treatments Inhibited Plant Growth
3.2. Photosynthetic Pigment Contents and Photosynthetic Parameter Observations Revealed the Effects of NaCl on Photosynthesis
3.3. Antioxidant Enzymes and Nonenzymatic Antioxidants Work Together
3.4. Osmotic Regulatory Substances Used to Resist NaCl Stress
3.5. Na+ and K+ Contents Changed under NaCl Stress
3.6. Principal Component, Heatmap, and Correlation Analyses Reveal the Salt Tolerance Mechanism
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Experimental Design
4.3. Plant Growth
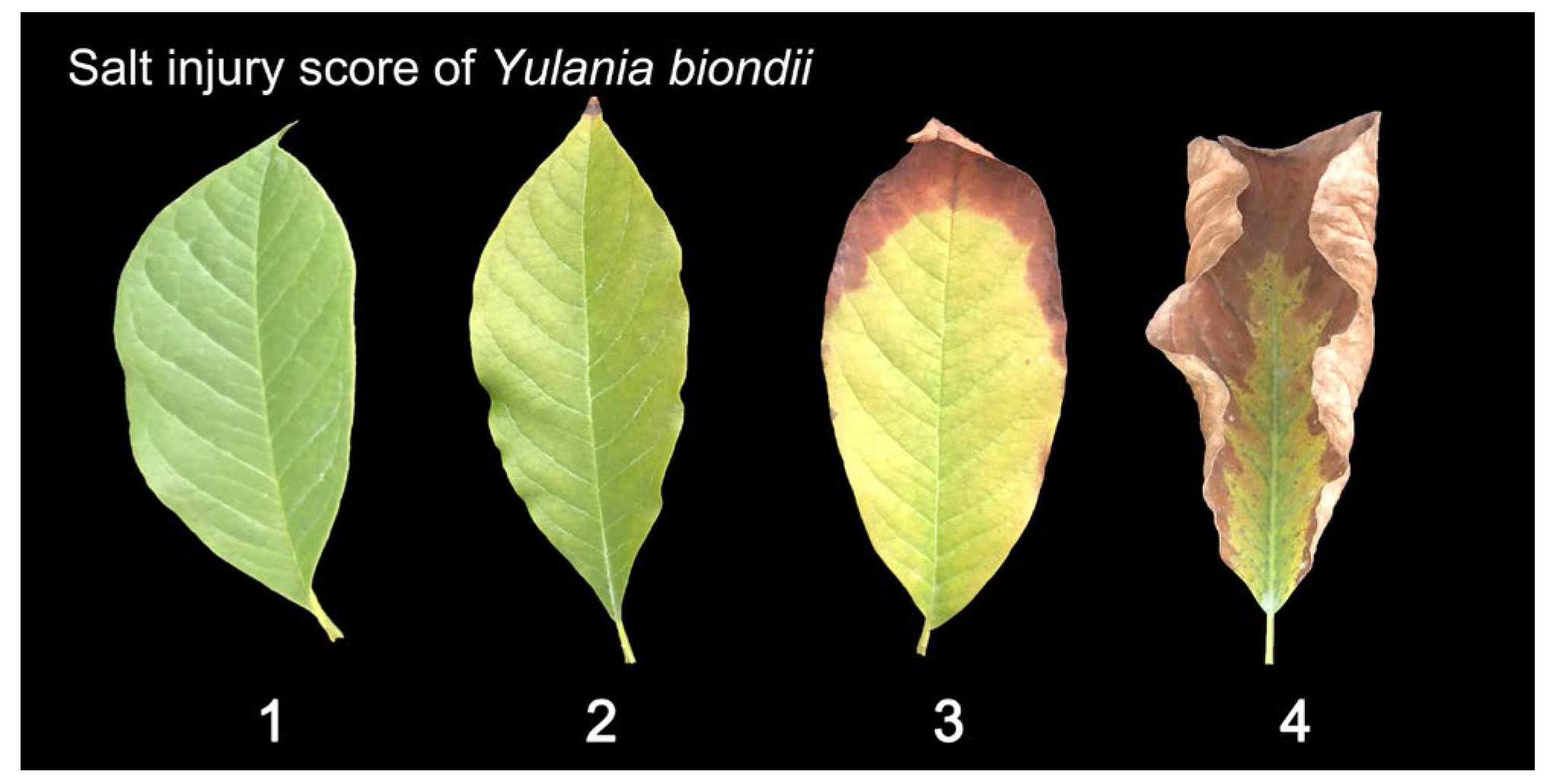
4.4. Photosynthetic Pigment Contents and Photosynthetic Parameters
4.5. Antioxidant Enzyme Activities, Nonenzymatic Antioxidants, and Membrane Lipid Peroxidation
4.6. Estimation of Osmotic Adjustment
4.7. Ion Contents
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Yang, F.; Yang, N.; Ja, W.; Cui, Y. Analysis and Prospects of Saline-alkali Land in China from the Perspective of Utilization. Chin. J. Soil Sci. 2023, 54, 489–494. [Google Scholar]
- Bai, R.Q.; Lin, Y.M.; Jiang, Y.W. Diverse Genotypic Variations of Photosynthetic Capacity, Transpiration and Antioxidant Enzymes of Lily hybrids to Increasing Salinity Stress. Sci. Hortic. 2021, 280, 109939. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Heyno, E.; Mary, V.; Schopfer, P.; Krieger-Liszkay, A. Oxygen Activation at the Plasma Membrane: Relation Between Superoxide and Hydroxyl Radical Production by Isolated Membranes. Planta 2011, 234, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Farooq, M.; Ahmad, R.; Shahzad, M.; Sajjad, Y.; Hassan, A.; Shah, M.M.; Naz, S.; Khan, S.A. Differential Variations in Total Flavonoid Content and Antioxidant Enzymes Activities in Pea under Different Salt and Drought Stresses. Sci. Hortic. 2021, 287, 110258. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of Proline, Lipid Peroxidation and Antioxidative Enzymes in Two Varieties of Pisum sativum L. under Salt Stress. Int. J. Plant Prod. 2008, 2, 353–366. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and Comparative Analysis of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Glutathione Peroxidase) in Selected Plants Employing Bioinformatics Approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles Enhances the Salinity Toxicity Tolerance in Linum usitatissimum L. by Modulating the Antioxidative Enzymes, Photosynthetic Efficiency, Redox Status and Cellular Damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Zhu, H.G.; Zhu, H.Y.; Tao, Y.Z.; Liu, C.Z.; Liu, J.Q.; Yang, F.Q.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2019, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Giri, J. Glycinebetaine and Abiotic Stress Tolerance in Plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.S. Enhancing Antioxidant–yield Relationship of Pea Plant under Drought at Different Growth Stages by Exogenously Applied Glycine Betaine and Proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Lodeyro, A.F.; Giró, M.; Poli, H.O.; Bettucci, G.; Cortadi, A.; Ferri, A.M.; Carrillo, N. Suppression of Reactive Oxygen Species Accumulation in Chloroplasts Prevents Leaf Damage but not Growth Arrest in Salt-stressed Tobacco Plants. PLoS ONE 2016, 11, e0159588. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, D.; Kumar, S.; Rampuria, S.; Reddy, A.R.; Kirti, P.B. Ectopic Expression of an Atypical Hydrophobic Group 5 LEA Protein from Wild Peanut, Arachis Diogoi Confers Abiotic Stress Tolerance in Tobacco. PLoS ONE 2016, 11, e0150609. [Google Scholar] [CrossRef]
- Ahmad, R.; Jamil, S.; Shahzad, M.; Zörb, C.; Irshad, U.; Khan, N.; Younas, M.; Khan, S.A. Metabolic Profiling to Elucidate Genetic Elements Due to Salt Stress. Clean–Soil Air Water 2017, 45, 1600574. [Google Scholar] [CrossRef]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y. Prediction of Potentially Suitable Areas for the Introduction of Magnolia Wufengensis under Climate Change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wang, L.R.; He, S.C.; Liu, X.; Wang, X.Q. A New Species of Magnolia (Magnoliaceae) from Hubei, China. Bull. Bot. Res. 2006, 26, 4–7. [Google Scholar]
- Ma, L.Y.; Wang, L.R.; He, S.C.; Liu, X.; Wang, X.Q. A New Variety of Magnolia (Magnoliaceae) from Hubei, China. Bull. Bot. Res. 2006, 26, 516–519. [Google Scholar]
- Huang, X.Y.; Soolanayakanahally, R.Y.; Guy, R.D.; Shunmugam, A.S.K.; Mansfield, S.D. Differences in Growth and Physiological and Metabolic Responses among Canadian Native and Hybrid Willows (Salix spp.) under Salinity Stress. Tree Physiol. 2020, 40, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.R.; Yang, X.Y.; Ren, J.Y.; Wu, H.W.; Zhu, J.F.; Zhang, H.X. Research Progress in Salt Tolerance Evaluation and Identification of Garden Plants in China. World For. Res. 2018, 31, 51–57. [Google Scholar] [CrossRef]
- Xue, F.L.; Liu, W.L.; Cao, H.L.; Song, L.J.; Ji, S.S.; Tong, L.; Ding, R.S. Stomatal Conductance of Tomato Leaves is Regulated by both Abscisic Acid and Leaf Water Potential under Combined Water and Salt Stress. Physiol. Plant. 2021, 172, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.T.; Liu, X.M.; Shan, L.; Wu, Q.; Liu, Y.; Zhang, Z.J.; Ma, F.W.; Li, C. Dopamine and Arbuscular Mycorrhizal Fungi Act Synergistically to Promote Apple Growth under Salt Stress. Environ. Exp. Bot. 2020, 178, 104159. [Google Scholar] [CrossRef]
- Pandolfi, C.; Bazihizina, N.; Giordano, C.; Mancuso, S.; Azzarello, E. Salt Acclimation Process: A Comparison between a Sensitive and a Tolerant Olea europaea Cultivar. Tree Physiol. 2017, 37, 380–388. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Hu, X.F.; Wang, D.; Ren, S.; Feng, S.; Zhang, H.Z.; Zhang, J.Z.; Qian, K. Inhibition of Root Growth by Alkaline Salts Due to Disturbed Ion Transport and Accumulation in Leymus chinensis. Environ. Exp. Bot. 2022, 200, 104907. [Google Scholar] [CrossRef]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.X.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 24.1–24.31. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress- Induced A. tricolor to Growth, Anatomy, Physiology, Non-enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Crawford, T.; Lehotai, N.; Strand, Å. The Role of Retrograde Signals During Plant Stress Responses. J. Exp. Bot. 2018, 69, 2783–2795. [Google Scholar] [CrossRef]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G. Arbuscular Mycorrhizal Fungi and Foliar Phosphorus Inorganic Supply Alleviate Salt Stress Effects in Physiological Attributes, but only Arbuscular Mycorrhizal Fungi Increase Biomass in Woody Species of a Semiarid Environment. Tree Physiol. 2017, 38, 25–36. [Google Scholar] [CrossRef]
- Musyimi, D.M.; Netondo, G.W.; Ouma, G. Effects of Salinity on Gas Exchange and Nutrients Uptake in Avocados. J. Biol. Sci. 2007, 7, 496–505. [Google Scholar] [CrossRef]
- Jayakumar, B.; Rana, M.; Sergey, S.; Matthew, G.; Barry, P.; Stephen, D.T. Chloroplast Function and Ion Regulation in Plants Growing on Saline Soils: Lessons from Halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Du, N.S.; Xue, L.; Xue, D.Q.; Dong, X.X.; Yang, Q.; Shah Jahan, M.; Guo, H.; Fu, R.; Wang, Y.H.; Piao, F.Z. The Transcription Factor SlNAP1 Increases Salt Tolerance by Modulating Ion Homeostasis and ROS Metabolism in Solanum lycopersicum. Gene 2023, 849, 146906. [Google Scholar] [CrossRef]
- Gohari, G.; Zareei, E.; Rostami, H.; Panahirad, S.; Kulak, M.; Farhadi, H.; Amini, M.; Martinez-Ballesta, M.D.C.; Fotopoulos, V. Protective Effects of Cerium Oxide Nanoparticles in Grapevine (Vitis vinifera L.) cv. Flame Seedless under Salt Stress Conditions. Ecotoxicol. Environ. Saf. 2021, 220, 112402. [Google Scholar] [CrossRef]
- Rabab, S.; Antonio, C.; Giuseppe, G.; Francesco, S. Osmoprotectants and Antioxidative Enzymes as Screening Tools for Salinity Tolerance in Radish (Raphanus sativus). Hortic. Plant J. 2020, 6, 14–24. [Google Scholar] [CrossRef]
- Patel, M.; Parida, A.K. Salinity Alleviates the Arsenic Toxicity in the Facultative Halophyte Salvadora persica L. by the Modulations of Physiological, Biochemical, and ROS Scavenging Attributes. J. Hazard. Mater. 2021, 401, 123368. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Aram, M.A.; Zia-ur-Rehman, M.; Wang, X.S.; Zhang, X.Q.; Huang, L.K. Physiological and Transcriptome Analyses Demonstrate the Silver Nanoparticles Mediated Alleviation of Salt Stress in Pearl Millet (Pennisetum glaucum L). Environ. Pollut. 2023, 318, 120863. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and Network Analysis Reveal the Potential Metabolites and Biological Pathways Involved in Salinity Tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Parida, A.K. Cross Talk between ROS Homeostasis and Antioxidative Machinery Contributes to Salt Tolerance of the Xero-halophyte Haloxylon salicornicum. Environ. Exp. Bot. 2019, 166, 103799. [Google Scholar] [CrossRef]
- Ran, X.; Huang, X.; Wang, X.; Liang, H.; Wang, Y.; Li, J.; Huo, Z.; Liu, B.; Ma, C. Ion Absorption, Distribution and Salt Tolerance Threshold of Three Willow Species under Salt Stress. Front. Plant Sci. 2022, 13, 969896. [Google Scholar] [CrossRef]
- Niu, M.L.; Xie, J.J.; Sun, J.Y.; Huang, Y.; Kong, Q.S.; Nawaz, M.A.; Bie, Z.L. A Shoot Based Na+ Tolerance Mechanism Observed in Pumpkin—An Important Consideration for Screening Salt Tolerant Rootstocks. Sci. Hortic. 2017, 218, 38–47. [Google Scholar] [CrossRef]
- Guo, Z.X.; Qin, Y.P.; Lv, J.L.; Wang, X.J.; Dong, H.; Dong, X.X.; Zhang, T.; Du, N.S.; Piao, F.Z. Luffa Rootstock Enhances Salt Tolerance and Improves Yield and Quality of Grafted Cucumber Plants by Reducing Sodium Transport to the Shoot. Environ. Pollut. 2023, 316, 120521. [Google Scholar] [CrossRef]
- Kotula, L.; Khan, H.A.; Quealy, J.; Turner, N.C.; Vadez, V.; Siddique, K.H.M.; Clode, P.L.; Colmer, T.D. Salt Sensitivity in Chickpea (Cicer arietinum L.): Ions in Reproductive Tissues and Yield Components in Contrasting Genotypes. Plant Cell Environ. 2015, 38, 1565–1577. [Google Scholar] [CrossRef]
- Ahmad, J.; Wang, H.; Song, J.; Nuerawuti, M.; Zhang, X.; Yang, W.; Ma, L.; Li, X.X. The Tolerance of an Extensive Collection of Garlic (Allium sativum L.) Germplasms to Salt Stress—A Sustainable Solution to Salt Stress. Appl. Ecol. Environ. Res. 2021, 19, 2281–2303. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b in Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kamran, M.; Wang, D.; Xie, K.Z.; Lu, Y.S.; Shi, C.H.; Sabagh, A.E.; Gu, W.J.; Xu, P.Z. Pre-sowing Seed Treatment with Kinetin and Calcium Mitigates Salt Induced Inhibition of Seed Germination and Seedling Growth of Choysum (Brassica rapa var. parachinensis). Ecotoxicol. Environ. Saf. 2021, 227, 112921. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of Antioxidant and Glyoxalase Systems by Exogenous Glycinebetaine and Proline in Mung Bean Confer Tolerance to Cadmium Stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.W.; Liang, D.; Ma, F.W. Long-term Exogenous Application of Melatonin Delays Drought-induced Leaf Senescence in Apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Quantities Microgram Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ringel, C.; Siebert, S.; Wienhaus, O. Photometric Determination of Proline in Quartz Microplates: Remarks on Specificity. Anal. Biochem. 2003, 313, 167–169. [Google Scholar] [CrossRef]




| Treatment | Soil NaCl Content (Weight Ratio) | Soil Electric Conductivity (ms·cm−1) | Soil Weight (kg) | NaCl Weight (g) | NaCl Solution Concentration (mM) | Total NaCl Solution Volume (L) |
|---|---|---|---|---|---|---|
| Na0 | 0‰ | 0.213 | 3.5 | 0 | 0 | 1 |
| Na1 | 1‰ | 0.421 | 3.5 | 3.5 | 60 | 1 |
| Na2 | 2‰ | 0.784 | 3.5 | 7.0 | 120 | 1 |
| Na3 | 3‰ | 1.592 | 3.5 | 10.5 | 180 | 1 |
| Na4 | 4‰ | 3.021 | 3.5 | 14.0 | 240 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Tian, L.; Zhu, Z.; Sang, Z.; Ma, L.; Jia, Z. Growth and Physiological Responses of Magnoliaceae to NaCl Stress. Plants 2024, 13, 170. https://doi.org/10.3390/plants13020170
Zhao X, Tian L, Zhu Z, Sang Z, Ma L, Jia Z. Growth and Physiological Responses of Magnoliaceae to NaCl Stress. Plants. 2024; 13(2):170. https://doi.org/10.3390/plants13020170
Chicago/Turabian StyleZhao, Xiuting, Ling Tian, Zhonglong Zhu, Ziyang Sang, Lvyi Ma, and Zhongkui Jia. 2024. "Growth and Physiological Responses of Magnoliaceae to NaCl Stress" Plants 13, no. 2: 170. https://doi.org/10.3390/plants13020170






