Functional Characterization of CsF3Ha and Its Promoter in Response to Visible Light and Plant Growth Regulators in the Tea Plant
Abstract
1. Introduction
2. Results
2.1. Changes of the Anthocyanin Content in Tea Plant under Different Treatments
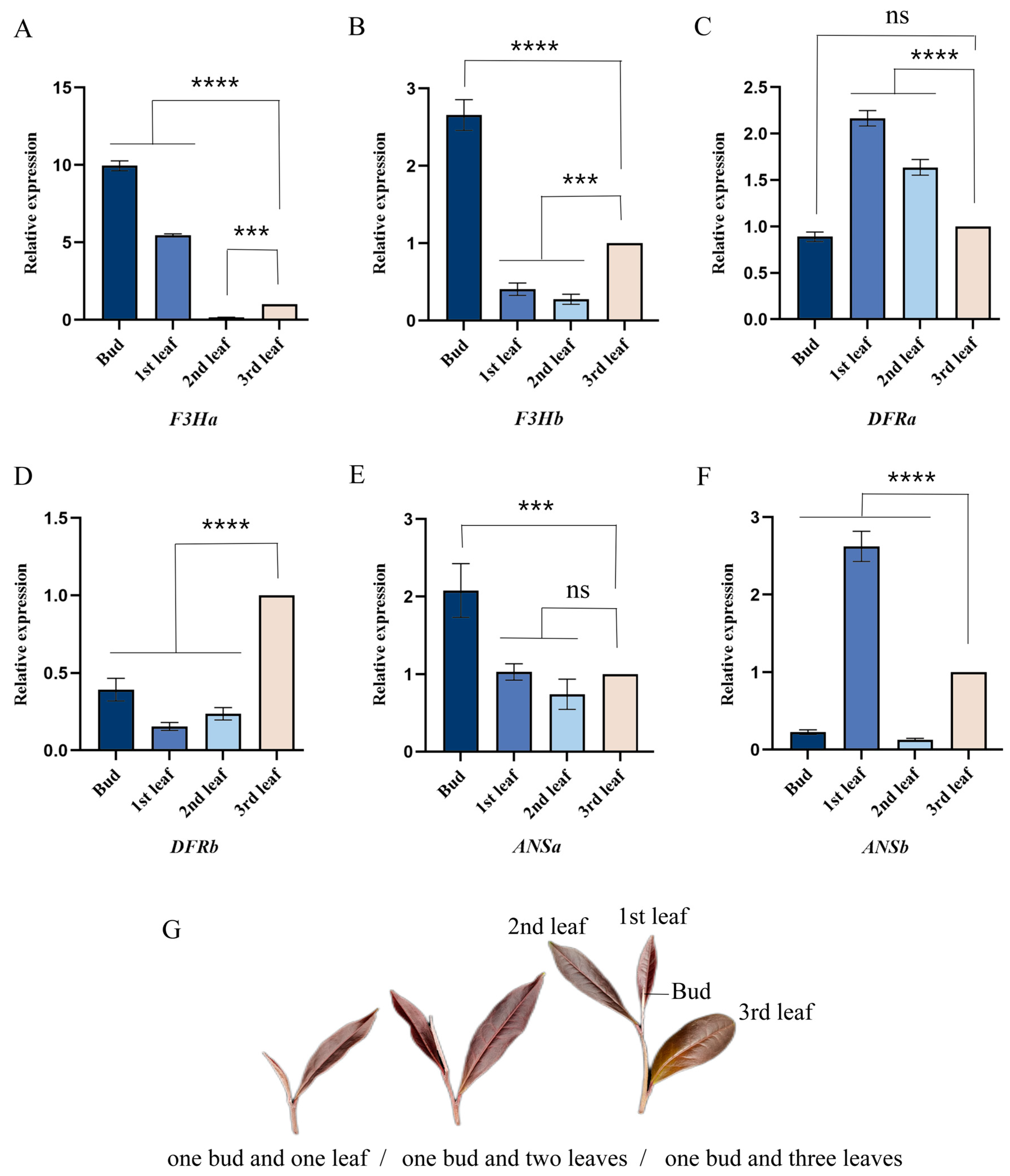
2.2. Analysis of the Quantitative Real-Time PCR for Candidate Genes
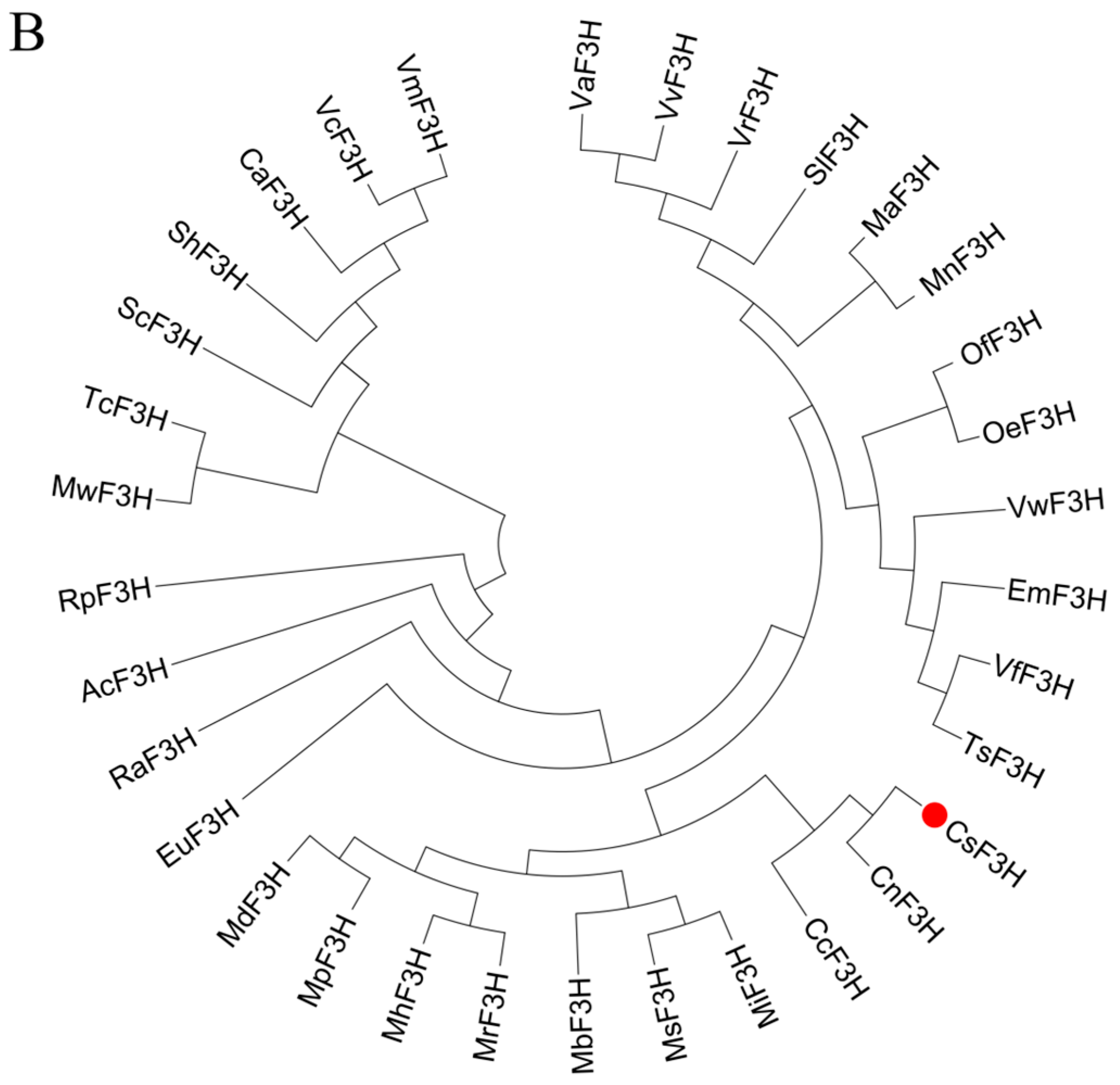
2.3. Characterization of CsF3Ha Gene and Phylogenetic Tree Analysis
2.4. Expression Analysis of CsF3Ha Gene
2.5. Cloning and Cis-Element Analysis of the CsF3Ha Promoter
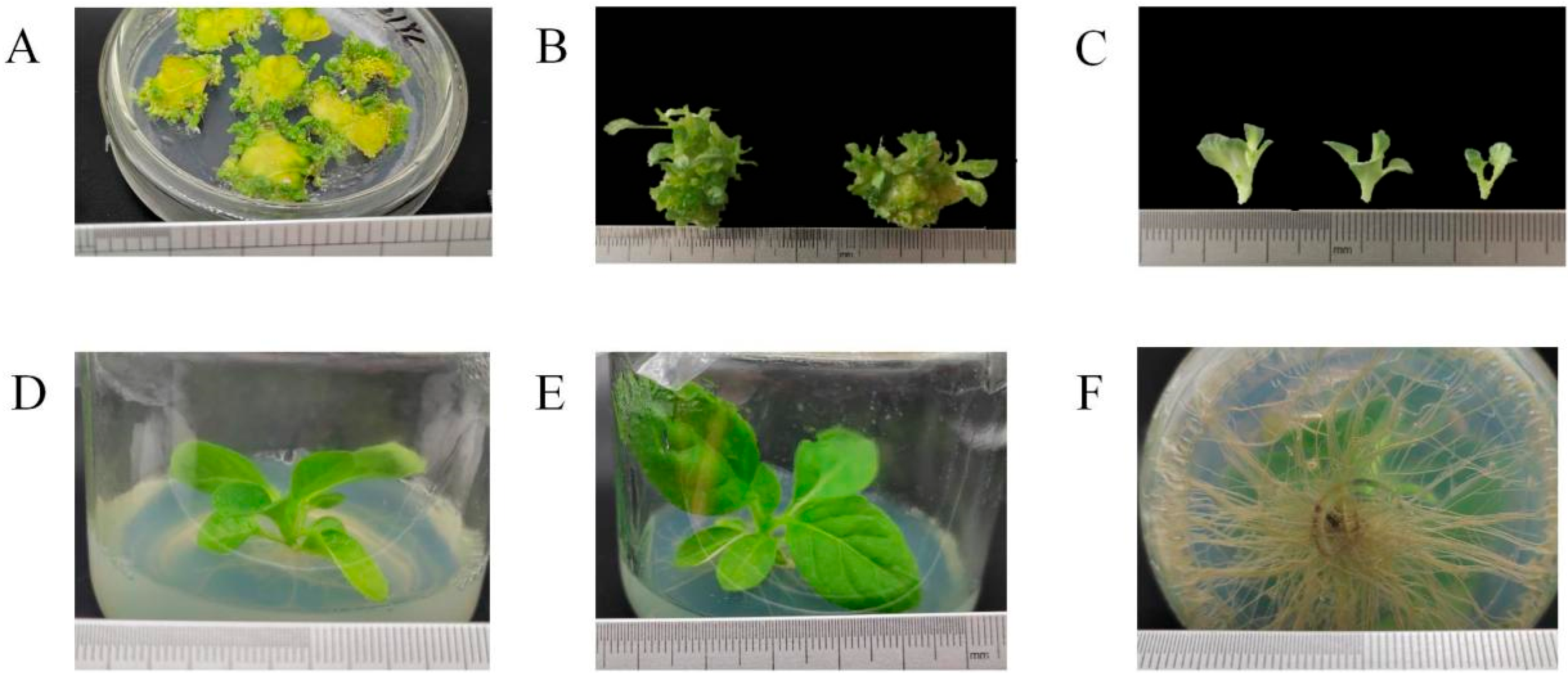
2.6. Transgenic Tobacco Lines with Hygromycin Resistance
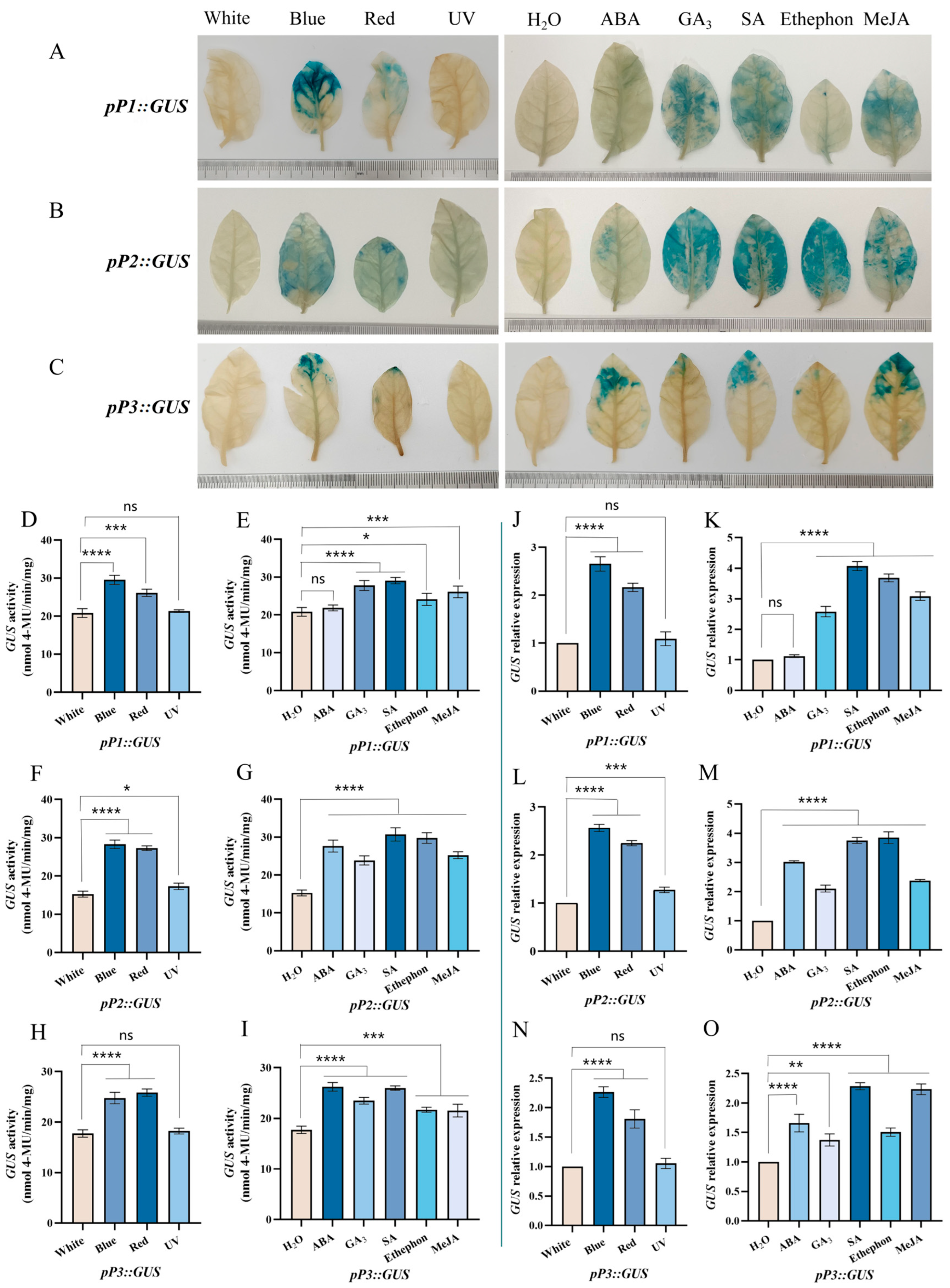
2.7. Histochemical GUS Staining
2.8. GUS Activity Assay
2.9. The GUS Gene’s Relative Expression
3. Discussion
3.1. The Key Cis-Elements of CsF3Ha Promoter
3.2. Differences of CsF3Ha Promoter the P1, P2 and P3
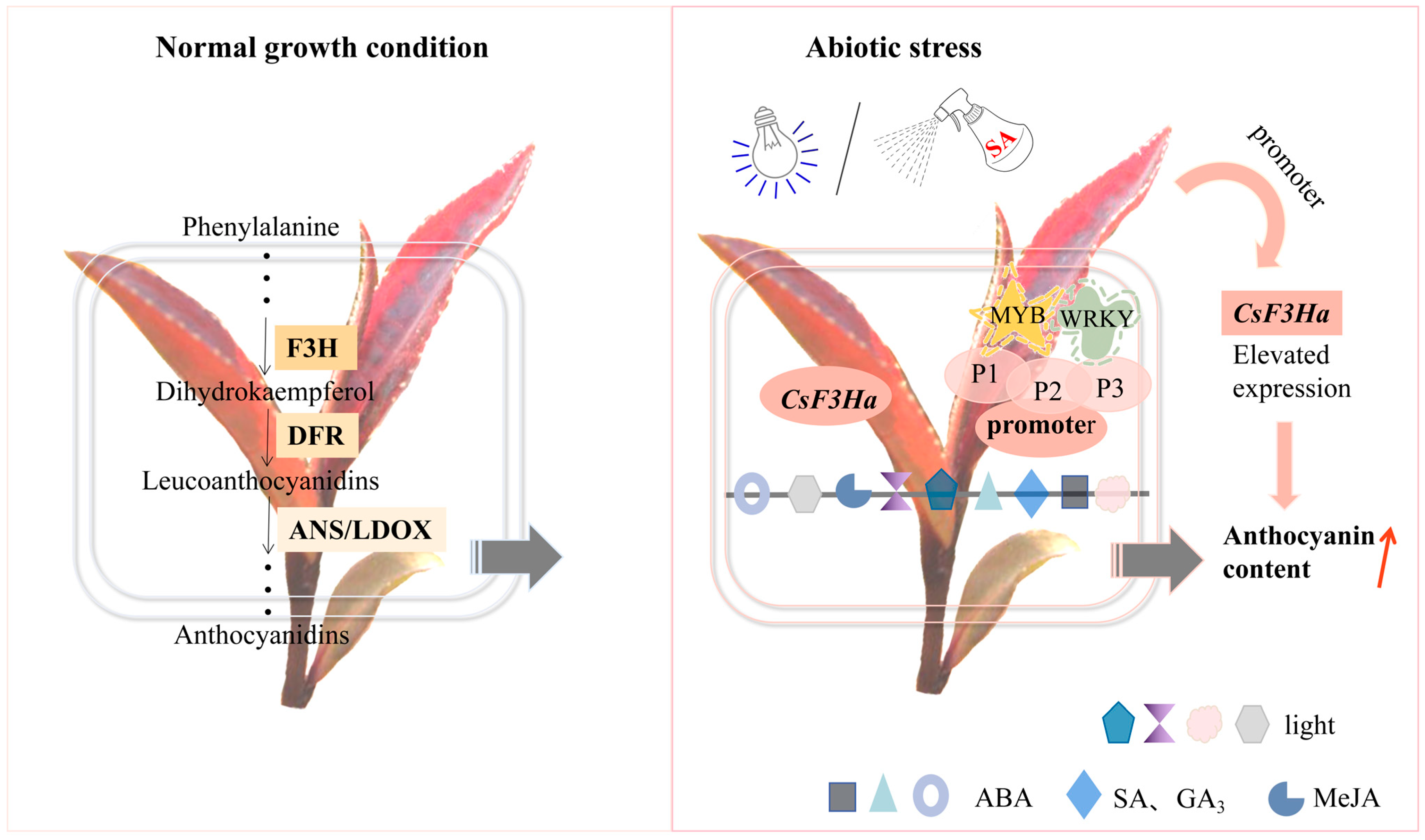
3.3. Molecular Regulatory Mechanism of CsF3Ha Promoter on Anthocyanin Synthesis in the Tea Plant
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Visible Light and PGRs Treatments on the Tea Plant
4.3. Assay of Anthocyanin Content
4.4. Screening of Candidate Genes
4.5. Analysis of the Quantitative Real-Time PCR for CsF3Ha Gene
4.6. Promoter Cloning and Sequence Analysis
4.7. Vector Construction of CsF3Ha Promotor and Transformation
4.8. Histochemical GUS Staining
4.9. GUS Activity Assay
4.10. The GUS Gene Relative Expression
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, H.P.; Dai, W.D.; Tan, J.F.; Guo, L.; Zhu, Y.; Lin, Z. Identification of the anthocyanins from the purple leaf coloured tea cultivar Zijuan (Camellia sinensis var. assamica) and characterization of their antioxidant activities. J. Funct. Foods 2015, 17, 449–458. [Google Scholar] [CrossRef]
- Khusnutdinov, E.; Sukhareva, A.; Panfilova, M.; Mikhaylova, E. Anthocyanin biosynthesis genes as model genes for genome editing in plants. Int. J. Mol. Sci. 2021, 22, 8752. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Salehi, J.; Sharifi-Rad, F.; Cappellini, Z.; Reiner, D.; Zorzan, M.; Imran, B.; Sener, M.; Kilic, M.; El-Shazly, N.M.; Fahmy, E.; et al. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Dzierzanowski, T. Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: An update. Molecules 2021, 26, 4380. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Rana, A.; Kumar, V.; Kumar, D.; Padwad, Y.S.; Yadav, S.K.; Gulati, A. Anthocyanins enriched purple tea exhibits antioxidant, immunostimulatory and anticancer activities. J. Food Sci. Technol. 2017, 54, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox. Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Guo, N.; Cheng, F.; Wu, J.; Liu, B.; Zheng, S.; Liang, J.; Wang, X. Anthocyanin biosynthetic genes in Brassica rapa. BMC Genom. 2014, 15, 426. [Google Scholar] [CrossRef]
- Goswami, G.; Nath, U.K.; Park, J.I.; Hossain, M.R.; Biswas, M.K.; Kim, H.T.; Kim, H.R.; Nou, I.S. Transcriptional regulation of anthocyanin biosynthesis in a high-anthocyanin resynthesized Brassica napus cultivar. J. Biol. Res. (Thessalon) 2018, 25, 19. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Carbone, F.; Preuss, A.; De Vos, R.C.; D’Amico, E.; Perrotta, G.; Bovy, A.G.; Martens, S.; Rosati, C. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant Cell Environ. 2009, 32, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Yang, L. Stability of anthocyanins and their degradation products from cabernet sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef]
- Han, Y.; Huang, K.; Liu, Y.; Jiao, T.; Ma, G.; Qian, Y.; Wang, P.; Dai, X.; Gao, L.; Xia, T. Functional analysis of two flavanone-3-hydroxylase genes from Camellia sinensis: A critical role in flavonoid accumulation. Genes 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Tao, R.; Bai, S.; Ni, J.; Yang, Q.; Zhao, Y.; Teng, Y. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tan, L.; Zou, Y.; Tan, X.; Huang, J.; Chen, W.; Tang, Q. The effects of ultraviolet A/B treatments on anthocyanin accumulation and gene expression in dark-purple tea cultivar ‘Ziyan’ (Camellia sinensis). Molecules 2020, 25, 354. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Shin, D.H.; Choi, S.B.; Park, Y.I. Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol. Cells 2012, 34, 501–507. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; Liu, Y.J.; Wang, X.F.; You, C.X.; Hao, Y.J. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple. J. Exp. Bot. 2020, 72, 1460–1472. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Liu, X.; Zhang, J.C.; Wang, X.F.; You, C.X.; Hao, Y.J. Jasmonate induces biosynthesis of anthocyanin and proanthocyanidin in apple by mediating the JAZ1-TRB1-MYB9 complex. Plant J. 2021, 106, 1414–1430. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Chen, H.; Yang, B.; Ge, M.; Zhang, Z. Effects of gibberellin applications before flowering on the phenotype, ripening, and flavonoid compounds of Syrah grape berries. J. Sci. Food Agric. 2022, 102, 6100–6111. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Li, Y.Y.; Song, L.Q.; Zhao, L.L.; You, C.X.; Hao, Y.J. EIN3-LIKE1, MYB1, and ethylene response factor3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, N.; Xu, H.F.; Jiang, S.H.; Fang, H.C.; Su, M.Y.; Zhang, Z.Y.; Zhang, T.L.; Chen, X.S. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef]
- Dong, G.; Fan, M.; Wang, H.; Leng, Y.; Sun, J.; Huang, J.; Zhang, H.; Yan, J. Functional characterization of TkSRPP promoter in response to hormones and wounding stress in transgenic tobacco. Plants 2023, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and suppressor of overexpression of constans. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Kim, W.Y.; Cha, J.Y.; Park, H.J.; Shin, G.; Park, J.; Lim, C.J.; Chun, H.J.; Li, N.; Kim, D.H.; et al. The GIGANTEA-ENHANCED EM LEVEL complex enhances drought tolerance via regulation of abscisic acid synthesis. Plant Physiol. 2020, 184, 443–458. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, Y.M.; Hwang, O.J.; Kim, J.I. Characterization of a small constitutive promoter from Arabidopsis translationally controlled tumor protein (AtTCTP) gene for plant transformation. Plant Cell Rep. 2015, 34, 265–275. [Google Scholar] [CrossRef]
- Wang, H.J.; Jiang, Y.H.; Qi, Y.W.; Dai, J.Y.; Liu, Y.L.; Zhu, X.B.; Liu, C.H.; Lü, Y.R.; Ren, X.L. Identification and functional characterization of the MdHB-1 gene promoter sequence from Malus×domestica. J. Integr. Agric. 2017, 16, 1730–1741. [Google Scholar] [CrossRef]
- Yan, H.; Xiong, Y.; Teixeira da Silva, J.A.; Pang, J.; Zhang, T.; Yu, X.; Zhang, X.H.; Niu, M.Y.; Ma, G.H. Molecular cloning and functional characterization of Bisabolene Synthetase (SaBS) promoter from Santalum album. Forests 2020, 11, 85. [Google Scholar] [CrossRef]
- Tang, W.; Wang, X.X.; Kou, M.; Yan, H.; Gao, R.F.; Li, C.; Song, W.H.; Zhang, Y.G.; Wang, X.; Liu, Y.J.; et al. The sweetpotato GIGANTEA gene promoter is co-regulated by phytohormones and abiotic stresses in Arabidopsis thaliana. Plant Physiol. Bioch. 2021, 168, 143–154. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Lu, S.X.; Webb, C.J.; Knowles, S.M.; Kim, S.H.; Wang, Z.; Tobin, E.M. CCA1 and ELF3 interact in the control of hypocotyl length and flowering time in Arabidopsis. Plant Physiol. 2012, 158, 1079–1088. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, J.; Wang, D.; Gao, F. Molecular cloning, functional characterization and transcriptional regulation of the promoter of dihydroflavonal-4-reductase gene from purple-fleshed sweet potato. S. Afr. J. Bot. 2020, 131, 206–213. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Qiu, F.; Chen, Y.; Zhang, F.; Lan, X.; Chen, M.; Zhang, H.; Liao, Z. Molecular cloning and characterization of the promoter of aldehyde dehydrogenase gene from Artemisia annua. Biotechnol. Appl. Biochem. 2017, 64, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Li, X.; Zhang, Z.; Ai, S.; Liu, C.; Ma, F.; Li, C. MdERF114 enhances the resistance of apple roots to Fusarium solani by regulating the transcription of MdPRX63. Plant Physiol. 2023, 31, kiad057. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of avonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant. Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002, 129, 706–716. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Abdel-Aal, J.; El-Sayed, M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Zhao, K.; Shen, X.; Yuan, H.; Liu, Y.; Liao, X.; Wang, Q.; Liu, L.; Li, F.; Li, T. Isolation and characterization of dehydration-responsive element-binding factor 2C (MsDREB2C) from Malus sieversii Roem. Plant Cell Physiol. 2013, 54, 1415–1430. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Hou, L.; Zhao, S.; Zhao, C.; Han, X.; Li, P.; Zhang, Y.; Bian, X.; Wang, X. Globalanalysis of WRKY genes and their response to dehydration and salt stress in soybean. Front. Plant Sci. 2016, 7, 9. [Google Scholar] [CrossRef]
- Wang, C.; Gao, G.; Cao, S.; Xie, Q.; Qi, H. Isolation and functional validation of the CmLOX08 promoter associated with signalling molecule and abiotic stress responses in oriental melon, Cucumis melo var. makuwa Makino. BMC Plant Biol. 2019, 19, 75. [Google Scholar] [CrossRef]




| Cis-Elements | Number | Function | Motif |
|---|---|---|---|
| W-BOX | 10 | transcription suppressor of the GA3 signaling pathway | TGAC |
| W-BOX | 5 | They were recognized specifically by salicylic acid (SA)-induced WRKY DNA-binding proteins | TTGAC |
| W-BOX | 3 | A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter | TGACT |
| MYB PZM | 1 | cis-acting regulatory element involved in plant red pigment regulation | CCWACC |
| MYB PLANT | 1 | Promoters of phenylpropanoid biosynthetic genes, such as PAL, CHS, CHI, DFR | MACCWAMC |
| MYB CORE | 3 | Cis-acting regulatory element involved in flavonoid biosynthesis | CNGTTR |
| MYB1AT | 2 | MYB recognition site found in the promoters of the dehydration-responsive gene rd22 and many other genes in Arabidopsis | WAACCA |
| Primer Name | Sequences (5′-3′) | Primer Name | Sequences (5′-3′) |
|---|---|---|---|
| F3HaF | CCGATTCTTGAAGAGCCAATCACG | F3HaR | TGAGAAGGCCAAGGTGGAAAT |
| F3HbF | TCAATAATGGAGGAACCAATCACC | F3HbR | GGAGTCCAAGCCAGTAGATGA |
| DFRaF | GAATCATTGAAACCTATCC | DFRaR | TAGAGAAAAGGGGATGTTGT |
| DFRbF | AAGGACTTGCCAGTTGTGT | DFRbR | CAGAAAACCCTGTCAATGGC |
| ANSaF | GTCTAGCAACAAAAGTCCTGTCG | ANSaR | GAGACGTCGGTGTGGGCTTCG |
| ANSbF | CGAGCCCTAACTACCAAGACG | ANSbR | GGGTTGAGGGCATTTTGGGT |
| β-actinF | GCCATCTTTGATTGGAATGG | β-actinR | GGTGCCACAACCTTGATCTT |
| PF | CCTGCCAACTGTCACCATACA | PR | TGAGGGACGAAGATGAACGT |
| P1F | CCGGAATTCTAAAATGTTGAGAATCG | P1R | GACGTCGACCTCTCAACTGAATCGAGCAA |
| P2F | CCGGAATTCAACCAAACTAAACTATT | P2R | GACGTCGACCTCTCTCTATCTCTCTCTCTCTCCTCTCTCCCTCTCTCTTCAA |
| P3F | CCGGAATTCCACGTTGATCGGACATCG | P3R | GACGTCGACGGTAGGACATTTTGGGGCAA |
| GUS-qF | GATCGCGAAAACTGTGGAAT | GUS-qR | TAATGAGTGACCGCATCGAA |
| EF1α-qF | TGGTTGTGACTTTTGGTCCCA | EF1α-qR | ACAAACCCACGCTTGAGATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Zou, R.; Zhang, H.; Li, J.; Wu, T. Functional Characterization of CsF3Ha and Its Promoter in Response to Visible Light and Plant Growth Regulators in the Tea Plant. Plants 2024, 13, 196. https://doi.org/10.3390/plants13020196
Bai Y, Zou R, Zhang H, Li J, Wu T. Functional Characterization of CsF3Ha and Its Promoter in Response to Visible Light and Plant Growth Regulators in the Tea Plant. Plants. 2024; 13(2):196. https://doi.org/10.3390/plants13020196
Chicago/Turabian StyleBai, Yan, Rui Zou, Hongye Zhang, Jiaying Li, and Tian Wu. 2024. "Functional Characterization of CsF3Ha and Its Promoter in Response to Visible Light and Plant Growth Regulators in the Tea Plant" Plants 13, no. 2: 196. https://doi.org/10.3390/plants13020196
APA StyleBai, Y., Zou, R., Zhang, H., Li, J., & Wu, T. (2024). Functional Characterization of CsF3Ha and Its Promoter in Response to Visible Light and Plant Growth Regulators in the Tea Plant. Plants, 13(2), 196. https://doi.org/10.3390/plants13020196






