Sucrose and Mannans Affect Arabidopsis Shoot Gravitropism at the Cell Wall Level
Abstract
1. Introduction
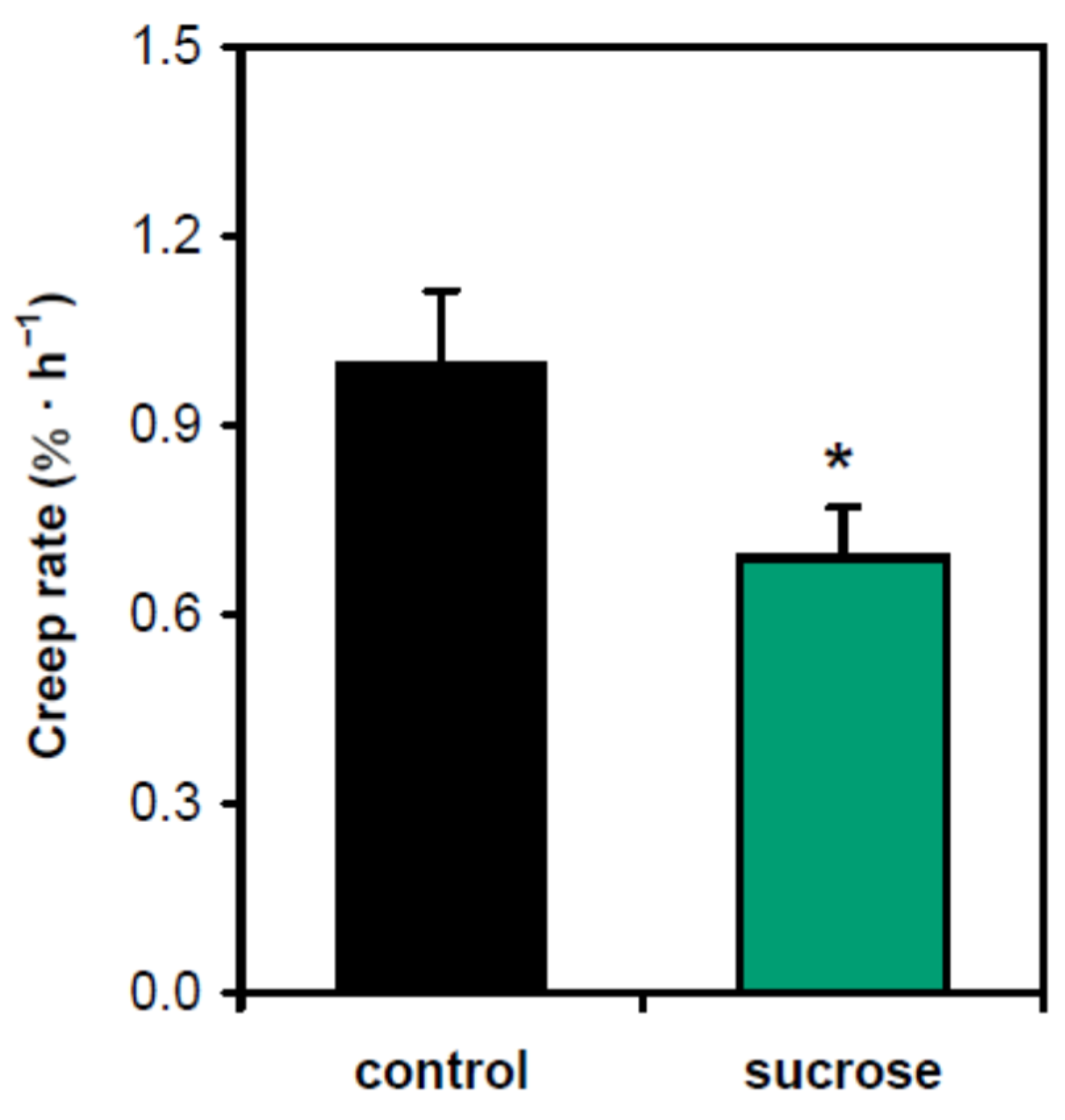
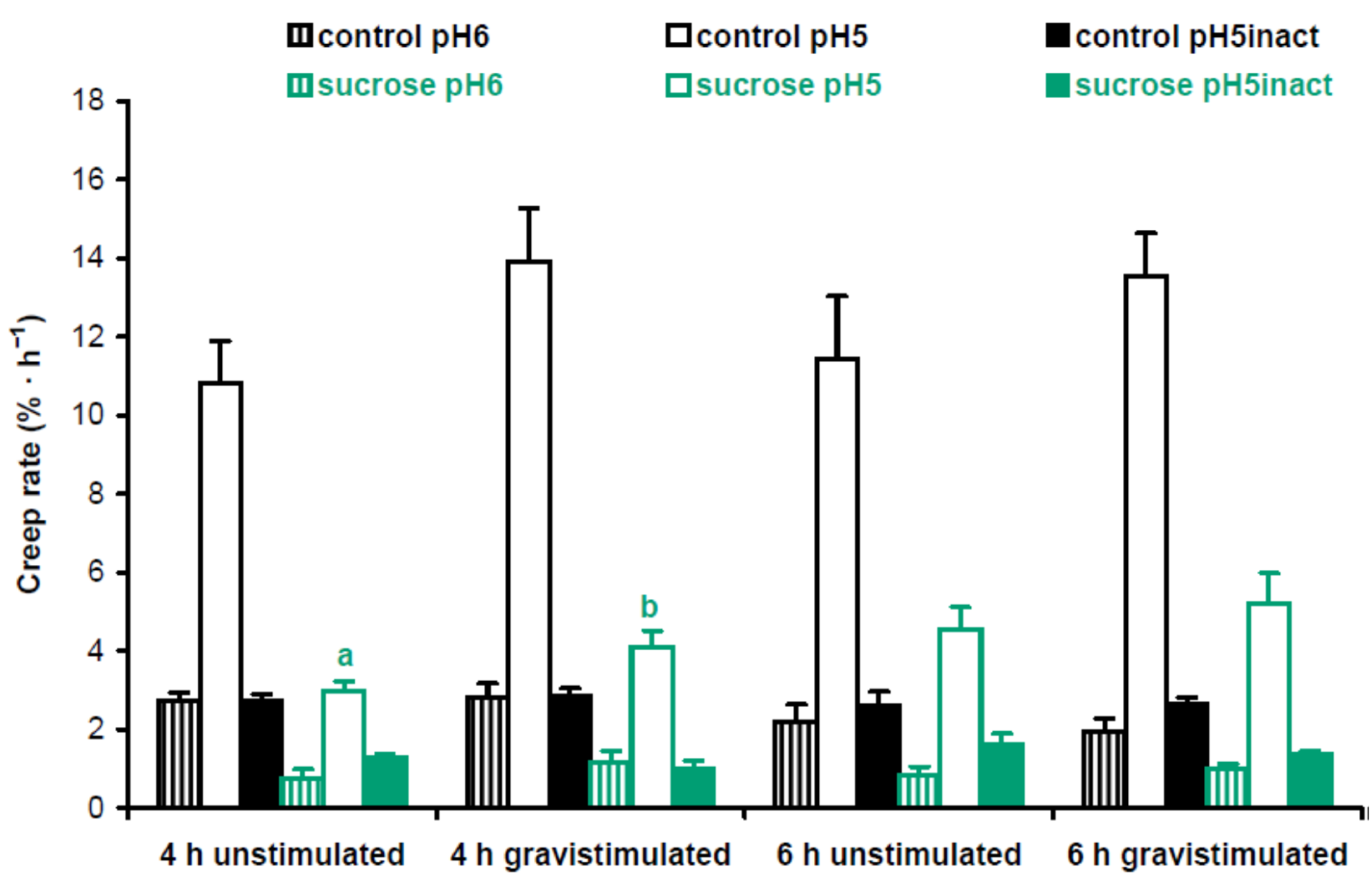
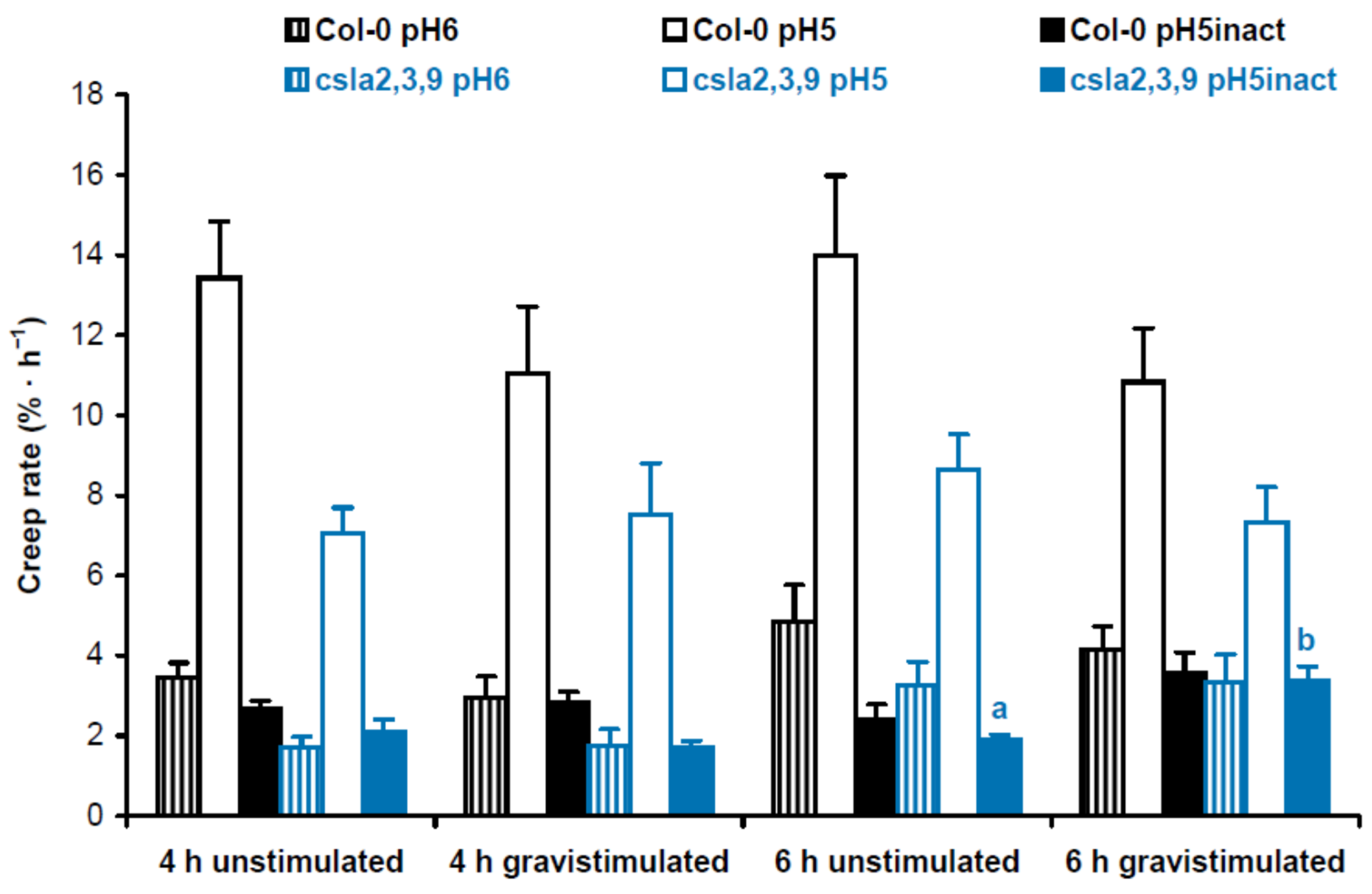
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Hypocotyl Length and Curvature Determination
4.3. Cell Wall Biochemical Analyses
4.4. Extensometry
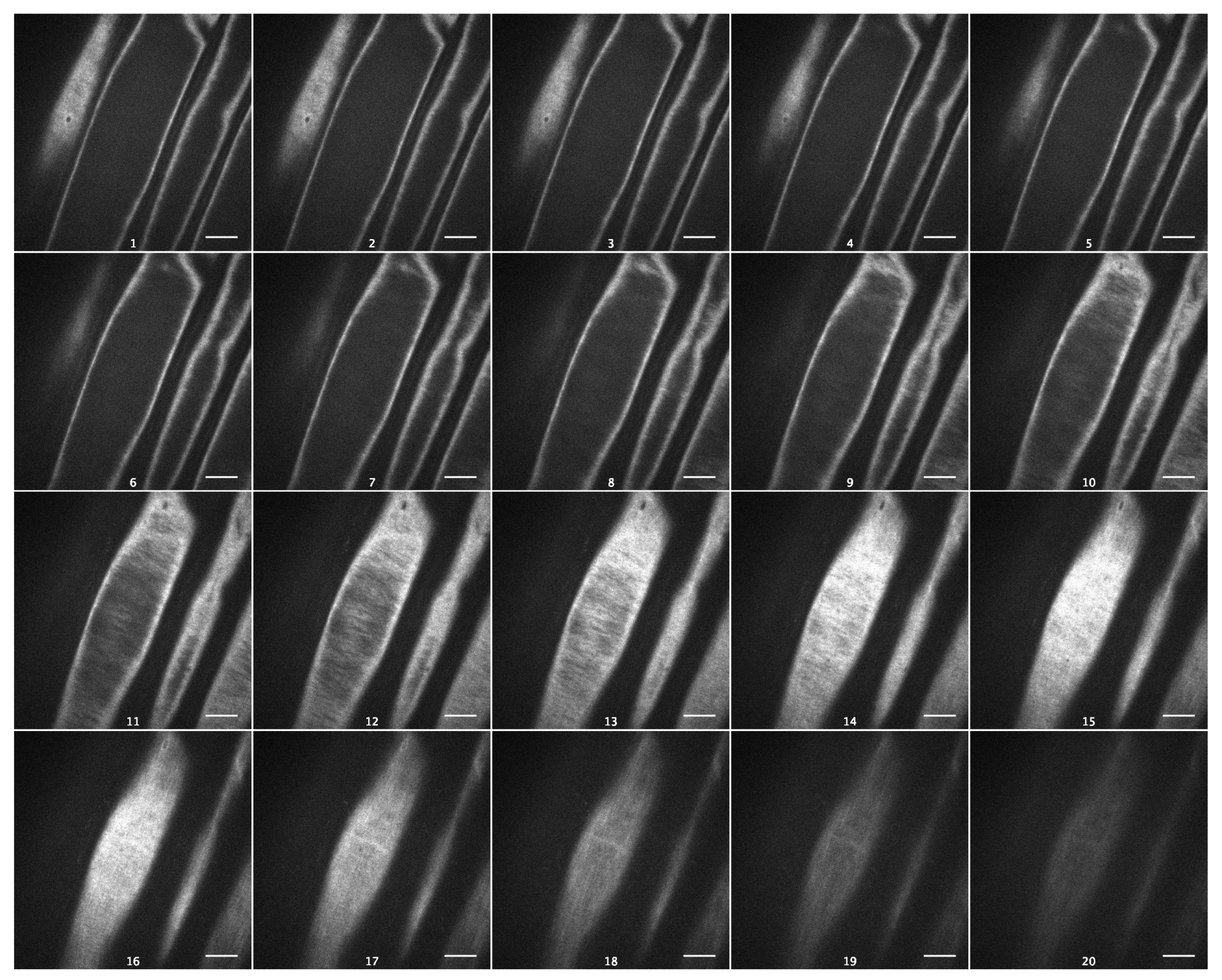
4.5. Spinning-Disc Confocal Microscopy
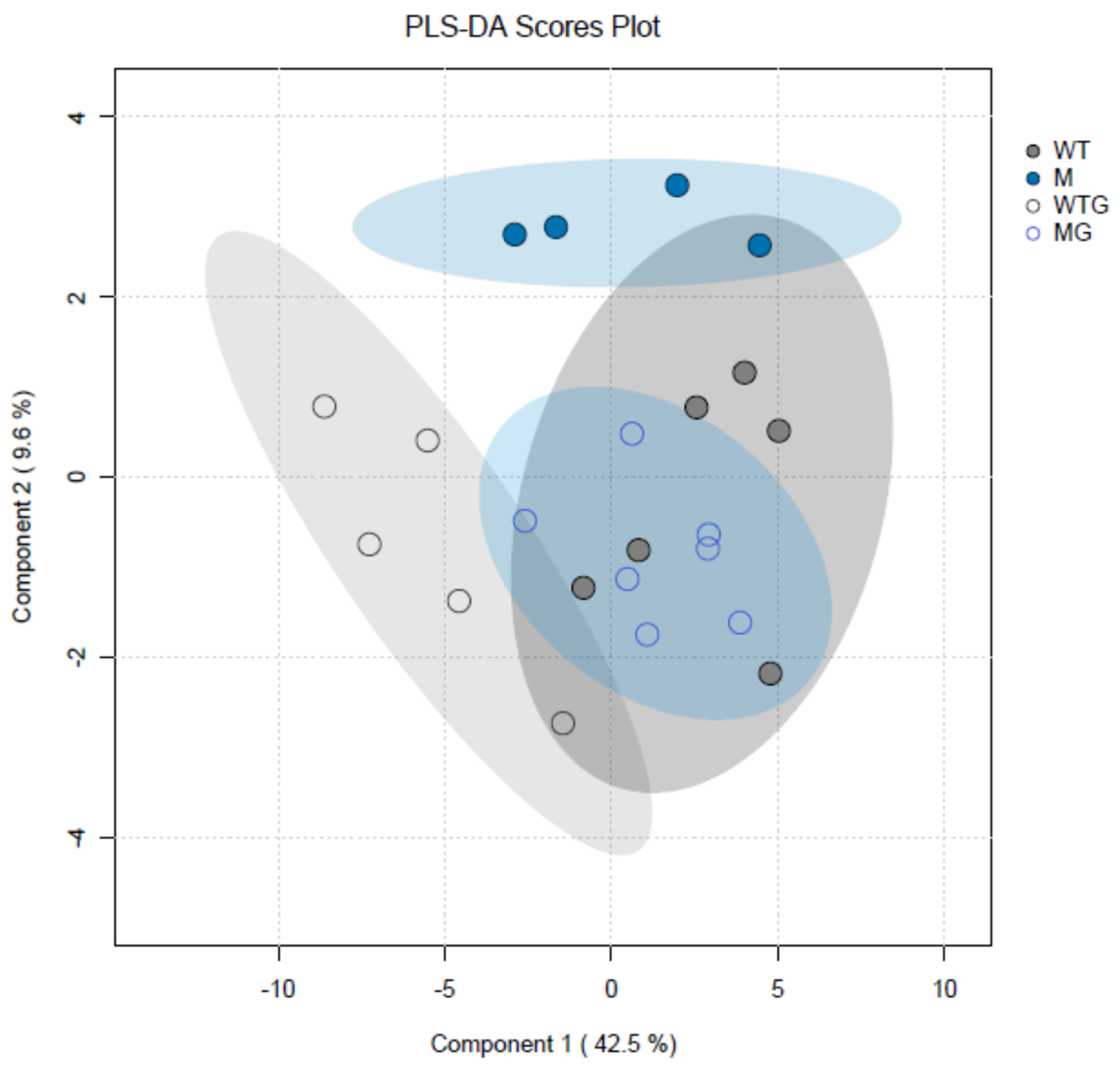
4.6. Metabolomics
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsugawa, S.; Miyake, Y.; Okamoto, K.; Toyota, M.; Yagi, H.; Morita, M.T.; Hara-Nishimura, I.; Demura, T.; Ueda, H. Shoot gravitropism and organ straightening cooperate to arrive at a mechanically favorable shape in Arabidopsis. Sci. Rep. 2023, 13, 11165. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.T. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 2010, 61, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Blancaflor, E.B. Plant Gravitropism: From Mechanistic Insights into Plant Function on Earth to Plants Colonizing Other Worlds. Methods Mol. Biol. 2022, 2368, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z. Mechanisms of the early phases of plant gravitropism. Crit. Rev. Plant Sci. 2000, 19, 551–573. [Google Scholar] [CrossRef]
- Nishimura, T.; Mori, S.; Shikata, H.; Nakamura, M.; Hashiguchi, Y.; Abe, Y.; Hagihara, T.; Yoshikawa, H.Y.; Toyota, M.; Higaki, T.; et al. Cell polarity linked to gravity sensing is generated by LZY translocation from statoliths to the plasma membrane. Science 2023, 381, 1006–1010. [Google Scholar] [CrossRef]
- Furutani, M.; Hirano, Y.; Nishimura, T.; Nakamura, M.; Taniguchi, M.; Suzuki, K.; Oshida, R.; Kondo, C.; Sun, S.; Kato, K.; et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 2020, 11, 76. [Google Scholar] [CrossRef]
- Ikushima, T.; Soga, K.; Hoson, T.; Shimmen, T. Role of xyloglucan in gravitropic bending of azuki bean epicotyl. Physiol. Plant. 2008, 132, 552–565. [Google Scholar] [CrossRef]
- Somssich, M.; Vandenbussche, F.; Ivakov, A.; Funke, N.; Ruprecht, C.; Vissenberg, K.; Van Der Straeten, D.; Persson, S.; Suslov, D. Brassinosteroids Influence Arabidopsis Hypocotyl Graviresponses through Changes in Mannans and Cellulose. Plant Cell Physiol. 2021, 62, 678–692. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Guo, X.; Gallemi, M.; Aryal, B.; Venhuizen, P.; Barbez, E.; Dünser, K.A.; Darino, M.; Pĕnčík, A.; Novák, O.; et al. Xyloglucan Remodeling Defines Auxin-Dependent Differential Tissue Expansion in Plants. Int. J. Mol. Sci. 2021, 22, 9222. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Guisinger, M.M.; Miller, A.J.; Stackhouse, K.S. Reduced Gravitropism in Hypocotyls of Starch-Deficient Mutants of Arabidopsis. Plant Cell Physiol. 1997, 38, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Suslov, D.; De Grauwe, L.; Leroux, O.; Vissenberg, K.; Van der Straeten, D. The role of brassinosteroids in shoot gravitropism. Plant Physiol. 2011, 156, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Ivakov, A.; Flis, A.; Apelt, F.; Fünfgeld, M.; Scherer, U.; Stitt, M.; Kragler, F.; Vissenberg, K.; Persson, S.; Suslov, D. Cellulose synthesis and cell expansion are regulated by different mechanisms in growing Arabidopsis hypocotyls. Plant Cell 2017, 29, 1305–1315. [Google Scholar] [CrossRef]
- Goubet, F.; Barton, C.J.; Mortimer, J.C.; Yu, X.; Zhang, Z.; Miles, G.P.; Richens, J.; Liepman, A.H.; Seffen, K.; Dupree, P. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J. 2009, 60, 527–538. [Google Scholar] [CrossRef] [PubMed]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [CrossRef]
- Maris, A.; Kaewthai, N.; Eklöf, J.M.; Miller, J.G.; Brumer, H.; Fry, S.C.; Verbelen, J.P.; Vissenberg, K. Differences in enzymic properties of five recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 261–271. [Google Scholar] [CrossRef]
- Boron, A.K.; Van Loock, B.; Suslov, D.; Markakis, M.N.; Verbelen, J.P.; Vissenberg, K. Over-expression of AtEXLA2 alters etiolated arabidopsis hypocotyl growth. Ann. Bot. 2015, 115, 67–80. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Wang, X.; Durachko, D.M.; Zhang, S.; Cosgrove, D.J. Molecular insights into the complex mechanics of plant epidermal cell walls. Science 2021, 372, 706–711. [Google Scholar] [CrossRef]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef]
- Shah, D.U.; Reynolds, T.P.S.; Ramage, M.H. The strength of plants: Theory and experimental methods to measure the mechanical properties of stems. J. Exp. Bot. 2017, 68, 4497–4516. [Google Scholar] [CrossRef]
- Ray, P.M.; Green, P.B.; Cleland, R. Role of turgor in plant cell growth. Nature 1972, 239, 163–164. [Google Scholar] [CrossRef]
- Stewart, J.L.; Maloof, J.N.; Nemhauser, J.L. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS ONE 2011, 6, e19894. [Google Scholar] [CrossRef]
- Yu, L.; Yoshimi, Y.; Cresswell, R.; Wightman, R.; Lyczakowski, J.J.; Wilson, L.F.L.; Ishida, K.; Stott, K.; Yu, X.; Charalambous, S.; et al. Eudicot primary cell wall glucomannan is related in synthesis, structure, and function to xyloglucan. Plant Cell 2022, 34, 4600–4622. [Google Scholar] [CrossRef]
- Yu, L.; Shi, D.; Li, J.; Kong, Y.; Yu, Y.; Chai, G.; Hu, R.; Wang, J.; Hahn, M.G.; Zhou, G. CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed. Plant Physiol. 2014, 164, 1842–1856. [Google Scholar] [CrossRef]
- Jonsson, K.; Ma, Y.; Routier-Kierzkowska, A.L.; Bhalerao, R.P. Multiple mechanisms behind plant bending. Nat. Plants 2023, 9, 13–21. [Google Scholar] [CrossRef]
- Edelmann, H.G. Gravistimulated asymmetries in the outer epidermal cell walls of graviresponding coleoptiles. Planta 1997, 203 (Suppl. S1), S123–S129. [Google Scholar] [CrossRef]
- Bagshaw, S.L.; Cleland, R.E. Wall extensibility and gravitropic curvature of sunflower hypocotyls: Correlation between timing of curvature and changes in extensibility. Plant Cell Environ. 1990, 13, 85–89. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Rapid, bilateral changes in growth rate and curvature during gravitropism of cucumber hypocotyls: Implications for mechanism of growth control. Plant Cell Environ. 1990, 13, 227–234. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Arsuffi, G.; Braybrook, S.A. Acid growth: An ongoing trip. J. Exp. Bot. 2018, 69, 137–146. [Google Scholar] [CrossRef]
- Fendrych, M.; Leung, J.; Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. Elife 2016, 5, e19048. [Google Scholar] [CrossRef]
- Yu, L.; Lyczakowski, J.J.; Pereira, C.S.; Kotake, T.; Yu, X.; Li, A.; Mogelsvang, S.; Skaf, M.S.; Dupree, P. The Patterned Structure of Galactoglucomannan Suggests It May Bind to Cellulose in Seed Mucilage. Plant Physiol. 2018, 178, 1011–1026. [Google Scholar] [CrossRef]
- Blancaflor, E.B.; Hasenstein, K.H. Time course and auxin sensitivity of cortical microtubule reorientation in maize roots. Protoplasma 1995, 185, 72–82. [Google Scholar] [CrossRef]
- Himmelspach, R.; Wymer, C.L.; Lloyd, C.W.; Nick, P. Gravity-induced reorientation of cortical microtubules observed in vivo. Plant J. 1999, 18, 449–453. [Google Scholar] [CrossRef]
- Zhang, Z.; Friedman, H.; Meir, S.; Rosenberger, I.; Halevy, A.H.; Philosoph-Hadas, S. Microtubule reorientation in shoots precedes bending during the gravitropic response of cut snapdragon spikes. J. Plant Physiol. 2008, 165, 289–296. [Google Scholar] [CrossRef]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef]
- Liu, X.; Wolfe, R.; Welch, L.R.; Domozych, D.S.; Popper, Z.A.; Showalter, A.M. Bioinformatic Identification and Analysis of Extensins in the Plant Kingdom. PLoS ONE 2016, 11, e0150177. [Google Scholar] [CrossRef]
- Fry, S.C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem. J. 1982, 204, 449–455. [Google Scholar] [CrossRef]
- De la Torre, F.; Cañas, R.A.; Pascual, M.B.; Avila, C.; Cánovas, F.M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 2014, 65, 5527–5534. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Ricardi, M.M.; Poulsen, C.P.; Oikawa, A.; Dilokpimol, A.; Halim, A.; Mangano, S.; Denita Juarez, S.P.; Marzol, E.; Salgado Salter, J.D.; et al. Complex regulation of prolyl-4-hydroxylases impacts root hair expansion. Mol. Plant. 2015, 8, 734–746. [Google Scholar] [CrossRef]
- Clore, A.M.; Doore, S.M.; Tinnirello, S.M. Increased levels of reactive oxygen species and expression of a cytoplasmic aconitase/iron regulatory protein 1 homolog during the early response of maize pulvini to gravistimulation. Plant Cell Environ. 2008, 31, 144–158. [Google Scholar] [CrossRef]
- Krieger, G.; Shkolnik, D.; Miller, G.; Fromm, H. Reactive Oxygen Species Tune Root Tropic Responses. Plant Physiol. 2016, 172, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.L.; Mukherjee, A.; Kar, R.K. Early axis growth during seed germination is gravitropic and mediated by ROS and calcium. J. Plant Physiol. 2017, 216, 181–187. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Yang, T.; Lian, Y.; Sun, Y.; Bian, Z.; Wang, C. Exogenous hydrogen peroxide inhibits primary root gravitropism by regulating auxin distribution during Arabidopsis seed germination. Plant Physiol. Biochem. 2018, 128, 126–133. [Google Scholar] [CrossRef]
- Suslov, D.; Verbelen, J.P. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. J. Exp. Bot. 2006, 57, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Suslov, D.; Vissenberg, K. Cell wall expansion as viewed by the creep method. In Plant Biomechanics: From Structure to Function at Multiple Scales; Geitmann, A., Gril, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 305–320. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef]
- Hummel, J.; Strehmel, N.; Bölling, C.; Schmidt, S.; Walther, D.; Kopka, J. Mass Spectral Search and Analysis Using the Golm Metabolome Database. In The Handbook of Plant Metabolomics; Weckwerth, W., Kahl, G., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 321–343. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozhvanov, G.; Suslov, D. Sucrose and Mannans Affect Arabidopsis Shoot Gravitropism at the Cell Wall Level. Plants 2024, 13, 209. https://doi.org/10.3390/plants13020209
Pozhvanov G, Suslov D. Sucrose and Mannans Affect Arabidopsis Shoot Gravitropism at the Cell Wall Level. Plants. 2024; 13(2):209. https://doi.org/10.3390/plants13020209
Chicago/Turabian StylePozhvanov, Gregory, and Dmitry Suslov. 2024. "Sucrose and Mannans Affect Arabidopsis Shoot Gravitropism at the Cell Wall Level" Plants 13, no. 2: 209. https://doi.org/10.3390/plants13020209
APA StylePozhvanov, G., & Suslov, D. (2024). Sucrose and Mannans Affect Arabidopsis Shoot Gravitropism at the Cell Wall Level. Plants, 13(2), 209. https://doi.org/10.3390/plants13020209





