Effects of Exogenous Isosteviol on the Physiological Characteristics of Brassica napus Seedlings under Salt Stress
Abstract
:1. Introduction
2. Results
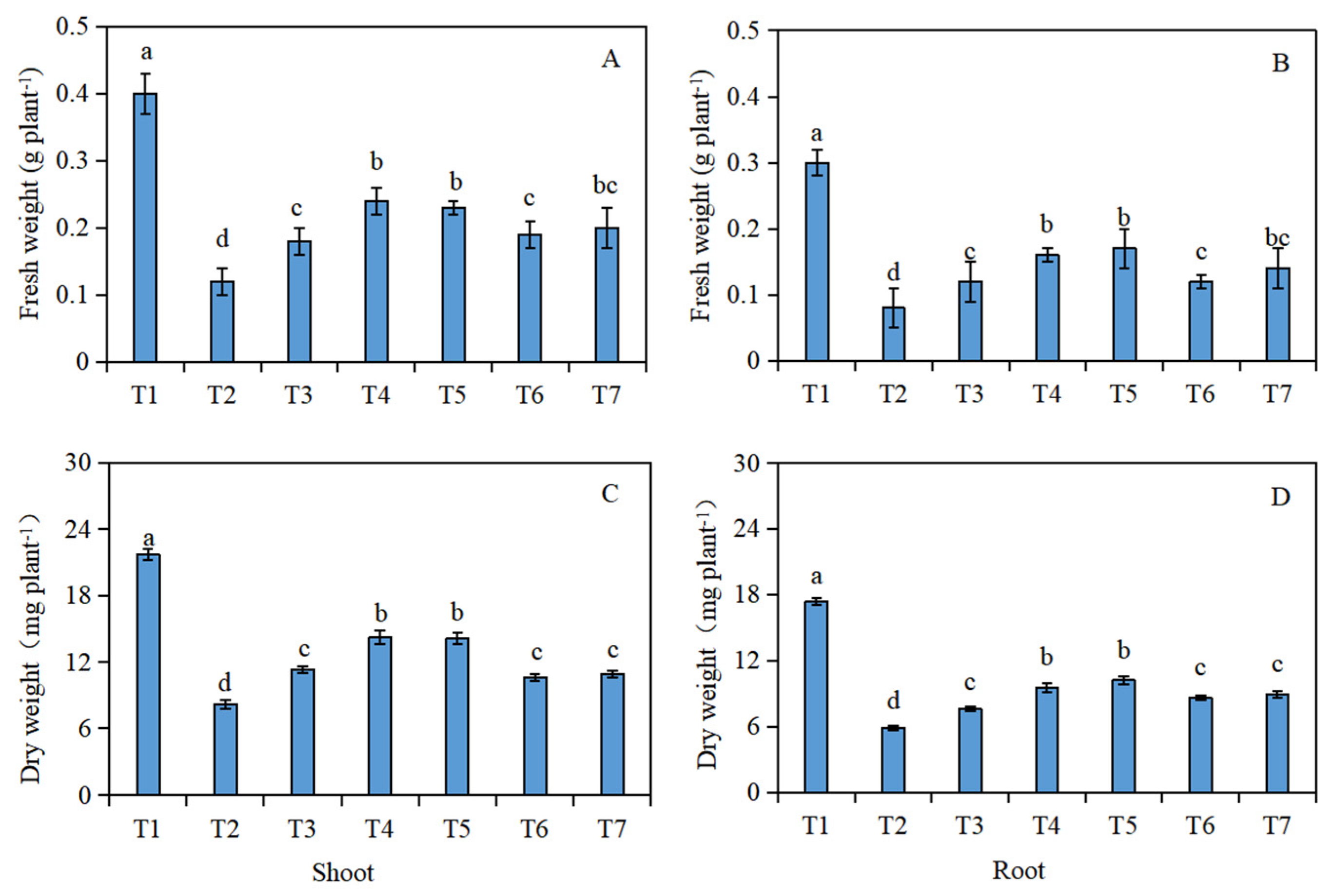
2.1. Biomass of Seedlings
2.2. Contents of Osmotic Substances in the Shoots and Roots
2.2.1. Glycine Betaine Contents in the Shoots and Roots
2.2.2. Proline Content in the Shoots and Roots
2.2.3. Soluble Protein Contents in the Shoots and Roots
2.3. Na+, K+, Cl− Contents in the Shoots and Roots
2.4. ROS Contents in the Shoots and Roots
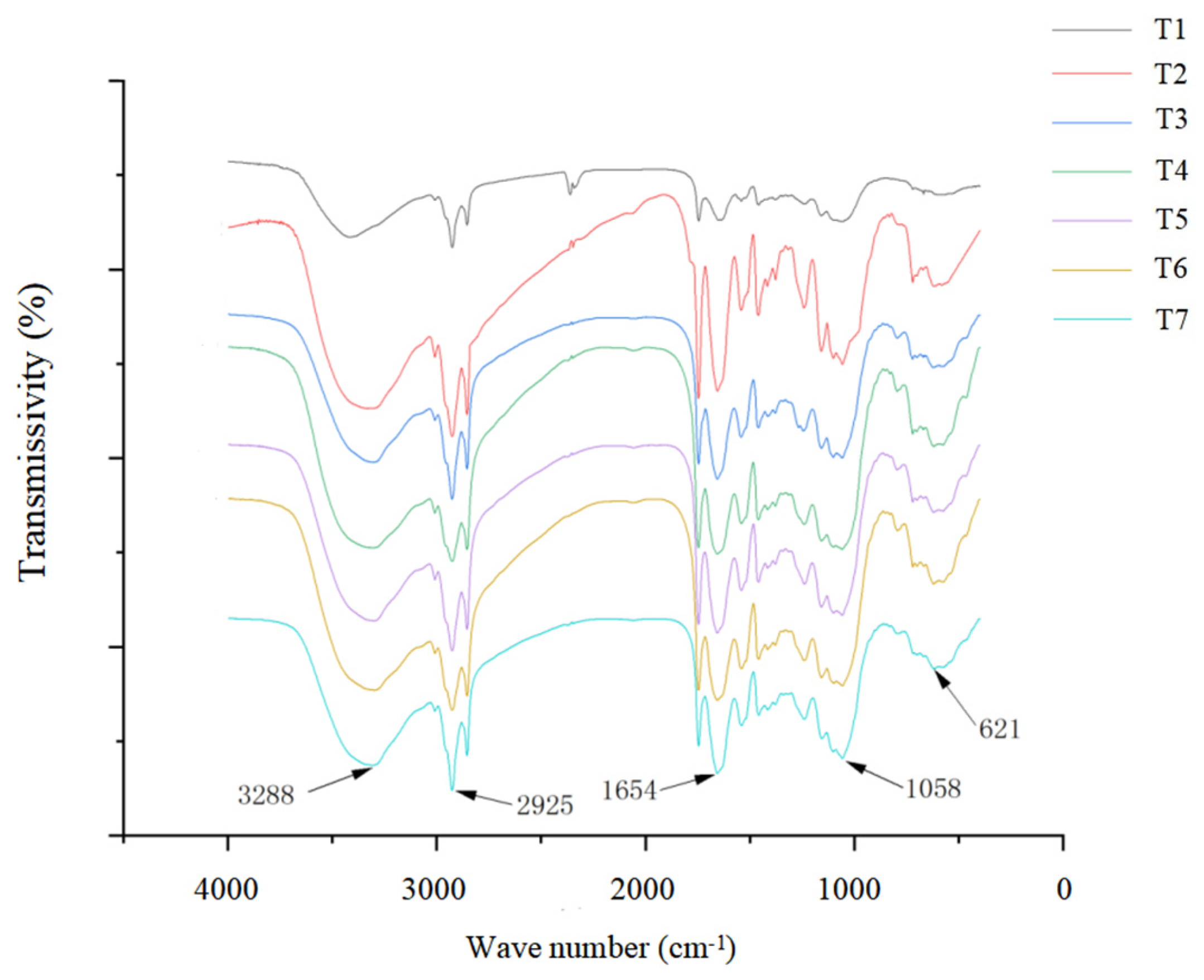
2.5. Spectroscopy Analysis of Seedling Tissues
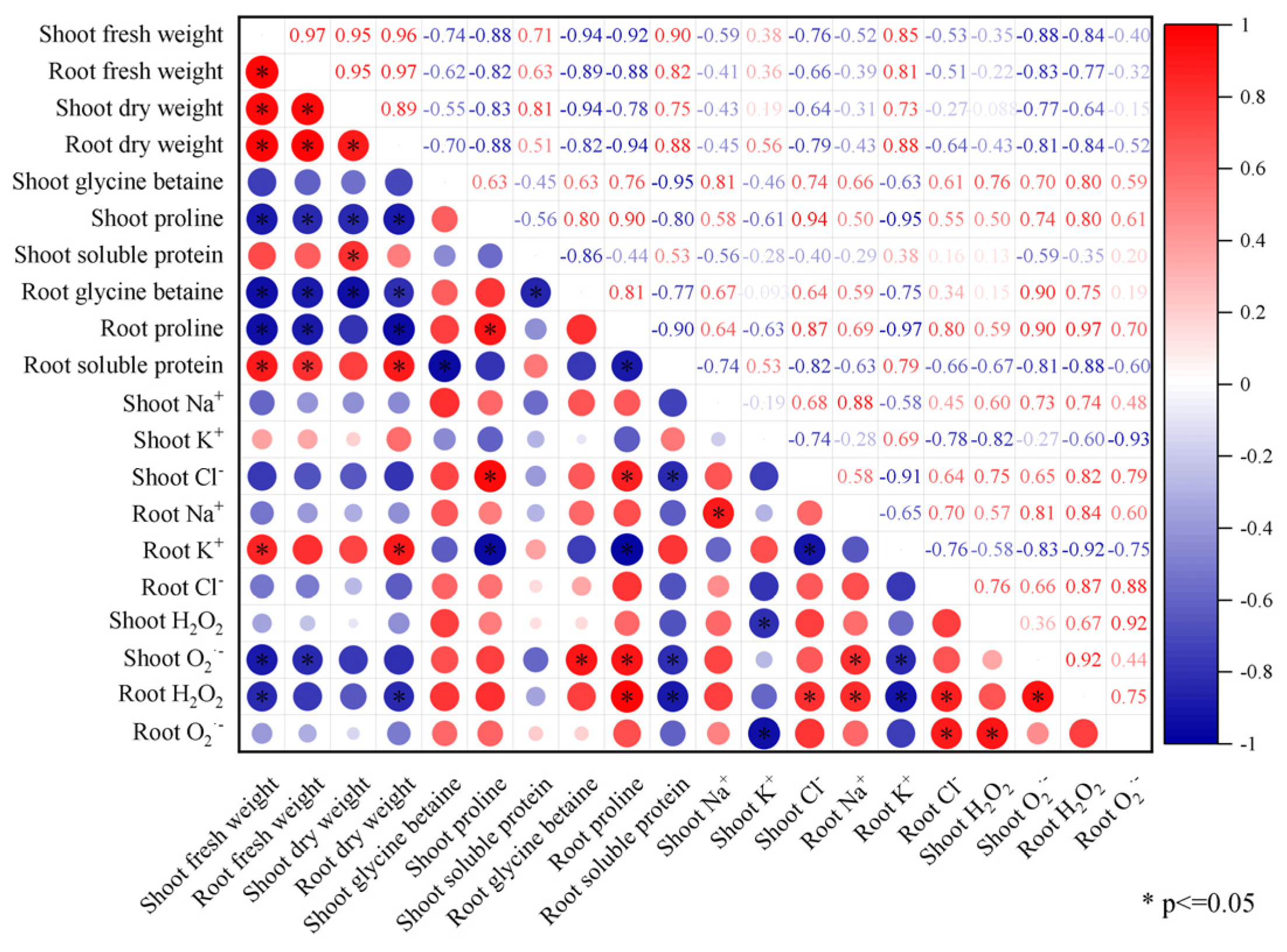
2.6. Correlation Analysis of Physiological Indicators
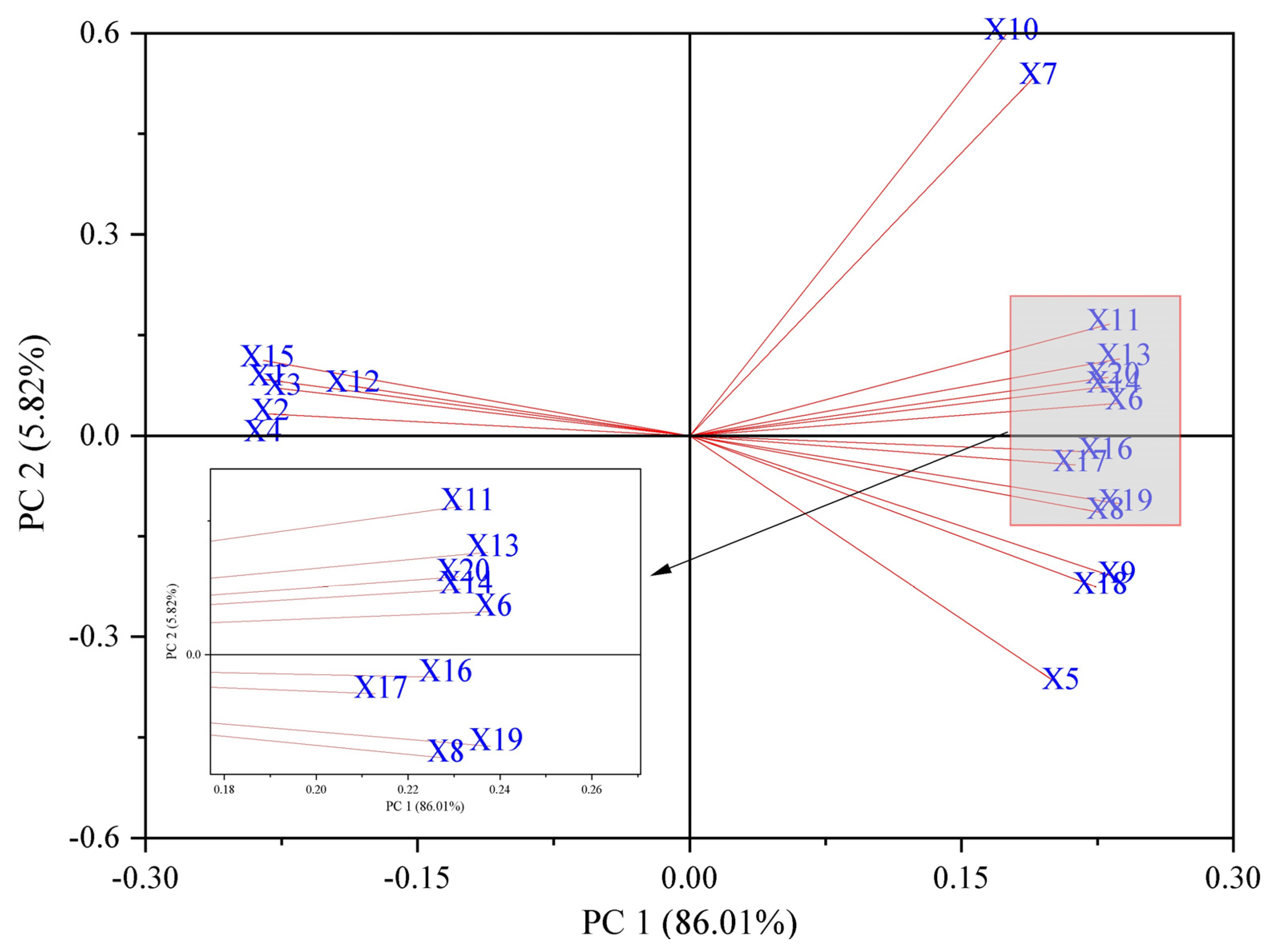
2.7. Principal Component Analysis
3. Discussion
3.1. Biomass
3.2. Osmotic Substance
3.3. Absorption and Distribution of Na+, K+, and Cl−
3.4. Generation of ROS
4. Conclusions
5. Materials and Methods
5.1. Test Materials
5.2. Experimental Design
5.3. Sampling and Sample Analysis
5.3.1. Determination of Seedling Biomass
5.3.2. Determination of Shoot and Root Osmotic Substances
5.3.3. Determination of Na+, K+, and Cl− in the Shoots and Roots
5.3.4. Determination of Shoot and Root ROS
5.3.5. Spectroscopy Analysis
5.4. Data Statistics and Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frukh, A.; Siddiqi, T.O.; Khan, M.I.R.; Ahmad, A. Modulation in growth, biochemical attributes and proteome profile of rice cultivars under salt stress. Plant Physiol. Biochem. 2020, 146, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Javed, Q.; Sun, J.; Azeem, A.; Ullah, I.; Huang, P.; Kama, R.; Jabran, K.; Du, D. The enhanced tolerance of invasive Alternanthera philoxeroides over native species under salt-stress in China. Appl. Ecol. Environ. Res. 2019, 17, 14767–14785. [Google Scholar] [CrossRef]
- Kawa, D.; Meyer, A.J.; Dekker, H.L.; Abd-El-Haliem, A.M.; Gevaert, K.; Van De Slijke, E.; Maszkowska, J.; Bucholc, M.; Dobrowolska, G.; De Jaeger, G.; et al. SnRK2 protein kinases and mRNA decapping machinery control root development and response to salt. Plant Physiol. 2020, 182, 361–377. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Biol. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Bao, J.; Yuan, F.; Liang, X.; Feng, Z.T.; Wang, B.S. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016, 79, 391–399. [Google Scholar] [CrossRef]
- Soares, T.F.S.N.; Dias, D.C.F.D.S.; Oliveira, A.M.S.; Ribeiro, D.M.; Dias, L.A.D.S. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rodrigues, V.; Verma, S.; Singh, M.; Hiremath, C.; Shanker, K.; Shukla, A.K.; Sundaresan, V. Effect of salt stress on seed germination, morphology, biochemical parameters, genomic template stability, and bioactive constituents of Andrographis paniculata Nees. Acta Physiol. Plant. 2021, 43, 68. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- El Hamdaoui, A.; Mechqoq, H.; El Yaagoubi, M.; Bouglad, A.; Hallouti, A.; El Mousadik, A.; El Aouad, N.; Aoumar, A.A.B.; Msanda, F. Effect of pretreatment, temperature, gibberellin (GA3), salt and water stress on germination of Lavandula mairei Humbert. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100314. [Google Scholar] [CrossRef]
- Ge, H.L.; Zhang, F.L. Growth-promoting ability of Rhodopseudomonas palustris G5 and its effect on induced resistance in cucumber against salt stress. J. Plant Growth Regul. 2019, 38, 180–188. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Khan, S.U.; Din, J.; Zhang, J.L. Alleviation of salt stress by seed treatment with abscisic acid (ABA), 6-benzylaminopurine (BA) and chlormequat chloride (CCC) optimizes ion and organic matter Accumulation and increases yield of rice (Oryza sativa L.). Aust. J. Crop Sci. 2011, 5, 1278–1285. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Du, W.; Zhao, L.; Davey, A.K.; Wang, J. The neuroprotective effects of isosteviol against focal cerebral ischemia injury induced by middle cerebral artery occlusion in rats. Planta Medica 2008, 74, 816–821. [Google Scholar] [CrossRef]
- An, Y.J.; Zhang, Y.X.; Wu, Y.; Liu, Z.M.; Pi, C.; Tao, J.C. Cheminform abstract: Simple amphiphilic isosteviol–proline conjugates as chiral catalysts for the direct asymmetric aldol reaction in the presence of water. Cheminform 2010, 21, 688–694. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.T.; Xia, W.J.; Xu, X.F. Effects of isosteviol soaking on seed germination and seedling growth of rapeseed under salt stress. Fujian Agric. J. 2020, 35, 883–890. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, B.B. Effect of isosteviol on wheat seed germination and seedling growth under cadmium stress. Plants 2021, 10, 1779. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, J.W.; Mou, Y.; Wen, S.; Rui, H.Y. Effects of isosteviol immersion seed on growth of wheat seedlings under chromium stress. J. Shandong Agric. Univ. 2022, 53, 711–718. [Google Scholar] [CrossRef]
- Du, Y.W.; Liu, L.; Feng, N.J.; Zheng, D.F.; Liu, M.L.; Zhou, H.; Deng, P.; Wang, Y.X.; Zhao, H.M. Combined transcriptomic and metabolomic analysis of alginate oligosaccharides alleviating salt stress in rice seedlings. BMC Plant Biol. 2023, 23, 455. [Google Scholar] [CrossRef]
- Hunpatin, O.S.; Yuan, G.; Nong, T.; Shi, C.; Wu, X.; Liu, H.; Ning, Y.; Wang, Q. The roles of calcineurin B-like proteins in plants under salt stress. Int. J. Mol. Sci. 2023, 24, 16958. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Siddiqi, E.H.; Ahmad, S.; Zeb, U.; Muhammad, I.; Khan, H.; Zhao, G.F.; Li, Z.H. Exogenous application of indole-3-acetic acid to ameliorate salt induced harmful effects on four eggplants (Solanum melongena L.) varieties. Sci. Hortic. 2022, 292, 110662. [Google Scholar] [CrossRef]
- Han, A.; Wang, C.; Li, J.; Xu, L.; Guo, X.; Li, W.; Zhou, F.; Liu, R. Physiological mechanism of sodium salicylate and folcisteine on alleviating salt stress in wheat seedlings. Sci. Rep. 2023, 13, 22869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.Y.; Wang, D.; Li, J.W.; Peng, W.L.; Rui, H.Y. Effects of isosteviol on growth of wheat seedlings under salt stress. Crops 2022, 38, 141–145. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Cheng, J.; Guo, W.; Li, Y.; Li, C. Physiological and proteomic analysis of seed germination under salt stress in mulberry. Front. Biosci. 2023, 28, 49. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Fan, G.; Li, X.; Deng, M.; Zhao, Z.; Yang, L. Comparative analysis and identification of miRNAs and their target genes responsive to salt stress in diploid and tetraploid Paulownia fortunei seedlings. PLoS ONE 2016, 11, e0149617. [Google Scholar] [CrossRef]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, Y.Z.; Xing, L.X.; Liu, J.X.; Wang, R.M. Effects of GA3 on root growth and osmotic regulation of Lübaonuo Broomcorn Millet seedlings under salt stress. Crops 2022, 38, 98–104. [Google Scholar] [CrossRef]
- Kamran, M.; Wang, D.; Xie, K.; Lu, Y.; Shi, C.; El Sabagh, A.; Gu, W.; Xu, P. Presowing seed treatment with kinetin and calcium mitigates salt induced inhibition of seed germination and seedling growth of choysum (Brassica rapa var. parachinensis). Ecotoxicol. Environ. Saf. 2021, 227, 112921. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Kareem, H.A.; Hussain, S.; Guo, Z.; Niu, J.; Roy, M.; Saleem, S.; Wang, Q. Enhanced salinity tolerance in Alfalfa through foliar nano-zinc oxide application: Mechanistic insights and potential agricultural applications. Rhizosphere 2023, 28, 100792. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Wang, Z.J.; Fang, Y.; Sun, W.C.; Yuan, J.H.; Mi, C.; Fang, Y.; Wu, J.Y.; Li, X.C. Effect of salt stress on seed germination and seedling physiology of winter rapeseed (Brassica rapa L.). Chin. J. Oil Crop Sci. 2017, 39, 351–359. [Google Scholar] [CrossRef]
- Zhu, Y.; He, C.; Du, W.; Hu, Y.; Chen, Y. Effects of exogenous calcum on the seed germination and seedling ions distribution of Festuca arundinacea under salt stress. Trans. Chin. Soc. Agric. Eng. 2007, 23, 133–137. [Google Scholar] [CrossRef]
- Shkolnik, D.; Finkler, A.; Pasmanik-Chor, M.; Fromm, H. Calmodulin-binding transcription activator 6: A key regulator of Na+ homeostasis during germination. Plant Physiol. 2019, 180, 1101–1118. [Google Scholar] [CrossRef]
- Chandran, A.E.J.; Finkler, A.; Hait, T.A.; Kiere, Y.; David, S.; Pasmanik-Chor, M.; Shkolnik, D. Calcium regulation of the Arabidopsis Na+/K+ transporter HKT1; 1 improves seed germination under salt stress. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Yıldıztugay, E.; Sekmen, A.H.; Turkan, I.; Kucukoduk, M. Elucidation of physiological and biochemical mechanisms of an endemic halophyte Centaurea tuzgoluensis under salt stress. Plant Physiol. Biochem. 2011, 49, 816–824. [Google Scholar] [CrossRef]
- Ghouri, F.; Shahid, M.J.; Liu, J.; Lai, M.; Sun, L.; Wu, J.; Liu, X.; Ali, S.; Shahid, M.Q. Polyploidy and zinc oxide nanoparticles alleviated Cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes. J. Hazard. Mater. 2023, 448, 130991. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, X.; Sun, H. Transcriptomic mechanism for foliar applied nano-ZnO alleviating phytotoxicity of nanoplastics in corn (Zea mays L.) plants. Sci. Total Environ. 2023, 905, 166818. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, L.; Jenifer, M.A.; Chandrasekaran, N.; Suraishkumar, G.K.; Mukherjee, A. Polystyrene nanoplastics diminish the toxic effects of Nano-TiO2 in marine algae Chlorella sp. Environ. Res. 2022, 204, 112400. [Google Scholar] [CrossRef]
- Wen, F.P.; Zhang, Z.H.; Bai, T.; Xu, Q.; Pan, Y.H. Proteomics reveals the effects of gibberellic acid (GA3) on salt-stressed rice (Oryza sativa L.) shoots. Plant Sci. 2010, 178, 170–175. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Liu, Z.; Chen, L.; Tang, S.; Ding, C.; Jiang, Y.; Li, G. Melatonin regulates antioxidant strategy in response to continuous salt stress in rice seedlings. Plant Physiol. Biochem. 2021, 165, 239–250. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Kim, H.J.; Tewolde, H.; Zhang, H. Fourier transform infrared spectral features of plant biomass components during cotton organ development and their biological implications. J. Cotton Res. 2022, 5, 11. [Google Scholar] [CrossRef]
- Ye, W.; Wu, F.; Zhang, G.; Fang, Q.; Hu, H. Calcium decreases cadmium concentration in root but facilitates cadmium translocation from root to shoot in rice. J. Plant Growth Regul. 2020, 39, 422–429. [Google Scholar] [CrossRef]
- Yan, L.; Jiang, C.; Riaz, M.; Wu, X.; Lu, X.; Du, C.; Wang, Y. Mitigative effect of different forms of boron on aluminum toxicity of rape seedlings and its FTIR characteristics. Acta Agron. Sin. 2017, 43, 1817–1826. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Charfeddine, G.; Hatem, F.; Douglas, G.; Faten, G. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef]
- Valadez-Bustos, M.G.; Aguado-Santacruz, G.A.; Tiessen-Favier, A.; Robledo-Paz, A.; Muñoz-Orozco, A.; Rascón-Cruz, Q.; Santacruz-Varela, A. A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal. Biochem. 2016, 498, 47–52. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Hassan, F.A.S. How salt stress-responsive proteins regulate plant adaptation to saline conditions. Plant Mol. Biol. 2022, 108, 175–224. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Bose, J.; Shabala, L.; Eyles, A.; Shabala, S. Evaluating relative contribution of osmotolerance and tissue tolerance mechanisms toward salinity stress tolerance in three Brassica species. Physiol. Plant. 2016, 158, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Velikova, M.; Bankova, V.; Sorkun, K.; Houcine, S.; Tsvetkova, I.; Kujumgiev, A. Propolis from the Mediterranean region: Chemical composition and antimicrobial activity. Z. Naturforschung C J. Biosci. 2000, 55, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Lin, Y.X.; Lin, H.T.; Zhang, S.; Chen, Y.H.; Shi, J. Inhibitory effects of propyl gallate on browning and its relationship to active oxygen metabolism in pericarp of harvested longan fruit. Food Sci. Technol. 2015, 60, 1122–1128. [Google Scholar] [CrossRef]
- Estevanes, J.; Day, J.; Balaraman, R.; Buzzini, P.; Monjardez, G. Forensic characterization and differentiation of automotive lubricating greases using Fourier transform infrared spectroscopy and scanning electron microscopy-energy dispersive X-ray spectroscopy. Forensic Chem. 2023, 34, 100502. [Google Scholar] [CrossRef]



| Treatment | Shoot (mg g−1 DW) | Root (mg g−1 DW) | ||||
|---|---|---|---|---|---|---|
| Na+ | K+ | Cl− | Na+ | K+ | Cl− | |
| T1 | 1.2 ± 0.1 e | 38.7 ± 0.8 a | 2.1 ± 0.2 e | 1.6 ± 0.1 e | 15.6 ± 0.7 a | 1.5 ± 0.1 f |
| T2 | 59.1 ± 2.7 a | 13.6 ± 0.4 f | 99.3 ± 1.8 a | 99.9 ± 1.5 a | 7.2 ± 0.3 d | 63.8 ± 1.9 a |
| T3 | 51.2 ± 1.7 c | 16.4 ± 0.6 e | 85.1 ± 1.4 b | 70.9 ± 0.9 c | 10.2 ± 0.5 c | 49.6 ± 0.9 b |
| T4 | 46.0 ± 1.2 d | 16.7 ± 0.5 e | 78.0 ± 2.1 c | 70.5 ± 1.3 c | 10.4 ± 0.4 bc | 49.6 ± 1.2 b |
| T5 | 56.5 ± 0.9 b | 28.8 ± 0.8 c | 78.0 ± 1.4 c | 89.4 ± 1.4 b | 11.2 ± 0.5 b | 42.5 ± 0.8 c |
| T6 | 48.6 ± 0.7 cd | 34.9 ± 1.0 b | 70.9 ± 1.5 d | 71.2 ± 1.2 c | 11.0 ± 0.6 b | 35.5 ± 0.7 d |
| T7 | 51.2 ± 1.4 c | 25.7 ± 0.7 d | 85.1 ± 1.9 b | 65.9 ± 1.8 d | 10.4 ± 0.3 bc | 28.4 ± 0.9 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Meng, W.; Peng, Y.; Qin, Y.; Zhang, L.; Zhu, N. Effects of Exogenous Isosteviol on the Physiological Characteristics of Brassica napus Seedlings under Salt Stress. Plants 2024, 13, 217. https://doi.org/10.3390/plants13020217
Xia W, Meng W, Peng Y, Qin Y, Zhang L, Zhu N. Effects of Exogenous Isosteviol on the Physiological Characteristics of Brassica napus Seedlings under Salt Stress. Plants. 2024; 13(2):217. https://doi.org/10.3390/plants13020217
Chicago/Turabian StyleXia, Wenjing, Wangang Meng, Yueqin Peng, Yutian Qin, Liang Zhang, and Nianqing Zhu. 2024. "Effects of Exogenous Isosteviol on the Physiological Characteristics of Brassica napus Seedlings under Salt Stress" Plants 13, no. 2: 217. https://doi.org/10.3390/plants13020217
APA StyleXia, W., Meng, W., Peng, Y., Qin, Y., Zhang, L., & Zhu, N. (2024). Effects of Exogenous Isosteviol on the Physiological Characteristics of Brassica napus Seedlings under Salt Stress. Plants, 13(2), 217. https://doi.org/10.3390/plants13020217






