NMR Metabolomics and Chemometrics of Commercial Varieties of Phaseolus vulgaris L. Seeds from Italy and In Vitro Antioxidant and Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolomic Analysis: NMR Assignment of the Aqueous Extracts
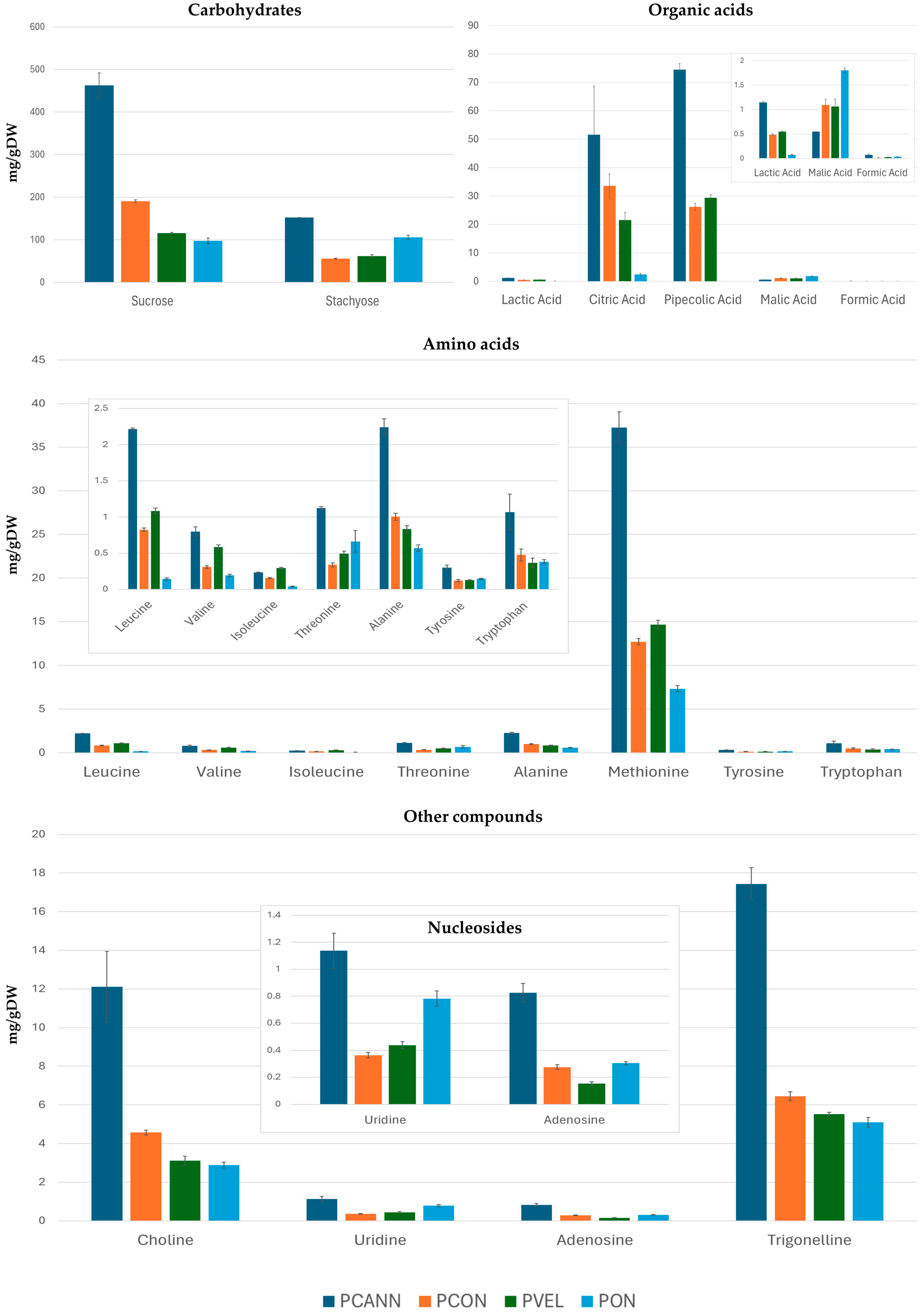
2.1.1. Free Amino Acids
2.1.2. Carbohydrates
2.1.3. Organic Acids
2.1.4. Other Components
2.2. NMR Assignment of the Organic Extracts
2.3. Quantitative Analysis
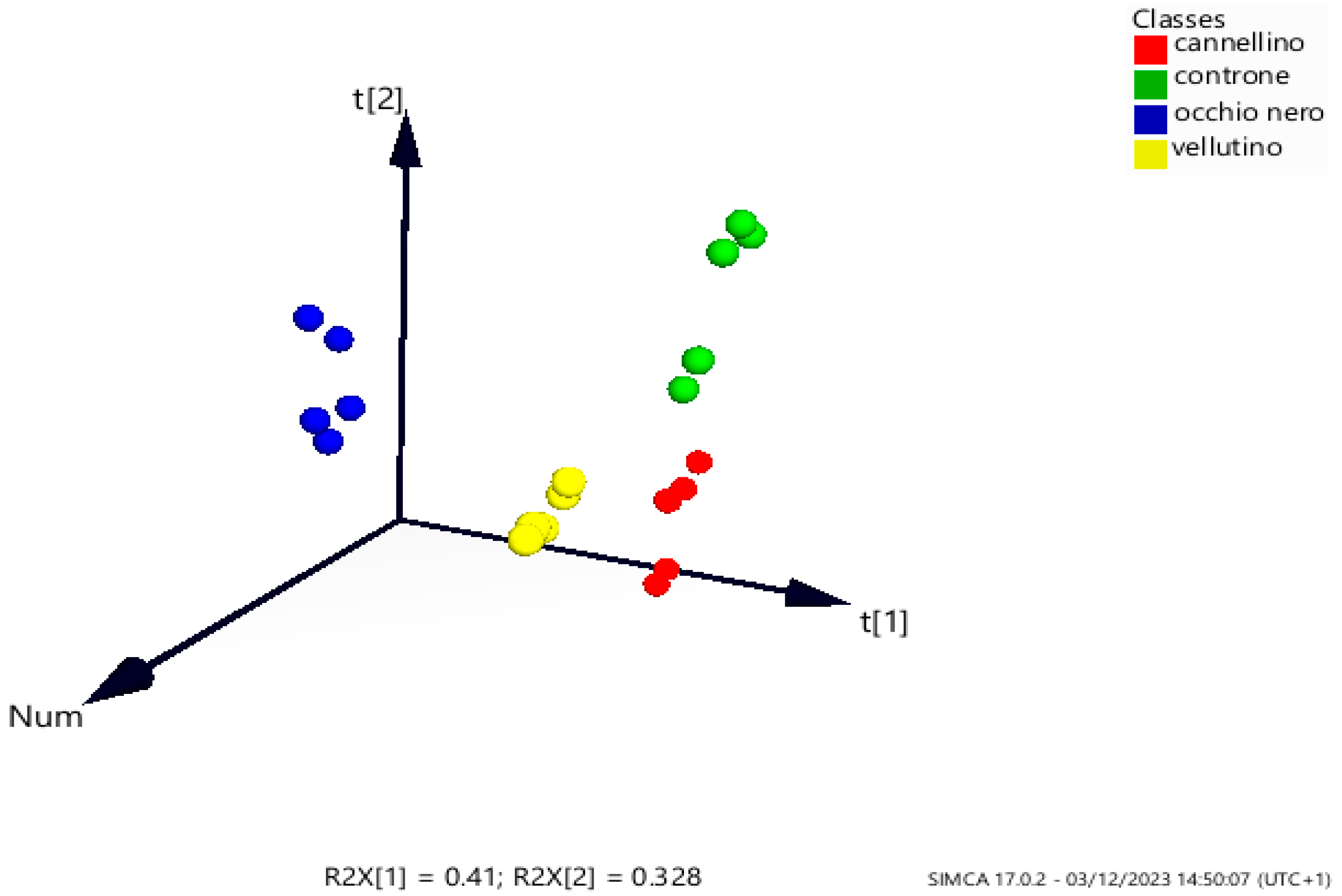
2.4. Chemometric Analysis
2.5. Phenolic Components and Antioxidant Activity
2.6. In Vitro Antioxidant Activity
2.7. Correlation between Phenolic Compounds and the Antioxidant Activity of the Extract of Phaseolus Seeds
2.8. Antifulgal Activity
3. Materials and Methods
3.1. General Information
3.2. Standards and Reagents
3.3. Plant Material and Extraction Procedure
3.4. Nuclear Magnetic Resonance (NMR) Experiments
3.5. Sample Preparation and NMR Analyses
3.6. Metabolite Identification
3.7. Quantification of Metabolites (qNMR)
3.8. Chemometric Analysis
3.9. Extraction of Bean Samples
3.9.1. Folin-Ciocalteu Assay: Determination of Total Phenolic, Flavonoid Content and Condensed Tannins Content
3.9.2. Total Flavonoid Content (TFC)
3.9.3. Condensed Tannins (TCT)
3.10. Antioxidant Capacity
3.10.1. DPPH Radical Scavenging Activity
3.10.2. ABTS Radical Scavenging Activity
3.10.3. FRAP Assay
3.10.4. Statistical Analysis
3.11. Determination of Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legume Sci. 2022, 5, e155. [Google Scholar] [CrossRef]
- Silva-Cristobal, L.; Osorio-Díaz, P.; Tovar, J.; Bello-Pérez, L.A. Chemical composition, carbohydrate digestibility, and antioxidant capacity of cooked black bean, chickpea, and lentil Mexican varieties Composición química, digestibilidad de carbohidratos, y capacidad antioxidante de variedades mexicanas cocidas de frijol negro, garbanzo, y lenteja. CyTA J. Food 2010, 8, 7–14. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sánchez, E. Bioactive Compounds from Mexican Varieties of the Common Bean (Phaseolus vulgaris): Implications for Health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physical and cooking characteristics of some Indian kidney bean (Phaseolus vulgaris L.) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 7–15. [Google Scholar] [CrossRef]
- Frahm, M.A.; Rosas, J.C.; Mayek-Pérez, N.; López-Salinas, E.; Acosta-Gallegos, J.A.; Kelly, J.D. Breeding beans for resistance to terminal drought in the Lowland tropics. Euphytica 2004, 136, 223–232. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The Nutritional Content of Common Bean (Phaseolus vulgaris L.) Landraces in Comparison to Modern Varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef]
- Singh, S.P. Broadening the Genetic Base of Common Bean Cultivars. Crop Sci. 2001, 41, 1659–1675. [Google Scholar] [CrossRef]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- Rehman, Z.-u.; Salariya, A.M.; Zafar, S.I. Effect of processing on available carbohydrate content and starch digestibility of kidney beans (Phaseolus vulgaris L.). Food Chem. 2001, 73, 351–355. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; García-Gasca, T.; Yousef, G.G.; Lila, M.A.; González de Mejia, E.; Loarca-Pina, G. Chemopreventive Activity of Polyphenolics from Black Jamapa Bean (Phaseolus vulgaris L.) on HeLa and HaCaT Cells. J. Agric. Food Chem. 2006, 54, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant Activity in Common Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef] [PubMed]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, 437S–442S. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 61, 85–96. [Google Scholar] [CrossRef]
- Kimothi, S.; Dhaliwal, Y.S. Nutritional and Health Promoting Attribute of Kidney Beans (Phaseolus vulgaris L.): A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1201–1209. [Google Scholar] [CrossRef]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases—Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef]
- Long, J.; Zhang, J.; Zhang, X.; Wu, J.; Chen, H.; Wang, P.; Wang, Q.; Du, C. Genetic Diversity of Common Bean (Phaseolus vulgaris L.) Germplasm Resources in Chongqing, Evidenced by Morphological Characterization. Front. Genet. 2020, 11, 697. [Google Scholar] [CrossRef]
- Prolla, I.R.D.; Barbosa, R.G.; Veeck, A.P.L.; Augusti, P.R.; Silva, L.P.d.; Ribeiro, N.D.; Emanuelli, T. Cultivar, harvest year, and storage conditions affecting nutritional quality of common beans (Phaseolus vulgaris L.). Ciênc. Tecnol. Aliment. 2010, 30, 96–102. [Google Scholar] [CrossRef]
- Delouche, J.C.; Baskin, C.C. Accelerated Aging Techniques for Predicting the Relative Storability of Seed Lots; Mississippi State University: Starkville, MS, USA, 2021. [Google Scholar]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Kotha, R.R.; Finley, J.W.; Luthria, D.L. Determination of Soluble Mono, Di, and Oligosaccharide Content in 23 Dry Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2020, 68, 6412–6419. [Google Scholar] [CrossRef]
- De Mejía, E.G.; Guzmán-Maldonado, S.H.; Acosta-Gallegos, J.A.; Reynoso-Camacho, R.; Ramírez-Rodríguez, E.; Pons-Hernández, J.L.; González-Chavira, M.M.; Castellanos, J.Z.; Kelly, J.D. Effect of Cultivar and Growing Location on the Trypsin Inhibitors, Tannins, and Lectins of Common Beans (Phaseolus vulgaris L.) Grown in the Semiarid Highlands of Mexico. J. Agric. Food Chem. 2003, 51, 5962–5966. [Google Scholar] [CrossRef] [PubMed]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; et al. Raffinose Family Oligosaccharides: Friend or Foe for Human and Plant Health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef]
- Hill, G.D. Plant Antinutritional Factors|Characteristics. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4578–4587. [Google Scholar]
- Acosta-Gallegos, J.A.; Kelly, J.D.; Gepts, P. Prebreeding in Common Bean and Use of Genetic Diversity from Wild Germplasm. Crop Sci. 2007, 47, S44–S59. [Google Scholar] [CrossRef]
- Sparvoli, F.; Giofré, S.; Cominelli, E.; Avite, E.; Giuberti, G.; Luongo, D.; Gatti, E.; Cianciabella, M.; Daniele, G.M.; Rossi, M.; et al. Sensory Characteristics and Nutritional Quality of Food Products Made with a Biofortified and Lectin Free Common Bean (Phaseolus vulgaris L.) Flour. Nutrients 2021, 13, 4517. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Bioactive phenolic compounds and functional properties of dehydrated bean flours. Food Res. Int. 2011, 44, 774–780. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Vignolini, P.; Falvino, M.; Heimler, D.J.I.J.o.F.S. Polyphenol content and antiradical activity of “Sarconi” beans (Phaseolus vulgaris L.) ecotype. Ital. J. Food Sci. 2013, 25, 251–373. [Google Scholar]
- Gan, R.-Y.; Deng, Z.-Q.; Yan, A.-X.; Shah, N.P.; Lui, W.-Y.; Chan, C.-L.; Corke, H. Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. LWT 2016, 73, 168–177. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G.J.F.C. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Liu, Z.; Xie, M.J.J.o.F.F. Antioxidant activities and anthocyanins composition of seed coats from twenty-six kidney bean cultivars. J. Funct. Foods 2016, 26, 622–631. [Google Scholar] [CrossRef]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; de Mejía, E.G.; Martín-Cabrejas, M.A. Black bean coats: New source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Emmambux, M.N. REVIEW: Developments in Our Understanding of Sorghum Polysaccharides and Their Health Benefits. Cereal Chem. 2010, 87, 263–271. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Qi, X.; Wu, G. Structural characterization and antioxidant activity of condensed tannins fractionated from sorghum grain. J. Cereal Sci. 2020, 92, 102918. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhang, X.; Chen, G.-L.; Yu, J.; Yang, L.-Q.; Gao, Y.-Q.J.J.o.F.F. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. Food Chem. 2016, 24, 359–372. [Google Scholar] [CrossRef]
- AquinoBolaos, E.; GarcaDaz, Y.; ChavezServia, J.; CarrilloRodrguez, J.; VeraGuzman, A.; HerediaGarcia, E. Anthocyanins, polyphenols, flavonoids and antioxidant activity in common bean (Phaseolus vulgaris L.) landraces. Emir. J. Food Agric. 2016, 28, 581. [Google Scholar] [CrossRef]
- Martínez-Alonso, C.; Taroncher, M.; Castaldo, L.; Izzo, L.; Rodríguez-Carrasco, Y.; Ritieni, A.; Ruiz, M.-J. Effect of Phenolic Extract from Red Beans (Phaseolus vulgaris L.) on T-2 Toxin-Induced Cytotoxicity in HepG2 Cells. Foods 2022, 11, 1033. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Campa Negrillo, A.; Suárez Valles, B.; Ferreira Fernández, J.J. Phenolic Content and Antioxidant Activity in Seeds of Common Bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of broad bean seed extract and its phenolic composition. J. Funct. Foods 2017, 38, 656–662. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; Reynoso-Camacho, R.; Castaño-Tostado, E.; García-Gasca, T.; González de Mejía, E.; Guzmán-Maldonado, S.H.; Elizondo, G.; Yousef, G.G.; Lila, M.A.; Loarca-Pina, G. Antiradical capacity and induction of apoptosis on HeLa cells by a Phaseolus vulgaris extract. Plant Foods Hum. Nutr. 2008, 63, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Longo-Mbenza, B.; Muaka, M. The protective effect of phaseolus vulgaris on cataract in type 2 diabetes: A profitable hypothesis. Med. Hypothesis Discov. Innov. Ophthalmol. 2013, 2, 105. [Google Scholar] [PubMed]
- Nosworthy, M.G.; Neufeld, J.; Frohlich, P.; Young, G.; Malcolmson, L.; House, J.D. Determination of the protein quality of cooked Canadian pulses. Food Sci. Nutr. 2017, 5, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Tomiyama, Y.; Kita, S.; Mizushina, Y. Lipid classes, fatty acid composition and triacylglycerol molecular species of kidney beans (Phaseolus vulgaris L.). Eur. J. Lipid Sci. Technol. 2005, 107, 307–315. [Google Scholar] [CrossRef]
- Cominelli, E.; Rodiño, A.P.; De Ron, A.M.; Sparvoli, F. Genetic approaches to improve common bean nutritional quality: Current knowledge and future perspectives. In Quality Breeding in Field Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 109–138. [Google Scholar]
- Rodhouse, J.; Haugh, C.; Roberts, D.; Gilbert, R.J. Red kidney bean poisoning in the UK: An analysis of 50 suspected incidents between 1976 and 1989. Epidemiol. Infect. 1990, 105, 485–491. [Google Scholar] [CrossRef]
- Van Damme, E.J.; Lannoo, N.; Fouquaert, E.; Peumans, W.J. The identification of inducible cytoplasmic/nuclear carbohydrate-binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconj. J. 2003, 20, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) Regulated Plant Defense: Mechanisms and Opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N.; et al. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2020, 16, 1853384. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.-Y. The Changes in GABA, GAD and DAO Activities, and Microbial Safety of Soaking- and High Voltage Electric Field-Treated Adzuki Bean Sprouts. Agriculture 2022, 12, 469. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Brites, C.; Rosa, E.A.S.; Barros, A.I.R.N.A. Comparison of near-infrared (NIR) and mid-infrared (MIR) spectroscopy for the determination of nutritional and antinutritional parameters in common beans. Food Chem. 2020, 306, 125509. [Google Scholar] [CrossRef]
- Hernández-Guerrero, C.J.; Villa-Ruano, N.; Zepeda-Vallejo, L.G.; Hernández-Fuentes, A.D.; Ramirez-Estrada, K.; Zamudio-Lucero, S.; Hidalgo-Martínez, D.; Becerra-Martínez, E.J.F.R.I. Bean cultivars (Phaseolus vulgaris L.) under the spotlight of NMR metabolomics. Food Res. Int. 2021, 150, 110805. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.J.B.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Prieto-Santiago, V.; del Mar Cavia, M.; Barba, F.J.; Alonso-Torre, S.R.; Carrillo, C.J.F.C. Multiple reaction monitoring for identification and quantification of oligosaccharides in legumes using a triple quadrupole mass spectrometer. Food Chem. 2022, 368, 130761. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frias, J.; Vidal-Valverde, C. Alpha-galactosides: Antinutritional factors or functional ingredients? Crit. Rev. Food Sci. Nutr. 2008, 48, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Hackbusch, S.; Franz, A.H. NMR solution geometry of saccharides containing the 6-O-(α-D-glucopyranosyl)-α/β-D-glucopyranose (isomaltose) or 6-O-(α-D-galactopyranosyl)-α/β-D-glucopyranose (melibiose) core. Carbohydr. Res. 2019, 473, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Schwachtje, J.; Fischer, A.; Erban, A.; Kopka, J.J.S.R. Primed primary metabolism in systemic leaves: A functional systems analysis. Sci. Rep. 2018, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Geissler, C. The New Oxford Book of Food Plants; OUP Oxford: Oxford, UK, 2009. [Google Scholar]
- Shan, L.; He, P. Pipped at the Post: Pipecolic Acid Derivative Identified as SAR Regulator. Cell 2018, 173, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Kim, D.; Bernsdorff, F.; Ajami-Rashidi, Z.; Scholten, N.; Schreiber, S.; Zeier, T.; Schuck, S.; Reichel-Deland, V.; Zeier, J. Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity. Plant Physiol. 2017, 174, 124–153. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Lim, G.-H.; de Lorenzo, L.; Yu, K.; Zhang, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 2018, 4, eaar4509. [Google Scholar] [CrossRef]
- Perera, T.; Young, M.R.; Zhang, Z.; Murphy, G.; Colburn, N.H.; Lanza, E.; Hartman, T.J.; Cross, A.J.; Bobe, G. Identification and monitoring of metabolite markers of dry bean consumption in parallel human and mouse studies. Mol. Nutr. Food Res. 2015, 59, 795–806. [Google Scholar] [CrossRef]
- Tramontano, W.A.; McGinley, P.A.; Ciancaglini, E.F.; Evans, L.S.J.E.; botany, e. A survey of trigonelline concentrations in dry seeds of the Dicotyledoneae. Environ. Exp. Bot. 1986, 26, 197–205. [Google Scholar] [CrossRef]
- Shimizu, M.; Mazzafera, P.J.P.B. A role for trigonelline during imbibition and germination of coffee seeds. Plant Biol. 2000, 2, 605–611. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A Plant Alkaloid with Therapeutic Potential for Diabetes and Central Nervous System Disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Tsiafoulis, C.; Papaemmanouil, C.; Alivertis, D.; Tzamaloukas, O.; Miltiadou, D.; Balayssac, S.; Malet-Martino, M.; Gerothanassis, I. NMR-Based Μetabolomics of the Lipid Fraction of Organic and Conventional Bovine Milk. Molecules 2019, 24, 1067. [Google Scholar] [CrossRef] [PubMed]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A.J.M. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 35. [Google Scholar] [CrossRef]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; de Freitas, V.; Soares, S.J.C.p. The role of wine polysaccharides on salivary protein-tannin interaction: A molecular approach. Carbohydr. Polym. 2017, 177, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zarin, M.A.; Wan, H.Y.; Isha, A.; Armania, N.J.F.S.; Wellness, H. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar] [CrossRef]
- Zeng, X.; Du, Z.; Sheng, Z.; Jiang, W.J.F.R.I. Characterization of the interactions between banana condensed tannins and biologically important metal ions (Cu2+, Zn2+ and Fe2+). Food Res. Int. 2019, 123, 518–528. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid Tests to Assess the Antioxidant Activity of Phaseolus vulgaris L. Dry Beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yuan, S.; Chang, S.J.J.O.F.S. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; McKey, D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. [2] Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Oxidants and Antioxidants Part A; Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 15–27. [Google Scholar]
- Ali, H.; El-Abeid, S.E.; Shaaban, S.A. Effect of some organic acids on growth, yield, oil production and enhancing anatomical changes to reduce fusarium wilt of Nigella sativa L. Plant Soil 2020, 20, 9231–9243. [Google Scholar]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Ingallina, C.; Spano, M.; Di Matteo, G.; Mannina, L. NMR-Based Approaches in the Study of Foods. Molecules 2022, 27, 7906. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Lam, S.; Shui, G. Metabolomics, a Powerful Tool for Agricultural Research. Int. J. Mol. Sci. 2016, 17, 1871. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Luthria, D. Recent applications of NMR in food and dietary studies. J. Sci. Food Agric. 2017, 97, 33–42. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid state 1H high field NMR in food analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef]
- de Falco, B.; Incerti, G.; Bochicchio, R.; Phillips, T.D.; Amato, M.; Lanzotti, V. Metabolomic analysis of Salvia hispanica seeds using NMR spectroscopy and multivariate data analysis. Ind. Crops Prod. 2017, 99, 86–96. [Google Scholar] [CrossRef]
- Wang, H.U.I.; Chen, J.; Feng, Y.U.N.; Zhou, W.; Zhang, J.; Yu, Y.U.; Wang, X.; Zhang, P. 1H nuclear magnetic resonance-based extracellular metabolomic analysis of multidrug resistant Tca8113 oral squamous carcinoma cells. Oncol. Lett. 2015, 9, 2551–2559. [Google Scholar] [CrossRef]
- Falasca, A.; Melck, D.; Paris, D.; Saviano, G.; Motta, A.; Iorizzi, M.J.M. Seasonal changes in the metabolic fingerprint of Juniperus communis L. berry extracts by 1 H NMR-based metabolomics. Metabolomics 2014, 10, 165–174. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Song, Y.-L.; Jing, W.-H.; Chen, Y.-G.; Yuan, Y.-F.; Yan, R.; Wang, Y.-T. 1H nuclear magnetic resonance based-metabolomic characterization of Peucedani Radix and simultaneous determination of praeruptorin A and praeruptorin B. J. Pharm. Biomed. Anal. 2014, 93, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Sullivan, H.; Wang, B.J.A.L. Principal component analysis (PCA) loading and statistical tests for nuclear magnetic resonance (NMR) metabolomics involving multiple study groups. Anal. Lett. 2022, 55, 1648–1662. [Google Scholar] [CrossRef]
- Falasca, A.; Caprari, C.; De Felice, V.; Fortini, P.; Saviano, G.; Zollo, F.; Iorizzi, M. GC-MS analysis of the essential oils of Juniperus communis L. berries growing wild in the Molise region: Seasonal variability and in vitro antifungal activity. Biochem. Syst. Ecol. 2016, 69, 166–175. [Google Scholar] [CrossRef]

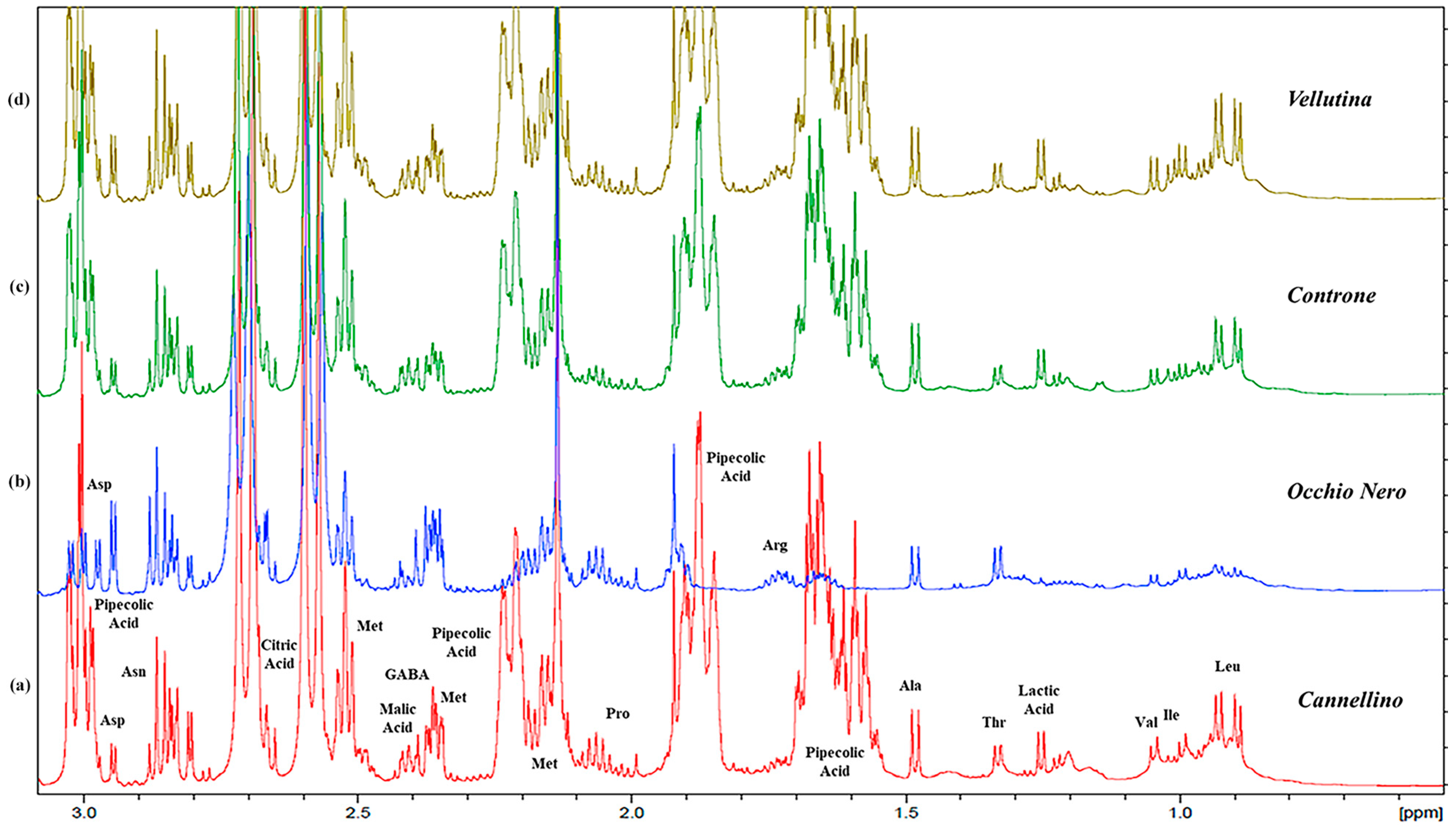
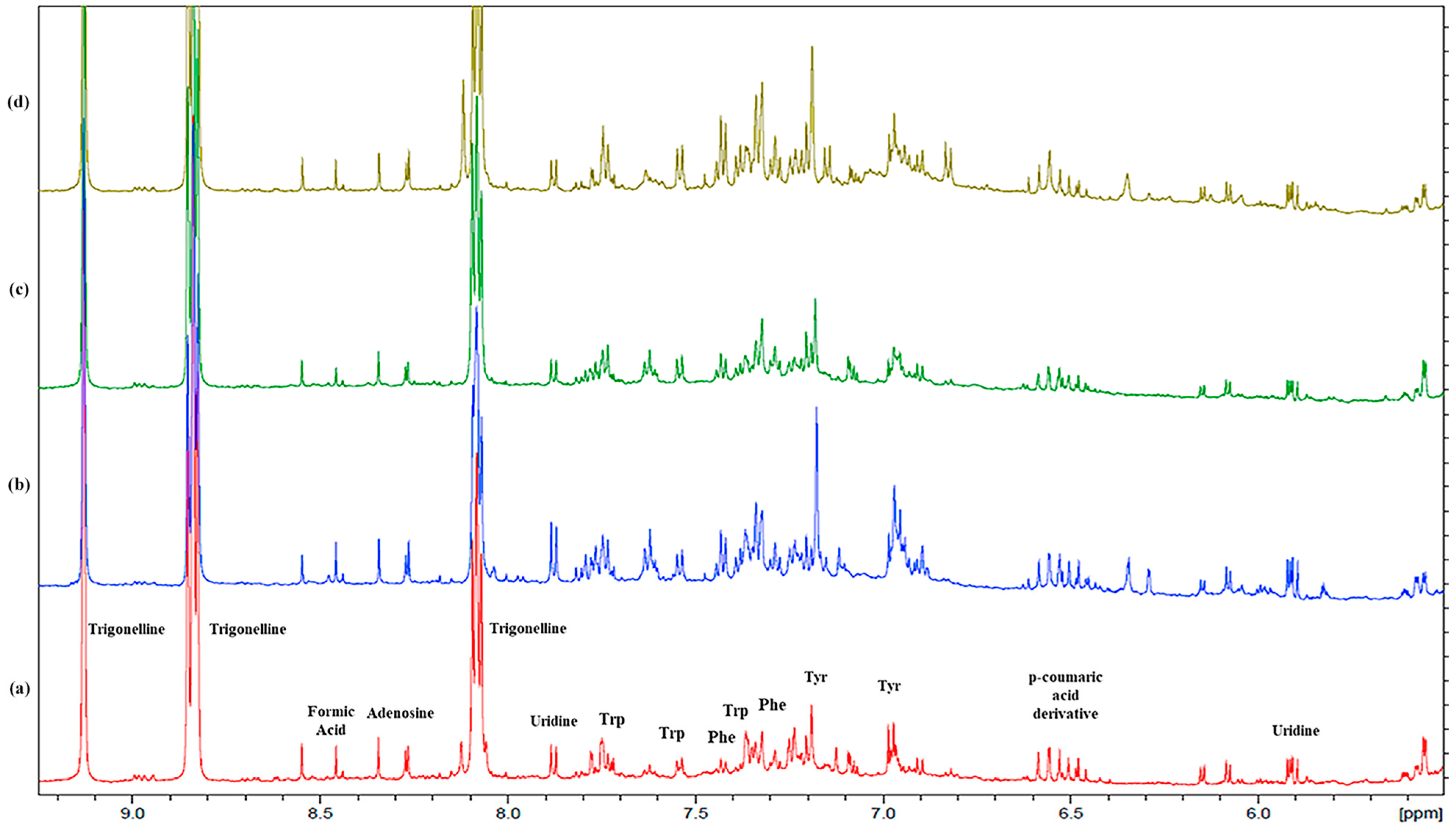
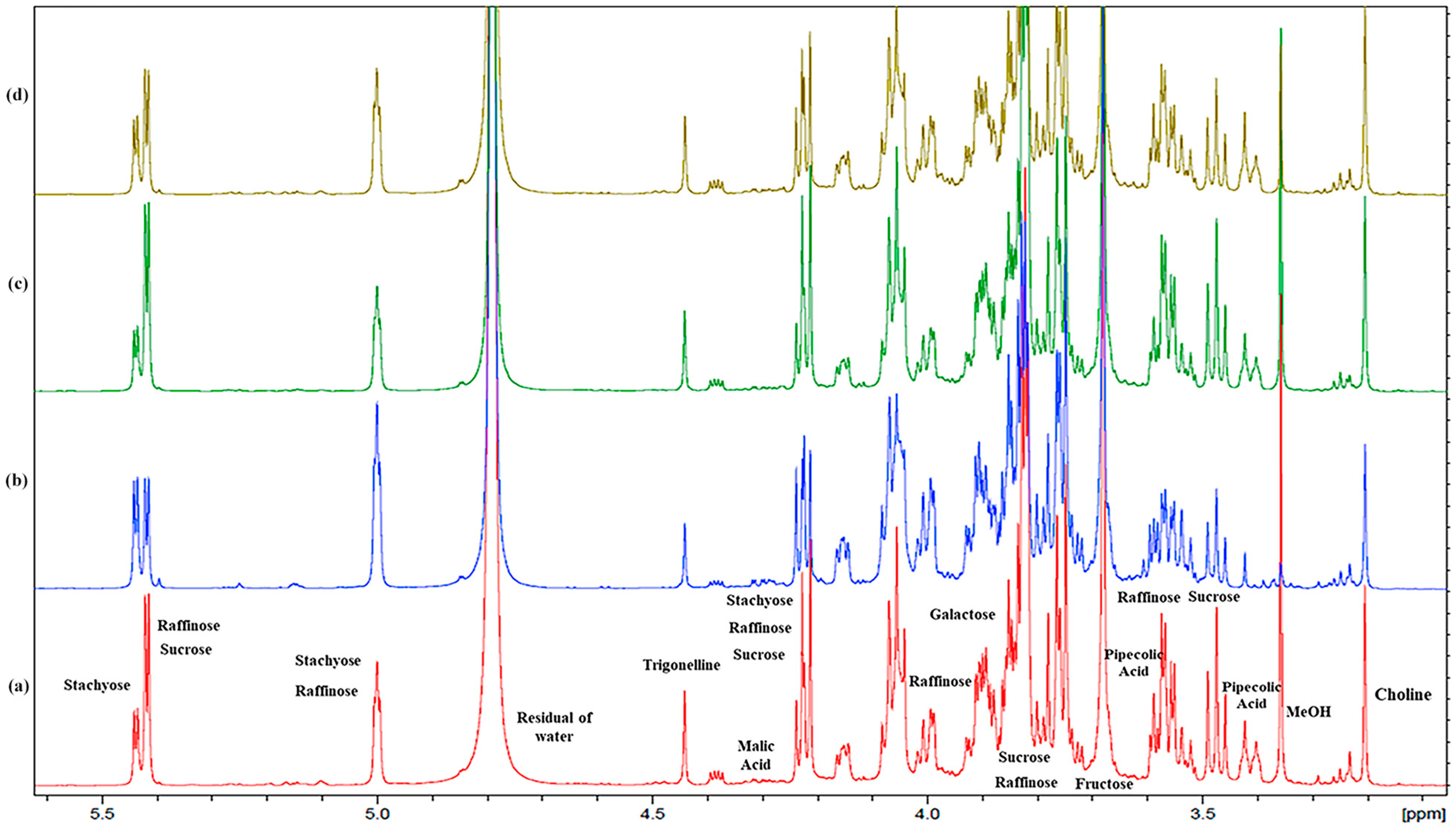

| Compound | Metabolite | Assignment | 1H (ppm) Multiplicity [J (Hz)] | 13C (ppm) | PON Occhio Nero |
|---|---|---|---|---|---|
| Aminoacids | |||||
| 1 | Alanine (Ala) | β-CH3 | 1.48 (d, 7) # | 16.00 | x |
| 2 | Arginine (Arg) | β,β′-CH2 γ-CH2 δ-CH2 | 1.91 m, 1.73 m 1.66 m # 3.23 (t, 7) | ||
| 3 | Asparagine (Asn) | β,β′-CH2 | 2.95 * 2.86 (dd, 7.3, 16.8) | 34.3 | 2.96 (dd, 4.3, 12.6) 2.87 (dd, 7.4, 16.8) |
| 4 | Aspartate (Asp) | β,β′-CH2 | 3.02 * 2.96 * | 35.6 | 3.01 (dd, 4.8, 17.3) # 2.95 * |
| 5 | γ-aminobutyrate (GABA) | α-CH2 γ-CH2 | 2.30 (t,7.3) 2.99 (t,7.3) | 34.4 35.9 | NOT PRESENT |
| 6 | Glutamate (Glu) | β,β′-CH2 γ-CH2 | 2.07 *; 2.14 * 2.36 m | x | |
| 7 | Isoleucine (Ile) | γ-CH3 | 1.01 (d, 7.3) # | 15.2 | x |
| 8 | Leucine (Leu) | δ-CH3 δ′-CH3 | 0.93 (d, 6.5) 0.90 (d, 6.5) # | 22.4 20.6 | |
| 9 | Methionine (Met) | α-CH β-CH2 γ-CH2 -S-CH3 | 2.52 * 2.38 * 2.17 * 2.14 s # | 33.4 26.6 14.5 | 2.52 t |
| 10 | Phenylalanine (Phe) | 2,6-CH 3,5-CH | 7.32 (d, 7.0) 7.42 (m) | - - | x |
| 11 | Proline (Pro) | γ-CH2 β-CH2 | 2.00 m 2.08 m | - - | |
| 12 | Threonine (Thr) | γ-CH3 α-CH β-CH | 1.33 (d, 6.0) # 3.51 * 4.23 * | 19.5 | x |
| 13 | Tyrosine (Tyr) | 2,6-CH 3,5-CH | 7.18 (d, 7.0) 6.89 (d, 7.0) # | - - | 7.16 6.90 |
| 14 | Tryptophan (Trp) | 7-CH 4-CH 2-CH α-CH β-CH2 | 7.54 (d, 8.0) # 7.74 (d, 8.0) 7.32 * 4.38 * 3.29 * | x | |
| 15 | Valine (Val) | γ-CH3 γ′-CH3 | 0.99 (d, 7) 1.05 (d, 7) # | 16.5 17.8 | x |
| Carbohydrates | |||||
| 16 | Sucrose | CH-1 (Glc) CH-2 (Glc) CH-3 (Glc) CH-4 (Glc) CH-3′ (Fru) CH-4′ (Fru) CH2-1′(Fru) CH-6′ (Fru) | 5.42 (d, 4.0) 3.57 3.76 (t) 3.47 (t, 9.6) # 4.23 (d, 9) 4.05 (t) 3.68 3.78 * | 92.7 69.0 76.3 74.0 61.6 | x |
| 17 | Raffinose | CH-1 (Glc) CH-1 (Gal) CH-3 (Fru) CH-3 (Glc) CH-5 (Glc) CH-5 (Gal) | 5.42 (d, 4.0) 5.00 (d, 4) 4.22 (d, 9) 3.77 (t) 4.04 * 4.01 * | 92.7 98.2 74.1 72.6 73.8 70.7 | x |
| 18 | Stachyose | CH-1 (Glc) CH-1 (Gal-T and Gal-I) CH-3 (Fru) CH2-1 (Fru) | 5.44 (d, 4) # 5.00 m 4.21 (d, 9) 3.68 | 92.0 98.3 76.3 61.6 | x |
| Organic acids | |||||
| 19 | Citric acid | α,γ-CH α’,γ′-CH | 2.58 (d, 16.0) # 2.70 (d, 16.0) | 44.5 | x |
| 20 | Formic acid | -CH | 8.44 s # | - | x |
| 21 | Lactic acid | -CH3 | 1.25 (d, 6.2) # | 22.8 | x |
| 22 | Malic acid | α-CH β-CH | 4.30 (dd, *) # 2.35 (dd, *) | 70.3 33.8 | x |
| 23 | Pipecolic acid (Pip) | CH-3,4,5 CH-3′,4′,5′ CH-6 CH-6′ CH-2 N-H | 2.22–1.67 m 1.73–1.87 m 3.00 (td, 12.5,3.2) 3.41 dd # 3.59 (dd, *) 2.17 m | 43.5 59.0 | NOT PRESENT |
| Other components | |||||
| 24 | Adenosine (Ade) | CH-1′ CH-2 CH-8 | 6.07 (d,5.5) 8.27 s 8.35 s # | x | |
| 25 | Choline (Cho) | -N(CH3)3+ | 3.21 s # | 53.6 | x |
| 26 | Guanosine (Gua) | CH-8 | 5.92 | x | |
| 27 | Uridine (Uri) | CH-6 | 5.91 d 7.87 d # | x | |
| 28 | Trigonelline (Tri) | H-2 H-4,6 H-5 -N-CH3 | 9.13 s # 8.83 m 8.09 m 4.44 s | 145.7 145.5 127.7 48.2 | x |
| 29 | p-coumaric acid derivative | 2,6-CH 3,5-CH CH=CH | 7.62 (d, 8.8) 6.97 (d,8.8) 7.79–6.51 (d, 16.1) | x | |
| Lipophilic extracts | |||||
| Fatty acids | |||||
| ω1-CH3 | 0.88 (t,7.5) | 13.5 | x | ||
| 30 | α-linolenic acid | ω3-CH3 | 0.97 (t, 7.5) | 14.2 | x |
| -(CH2)n- | 1.25, 1.30 | 29.0 | x | ||
| -CH2-CH2COO− | 1.61 | 24.4 | x | ||
| allylic-CH2 | 2.05 m | 27.1 | x | ||
| -CH2COO− | 2.31 | 34–35 | x | ||
| 31 | α-linolenic acid | bis-allylic -CH2 | 2.81 m | 24.9 | x |
| 32 | α-linoleic acid | bis-allylic -CH2 | 2.77 m | 25.1 | x |
| Glicerol | |||||
| TAG (glycerol backbone) | sn-1 and sn-3 | -CH2-O-CO- | 4.29 (dd, 11.9, 4.4) 4.15 (dd, 11.9, 5.7) | 61.8 | x |
| sn-2 | 2′-CH-O-CO- | 5.27 m | 68.1 | x | |
| sn-1,2/2,3 DAG | OH-CH2-CH- | 3.74 m | 62.2 | x | |
| sn-1,2 DAG | 1′ b-CH2-O-CO- | 4.36 dd | |||
| -CH=CH- | 5.37 m | 127.6 129.6 | x | ||
| SAMPLE | Total Phenolic | Flavonoids | Condensed Tannins |
|---|---|---|---|
| (mg GAE/g d.w.) | (mg QUE/g d.w.) | (mg CAE/g d.w.) | |
| PVEL | 1.35 ± 0.05 | 7.58 ± 0.13 | 2.12 ± 0.02 |
| PON | 1.11 ± 0.02 | 6.28 ± 0.40 | 1.22 ± 0.01 |
| PCON | 0.47 ± 0.01 | 2.65 ± 0.05 | 0.21 ± 0.01 |
| PCANN | 0.48 ± 0.02 | 2.72 ± 0.10 | 0.08 ± 0.01 |
| SAMPLE | DPPH | ABTS | FRAP |
|---|---|---|---|
| IC50 (mg/mL) | (μmol TE/mL) | (μmol TE/mL) | |
| PVEL | 1.43 ± 0.06 | 0.56 ± 0.06 | 15.18 ± 0.53 |
| PON | 2.04 ± 0.09 | 0.50 ± 0.04 | 12.86 ± 0.53 |
| PCON | 8.49 ± 0.23 | 0.17 ± 0.02 | 4.27 ± 0.23 |
| PCANN | 9.70 ± 0.37 | 0.18 ± 0.01 | 3.78 ± 0.15 |
| Correlation Coefficients (R) | Total Phenolic | Flavonoids | Condensed Tannin | DPPH | ABTS | FRAP |
|---|---|---|---|---|---|---|
| Total Phenolic | 1 | |||||
| Flavonoids | 1.000 ** | 1 | ||||
| Condensed Tannin | 0.983 * | 0.982 * | 1 | |||
| DPPH | −0.979 * | −0.979 * | −0.942 * | 1 | ||
| ABTS | 0.993 ** | 0.994 ** | 0.957 * | −0.991 ** | 1 | |
| FRAP | 0.985 * | 0.985 * | 0.963 * | −0.997 ** | 0.988 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samukha, V.; Fantasma, F.; D’Urso, G.; Caprari, C.; De Felice, V.; Saviano, G.; Lauro, G.; Casapullo, A.; Chini, M.G.; Bifulco, G.; et al. NMR Metabolomics and Chemometrics of Commercial Varieties of Phaseolus vulgaris L. Seeds from Italy and In Vitro Antioxidant and Antifungal Activity. Plants 2024, 13, 227. https://doi.org/10.3390/plants13020227
Samukha V, Fantasma F, D’Urso G, Caprari C, De Felice V, Saviano G, Lauro G, Casapullo A, Chini MG, Bifulco G, et al. NMR Metabolomics and Chemometrics of Commercial Varieties of Phaseolus vulgaris L. Seeds from Italy and In Vitro Antioxidant and Antifungal Activity. Plants. 2024; 13(2):227. https://doi.org/10.3390/plants13020227
Chicago/Turabian StyleSamukha, Vadym, Francesca Fantasma, Gilda D’Urso, Claudio Caprari, Vincenzo De Felice, Gabriella Saviano, Gianluigi Lauro, Agostino Casapullo, Maria Giovanna Chini, Giuseppe Bifulco, and et al. 2024. "NMR Metabolomics and Chemometrics of Commercial Varieties of Phaseolus vulgaris L. Seeds from Italy and In Vitro Antioxidant and Antifungal Activity" Plants 13, no. 2: 227. https://doi.org/10.3390/plants13020227
APA StyleSamukha, V., Fantasma, F., D’Urso, G., Caprari, C., De Felice, V., Saviano, G., Lauro, G., Casapullo, A., Chini, M. G., Bifulco, G., & Iorizzi, M. (2024). NMR Metabolomics and Chemometrics of Commercial Varieties of Phaseolus vulgaris L. Seeds from Italy and In Vitro Antioxidant and Antifungal Activity. Plants, 13(2), 227. https://doi.org/10.3390/plants13020227









